Abstract
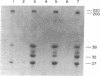
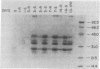
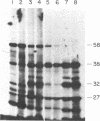
Feeding of sorghum with a high level of tannin (high-tannin sorghum) to rats caused changes in gene expression in parotid glands similar to isoproterenol treatment. Within 3 days the parotid glands were enlarged about 3-fold and a series of proline-rich proteins were increased about 12-fold. Unlike isoproterenol treatment, no changes were observed in the submandibular glands, and a Mr 220,000 glycoprotein in parotid glands was not induced. Amino acid analyses, electrophoretic patterns, and cell-free translations of mRNAs all confirmed that the proline-rich proteins induced by feeding high-tannin sorghum were identical to those induced by isoproterenol treatment. Binding curves for proline-rich proteins to tannins showed affinities 10-fold greater than bovine serum albumin and tannins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennick A., Connell G. E. Purification and partial characterization of four proteins from human parotid saliva. Biochem J. 1971 Jul;123(3):455–464. doi: 10.1042/bj1230455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A. Salivary proline-rich proteins. Mol Cell Biochem. 1982 Jun 11;45(2):83–99. doi: 10.1007/BF00223503. [DOI] [PubMed] [Google Scholar]
- Cousins B. W., Tanksley T. D., Jr, Knabe D. A., Zebrowska T. Nutrient digestibility and performance of pigs fed sorghums varying in tannin concentration. J Anim Sci. 1981 Dec;53(6):1524–1537. doi: 10.2527/jas1982.5361524x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sorensen A., Carlson D. M. Isolation of a "proline-rich" protein from rat parotid glands following isoproterenol treatment. Biochem Biophys Res Commun. 1974 Sep 9;60(1):249–256. doi: 10.1016/0006-291x(74)90198-3. [DOI] [PubMed] [Google Scholar]
- Hagerman A. E., Butler L. G. Condensed tannin purification and characterization of tannin-associated proteins. J Agric Food Chem. 1980 Sep-Oct;28(5):947–952. doi: 10.1021/jf60231a011. [DOI] [PubMed] [Google Scholar]
- Hagerman A. E., Butler L. G. The specificity of proanthocyanidin-protein interactions. J Biol Chem. 1981 May 10;256(9):4494–4497. [PubMed] [Google Scholar]
- Harding J. D., Przybyla A. E., MacDonald R. J., Pictet R. L., Rutter W. J. Effects of dexamethasone and 5-bromodeoxyuridine on the synthesis of amylase mRNA during pancreatic development in vitro. J Biol Chem. 1978 Oct 25;253(20):7531–7537. [PubMed] [Google Scholar]
- Hartman B. K., Udenfriend S. A method for immediate visualization of proteins in acrylamide gels and its use for preparation of antibodies to enzymes. Anal Biochem. 1969 Sep;30(3):391–394. doi: 10.1016/0003-2697(69)90132-8. [DOI] [PubMed] [Google Scholar]
- Isemura S., Saitoh E., Sanada K. Fractionation and characterization of basic proline-rich peptides of human parotid saliva and the amino acid sequence of proline-rich peptide P-E. J Biochem. 1982 Jun;91(6):2067–2075. doi: 10.1093/oxfordjournals.jbchem.a133900. [DOI] [PubMed] [Google Scholar]
- Jabbal I., Kells D. I., Forstner G., Forstner J. Human intestinal goblet cell mucin. Can J Biochem. 1976 Aug;54(8):707–716. doi: 10.1139/o76-102. [DOI] [PubMed] [Google Scholar]
- Jambunathan R., Mertz E. T. Relationship between tannin levels, rat growth, and distribution of proteins in sorghum. J Agric Food Chem. 1973 Jul-Aug;21(4):692–696. doi: 10.1021/jf60188a027. [DOI] [PubMed] [Google Scholar]
- Kauffman D. L., Keller P. J. The basic proline-rich proteins in human parotid saliva from a single subject. Arch Oral Biol. 1979;24(4):249–256. doi: 10.1016/0003-9969(79)90085-2. [DOI] [PubMed] [Google Scholar]
- Kauffman D., Wong R., Bennick A., Keller P. Basic proline-rich proteins from human parotid saliva: complete covalent structure of protein IB-9 and partial structure of protein IB-6, members of a polymorphic pair. Biochemistry. 1982 Dec 7;21(25):6558–6562. doi: 10.1021/bi00268a036. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Muenzer J., Bildstein C., Gleason M., Carlson D. M. Properties of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979 Jul 10;254(13):5629–5634. [PubMed] [Google Scholar]
- Muenzer J., Bildstein C., Gleason M., Carlson D. M. Purification of proline-rich proteins from parotid glands of isoproterenol-treated rats. J Biol Chem. 1979 Jul 10;254(13):5623–5628. [PubMed] [Google Scholar]
- Oppenheim F. G., Hay D. I., Franzblau C. Proline-rich proteins from human parotid saliva. I. Isolation and partial characterization. Biochemistry. 1971 Nov;10(23):4233–4238. doi: 10.1021/bi00799a013. [DOI] [PubMed] [Google Scholar]
- Schultz J. C., Baldwin I. T. Oak leaf quality declines in response to defoliation by gypsy moth larvae. Science. 1982 Jul 9;217(4555):149–151. doi: 10.1126/science.217.4555.149. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Auffret A. D., Carne A., Gurnett A., Hanisch P., Hill D., Saraste M. Solid-phase sequence analysis of polypeptides eluted from polyacrylamide gels. An aid to interpretation of DNA sequences exemplified by the Escherichia coli unc operon and bacteriophage lambda. Eur J Biochem. 1982 Apr 1;123(2):253–260. doi: 10.1111/j.1432-1033.1982.tb19761.x. [DOI] [PubMed] [Google Scholar]
- Wong R. S., Bennick A. The primary structure of a salivary calcium-binding proline-rich phosphoprotein (protein C), a possible precursor of a related salivary protein A. J Biol Chem. 1980 Jun 25;255(12):5943–5948. [PubMed] [Google Scholar]
- Ziemer M. A., Mason A., Carlson D. M. Cell-free translations of proline-rich protein mRNAs. J Biol Chem. 1982 Sep 25;257(18):11176–11180. [PubMed] [Google Scholar]