Abstract
Substantial experimental evidence suggests the usefulness of antioxidants for the treatment of various forms of pulmonary hypertension. However, no recommendations have yet been made if patients with pulmonary hypertension should receive pharmacologic and/or dietary antioxidants. Our understanding of antioxidants has evolved greatly over the last two decades, from the primitive use of natural antioxidant vitamins to the modulation of vascular oxidases, such as NAD(P)H oxidases. These oxidases and their products not only regulate pulmonary vascular tone and intimal and smooth muscle thickening, but also modulate the adaptation of the right ventricle to increased afterload. It is important that well-designed randomized clinical trials be conducted to test the importance of oxidase-reactive oxygen species activation in the pathogenesis and treatment of pulmonary hypertension. The purpose of this Forum on Pulmonary Hypertension is to summarize the available preclinical information, which may aid in designing and conducting future randomized clinical trials for evaluating the efficacy of antioxidants for the treatment of pulmonary hypertension. The complexity of oxidative pathways contributed to the tremendous difficulties and challenges in selecting agents, doses, and designing clinical trials. Further studies using human, animal, and cell culture models may be needed to define optimal trials. This Forum on Pulmonary Hypertension should stimulate new thinking and provide essential background information to better define the challenges of developing successful randomized clinical trials in the near future. Antioxid. Redox Signal. 18, 1723–1726.
Many studies suggest a pathogenic role for reactive oxygen species (ROS) and oxidative stress in pulmonary arterial hypertension (PAH). Experimental animal models have demonstrated the effectiveness of various antioxidant therapeutics in modulating PAH development and their progression. Moreover, PAH patients have increased oxidative stress and upregulation of critical oxidase enzyme systems. Despite substantial experimental evidence suggesting the usefulness of antioxidants for the treatment of pulmonary hypertension at various aspects of this disease (Fig. 1), it is unclear if patients with pulmonary hypertension should receive pharmacologic and/or dietary antioxidants. Furthermore, our conceptual understanding of antioxidants has evolved greatly over the last two decades, from the primitive use of natural antioxidant vitamins, with very low radical reactivities, to the current emphasis on the modulation of vascular oxidases, such as xanthine oxidase, NAD(P)H oxidases, and pathological mitochondrial ROS formation. These oxidases and their products appear to regulate not only pulmonary vascular tone and maladaptive intimal and smooth muscle proliferation, but also modulate the adaptation of the right ventricle to chronically increased afterload stress. It is important that well-designed randomized clinical trials be conducted to test the importance of oxidase-ROS activation in the pathogenesis and treatment of PAH. The purpose of this Antioxidants and Redox Signaling Forum on Pulmonary Hypertension is to summarize the available preclinical information, which may aid in designing and conducting future randomized clinical trials for evaluating the efficacy of antioxidants for the treatment of pulmonary hypertension.
FIG. 1.
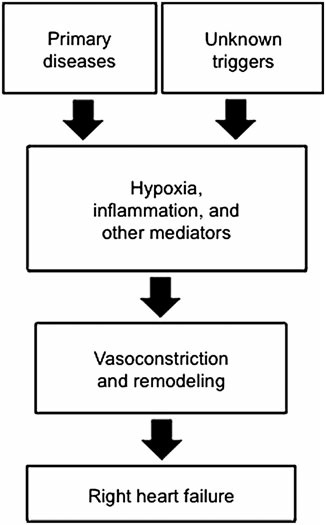
Potential therapeutic targets of antioxidants for the treatment of pulmonary hypertension. Reactive oxygen species are involved in all of these steps for the development and progression of various forms of pulmonary hypertension and right heart failure, suggesting the usefulness of antioxidants as therapeutic agents.
Because of the complexity associated with primary lung and heart diseases which cause secondary pulmonary hypertension, samples from idiopathic PAH (IPAH) patients are often used to investigate biological changes that are more directly associated with pulmonary hypertension. Several studies have identified oxidative modifications of DNA, lipids, and proteins in IPAH patients, providing strong evidence for the occurrence of oxidative stress in these patients (7, 9). It is reasonable to suggest that correcting this altered redox balance may be beneficial and may normalize the pathological conditions associated with IPAH, which is a devastating disease that has a median survival of ∼5 years. However, to date, no recommendations on whether patients with IPAH should take dietary antioxidant supplements have been provided by the medical and scientific community. Similarly, new specific oxidase inhibitors are now available but have not been tested, such as febuxistat, a specific inhibitor of xanthine oxidase used for gout and the 3-hydroxy-3-methyl-glutaryl-co-enzyme A reductase inhibitors (statins) which have broad effects on modulating inflammation and activation of vascular oxidases. Whether a pharmacologic approach to alter various stages of ROS generation by scavenging or direct inhibition of specific oxidases will modulate clinical human PAH remains unknown.
Preclinical animal models of pulmonary hypertension, which provide strong evidence for the effectiveness of antioxidants, have largely relied on the use of the mouse or rat chronic hypoxia models. These models most closely resemble human pulmonary hypertension associated with chronic hypoxic lung diseases, such as pulmonary fibrosis and chronic obstructive pulmonary disease (COPD). However, important limitations to these models include the reversibility of chronic hypoxic effects after exposure to normoxic conditions and the lack of severe obliterative plexogenic vasculopathy, the pathological hallmark of the human disease PAH. Pulmonary hypertension that develops secondary to chronic hypoxic pulmonary disease significantly worsens the prognosis of these primary diseases. Thus, at the time of initial diagnosis of the primary disease, treating patients with agents that can prevent pulmonary hypertension and pulmonary vascular remodeling may increase the survival of these patients. Published results using the chronic hypoxia model of pulmonary hypertension in animals (9) have provided strong evidence that antioxidants are useful in such a treatment strategy. However, whether these patients should receive dietary and/or pharmacologic antioxidants has not been defined. Furthermore, more relevant animal models that more closely resemble human diseases are needed.
Antioxidants have been previously considered for the treatment of primary diseases associated with pulmonary hypertension. For example, the Idiopathic Pulmonary Fibrosis International Group Exploring N-Acetylcysteine I Annual (IFIGENIA) trial suggested that the addition of N-acetylcysteine to standard therapy with prednisone plus azathioprine may be beneficial for the treatment of idiopathic pulmonary fibrosis. However, a recent report on the randomized, double-blind, placebo-controlled Prednisone, Azathioprine, and N-Acetylcysteine: A Study That Evaluates Response in Idiopathic Pulmonary Fibrosis (PANTHER-IPF) trial indicates that a combination of prednisone, azathioprine, and N-acetylcysteine increases the risk of death and hospitalization. While N-acetylcysteine is a very poor chemical ROS scavenger and antioxidant, and the effects of N-acetylcysteine alone have not yet been reported, such studies will likely discourage the use of antioxidants for the treatment of PAH. New therapies that directly target oxidase enzymes or enzymatically scavenge ROS are likely to more directly address the role of ROS in PAH pathogenesis.
In contrast to the forms of pulmonary hypertension that affect adult patients, persistent pulmonary hypertension of the newborn (PPHN) is a disease for which inhaled nitric oxide (NO) has been demonstrated to decrease the need for extracorporeal membrane oxygenation in newborns with PPHN (4). This treatment, however, has not reduced mortality or neurodevelopment morbidity in critically ill infants with hypoxic respiratory failure. Furthermore, up to 40% of infants do not respond or sustain a response to inhaled NO (4), an effect that may in part be mediated by the NO scavenging effects of ROS, which can divert NO from therapeutic signaling to deleterious reactive nitrogen species injury (with formation of nitrogen dioxide or peroxynitrite, for example). As supported by published reports, including some of the articles in this Forum of Pulmonary Hypertension, antioxidants may be particularly promising for the treatment of neonatal pulmonary hypertension, including PPHN (8) as well as pulmonary hypertension associated with bronchopulmonary dysplasia, which is emerging as a relatively common complication of preterm birth (2). The currently available data support the clear need for randomized clinical trials to investigate antioxidant therapies for these populations of patients.
Many inflammatory diseases, such as scleroderma, HIV infection, and lung diseases of smokers like COPD, are associated with the development of pulmonary hypertension. Since inflammation and smoking are known to cause oxidative stress, antioxidants could be beneficial for these patient populations. However, no recommendations have yet been provided for the use of antioxidants.
The major cause of death among patients with pulmonary hypertension is right heart failure. In addition to the role of ROS in pathogenesis of pulmonary vasculopathy that leads to pulmonary hypertension, animal studies also point to the importance of ROS production and the reduction of antioxidant defenses in the transition from cardiac hypertrophy to right heart failure. Two review articles in this Forum discuss a potential role for antioxidant therapy in preventing or reversing right heart failure (7, 9). Because many patients, particularly those with IPAH, already have developed a significant degree of pulmonary hypertension at the time of diagnosis, the preventative use of antioxidants for this patient group may not be realistic. However, the antioxidant supplementation and/or pharmacologic treatment with redox agents may play critical roles in reducing the death of PAH patients from right heart failure. Therefore, randomized clinical trials are needed to assess if the incidence of right heart failure and death may be influenced by antioxidants.
Considering the central role of ROS and oxidase activation in pulmonary vasculopathy and right heart failure, which ROS species and oxidases should be targeted for specific therapy? To address this question, we should first define and classify the available antioxidant strategies (Table 1), which can target various components of oxidative pathways (Fig. 2). Classical endogenous catalytic antioxidant enzymes include superoxide dismutase, catalase, and glutathione peroxidase. More recently, enzymes, such as peroxiredoxin and thioredoxin have been identified as important antioxidants systems as well. The activities of these enzymes can be mimicked by designing small molecules, such as a superoxide dismutase mimetics, which are discussed in this Forum of Pulmonary Hypertension (6). Small molecule antioxidants that we consume in our normal diet traditionally include vitamin E, vitamin C, beta-carotene, and selenium. However, there are hundreds of other agents that are considered to have “free radical scavenging” activities, such as flavonoids, resveratrol, and lipoic acid. Many of these antioxidants are defined as nutraceuticals, rather than pharmaceuticals. Agents that can prevent the formation of ROS would also qualify as “antioxidants” for the purpose of treating pulmonary hypertension. A number of studies have suggested that NAD(P)H oxidase plays critical roles in both vasoconstrictive and remodeling aspects of pulmonary hypertension (3, 8). Agents that may increase NO, such as nitrite (1) and l-arginine (5), should also ultimately act as antioxidants. Furthermore, agents that may activate a panel of antioxidant defenses through mechanisms involving molecules, such as Nrf2 could also be considered as “antioxidants.”
Table 1.
Classifications of Antioxidant Strategies for the Treatment of Pulmonary Hypertension
| Enzymatic ROS scavengers and regulators |
| Superoxide dismutase |
| Catalase |
| Glutathione peroxidase |
| Glutathione reductase |
| Glutaredoxin |
| Thioredoxin |
| Thioredoxin reductase |
| Peroxiredoxin |
| Sulfiredoxin |
| Small chemical ROS scavengers |
| Dietary antioxidants, such as ascorbic acid, tocopherols, carotenoids, selenium, flavonoids, resveratrol, and lipoic acid |
| Gases, such as NO, H2, H2S, and CO |
| Antioxidant enzyme mimetics, such as MnTE-2-PyP, tempol, and ebselen |
| Inhibitors of ROS generation |
| Inhibitor of oxidases, such as NAD(P)H oxidase, xanthine oxidase, monoamine oxidase |
| Iron chelators |
| Nrf2 activators |
ROS, reactive oxygen species.
FIG. 2.
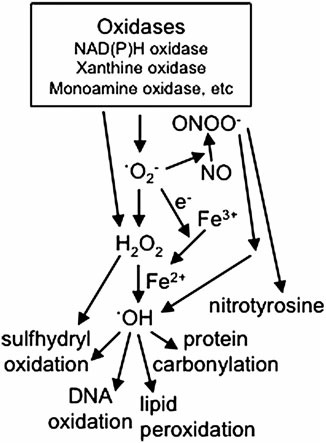
Potential molecular targets for the treatment of pulmonary hypertension. These molecular components can be targeted to inhibit the actions mediated by ROS. These include inhibitors of oxidases which generate ROS. ROS include •O2−, H2O2, •OH, and ONOO−. Some oxidases perform one-electron reduction to form .O2−, and others perform two-electron reduction to directly generate H2O2. Scavengers of ROS are good candidates for treatment strategies. H2O2 is formed by two .O2− molecules interacting with each other in a reaction called dismutation, that can be catalyzed by SOD. The success of SOD in experimental models to inhibit pulmonary hypertension suggests that the role of .O2− is not just serving as substrates for dismutation to produce H2O2. •OH can be generated by Fenton reaction that is catalyzed by ferrous iron (Fe2+), which can be produced by the reduction of ferric iron (Fe3+) by .O2−. •O2− also interact with NO and in turn decreases the availability of NO and produces ONOO− which could also produce •OH. •OH participates in oxidation of proteins, lipids, and DNA. Iron chelators can inhibit the production of .OH. •O2−, superoxide; •OH, hydroxyl radical; H2O2, hydrogen peroxide; NO, nitric oxide; ONOO−, peroxynitrite; ROS, reactive oxygen species; SOD, superoxide dismutase.
ROS include superoxide, hydrogen peroxide, hydroxyl radical, peroxynitrite, singlet oxygen, and others. Which ROS should be targeted to treat pulmonary hypertension? The answer to this question may well depend on the forms and stages of pulmonary hypertension. We should also note that the concentrations of ROS are also of importance, with low levels of hydrogen peroxide likely serving a homeostatic signaling role, and higher levels driving harmful Fenton and peroxidase chemistry. Such complexity may have contributed to the tremendous difficulties and challenges in selecting agents, doses and designing successful clinical trials. Further studies using human, animal, and cell culture models may be needed to define optimal randomized clinical trials that can address this important question on whether antioxidants should be given to patients with pulmonary hypertension. We hope that this Forum on Pulmonary Hypertension has stimulated new thinking, provided essential background information, and better defined the challenges we face, all of which may contribute to developing well-designed randomized clinical trials in the near future.
Abbreviations Used
- COPD
chronic obstructive pulmonary disease
- H2O2
hydrogen peroxide
- IPAH
idiopathic PAH
- NO
nitric oxide
- •O2−
superoxide
- •OH
hydroxyl radical
- ONOO−
peroxynitrite
- PAH
pulmonary arterial hypertension
- PPHN
persistent pulmonary hypertension of the newborn
- ROS
reactive oxygen species
- SOD
superoxide dismutase
References
- 1.Bueno M. Wang J. Mora AL. Gladwin MT. Nitrite signaling in pulmonary hypertension: mechanisms of bioactivation, signaling and therapeutics. Antioxid Redox Signal. 2013;18:1797–1809. doi: 10.1089/ars.2012.4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fike CD. Dikalova A. Slaughter JC. Kaplowitz MR. Zhang Y. Aschner JL. Reactive oxygen species reducing strategies improve pulmonary arterial responses to nitric oxide in piglets with chronic hypoxia-induced pulmonary hypertension. Antioxid Redox Signal. 2013;18:1727–1738. doi: 10.1089/ars.2012.4823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norton CE. Broughton BRS. Jernigan NL. Walker BR. Resta TC. Enhanced depolarization-induced pulmonary vasoconstriction following chronic hypoxia requires EGFR-dependent activation of NAD(P)H oxidase 2. Antioxid Redox Signal. 2013;18:1777–1788. doi: 10.1089/ars.2012.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med. 2010;11:S79–S84. doi: 10.1097/PCC.0b013e3181c76cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sun X. Sharma S. Fratz S. Kumar S. Rafikov R. Aggarwal S. Rafikova O. Lu Q. Burns T. Dasarathy S. Wright J. Schreiber C. Radman M. Fineman JR. Black SM. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid Redox Signal. 2013;18:1739–1752. doi: 10.1089/ars.2012.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Villegas LR. Kluck D. Field C. Oberley-Deegan RE. Woods C. Yeager ME. El Kasmi KC. Savani RC. Bowler RP. Nozik-Grayck E. Superoxide dismutase mimetic, MnTE-2-PyP, attenuates chronic hypoxia-induced pulmonary hypertension, pulmonary vascular remodeling, and activation of the NALP3 inflammasome. Antioxid Redox Signal. 2013;18:1753–1764. doi: 10.1089/ars.2012.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Voelkel NF. Bogaard HJ. al Husseini A. Farkas L. Gomez-Arroyo J. Natarajan R. Antioxidants for the treatment of patients with severe angioproliferative pulmonary hypertension? Antioxid Redox Signal. 2013;18:1810–1817. doi: 10.1089/ars.2012.4828. [DOI] [PubMed] [Google Scholar]
- 8.Wedgwood S. Lakshminrusimha S. Czech L. Schumacker PT. Steinhorn RH. Increased p22phox/Nox4 expression is involved in remodeling through hydrogen peroxide signaling in experimental persistent pulmonary hypertension of the newborn. Antioxid Redox Signal. 2013;18:1765–1776. doi: 10.1089/ars.2012.4766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong C. Bansal G. Pavlickova L. Marcocci L. Suzuki YJ. Reactive oxygen species and antioxidants in pulmonary hypertension. Antioxid Redox Signal. 2013;18:1789–1796. doi: 10.1089/ars.2012.4568. [DOI] [PMC free article] [PubMed] [Google Scholar]


