Abstract
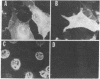
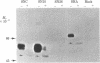
We have replaced the late genes of simian virus 40 (SV40) with a cloned cDNA copy of the neuraminidase (NA; EC 3.2.1.18) gene of the WSN (H1N1) strain of human influenza virus. When the SV40-NA recombinant virus was complemented in a lytic infection of monkey cells with a helper virus containing an early region deletion mutant, influenza NA was expressed and readily detected by immunofluorescence as well as by immunoprecipitation of in vivo labeled proteins with monoclonal antibodies against NA. In addition, the expressed NA exhibited enzymatic activity by cleaving the sialic acid residue from alpha-2,3-sialyllactitol. The expressed protein was glycosylated and transported to the cell surface, and it possessed the same molecular weight as the NA of WSN virus grown in monkey cells. Because the structure of NA is quite different from that of other integral membrane proteins and includes an anchoring region at the NH2 terminus consisting of hydrophobic amino acids, we also constructed deletion mutants of NA in this region. Replacement of DNA coding for the first 10 NH2-terminal amino acids with SV40 and linker sequences had no apparent effect on NA expression, glycosylation, transport to the cell surface, or enzymatic activity. However, further deletion of NA DNA encoding the first 26 amino acids abolished NA expression. These data suggest that the hydrophobic NH2-terminal region is multifunctional and is important in biosynthesis and translocation of NA across the membrane as well as in anchoring the protein.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blok J., Air G. M. Comparative nucleotide sequences at the 3' end of the neuraminidase gene from eleven influenza type A viruses. Virology. 1980 Nov;107(1):50–60. doi: 10.1016/0042-6822(80)90271-8. [DOI] [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Brunner J., Hauser H., Braun H., Wilson K. J., Wacker H., O'Neill B., Semenza G. The mode of association of the enzyme complex sucrase.isomaltase with the intestinal brush border membrane. J Biol Chem. 1979 Mar 25;254(6):1821–1828. [PubMed] [Google Scholar]
- Bucher D. J., Kilbourne E. D. A 2 (N2) neuraminidase of the X-7 influenza virus recombinant: determination of molecular size and subunit composition of the active unit. J Virol. 1972 Jul;10(1):60–66. doi: 10.1128/jvi.10.1.60-66.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A. R., Bos T., Ueda M., Nayak D. P., Dowbenko D., Compans R. W. Immune response to human influenza virus hemagglutinin expressed in Escherichia coli. Gene. 1983 Mar;21(3):273–284. doi: 10.1016/0378-1119(83)90011-2. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Hiti A. L., Nayak D. P. Construction and characterization of a bacterial clone containing the hemagglutinin gene of the WSN strain (HON1) of influenza virus. Gene. 1980 Aug;10(3):205–218. doi: 10.1016/0378-1119(80)90050-5. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Nayak D. P., Ueda M., Hiti A. L., Dowbenko D., Kleid D. G. Expression of antigenic determinants of the hemagglutinin gene of a human influenza virus in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5376–5380. doi: 10.1073/pnas.78.9.5376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Frisch A., Neufeld E. F. A rapid and sensitive assay for neuraminidase: application to cultured fibroblasts. Anal Biochem. 1979 May;95(1):222–227. doi: 10.1016/0003-2697(79)90209-4. [DOI] [PubMed] [Google Scholar]
- Gething M. J., Sambrook J. Construction of influenza haemagglutinin genes that code for intracellular and secreted forms of the protein. Nature. 1982 Dec 16;300(5893):598–603. doi: 10.1038/300598a0. [DOI] [PubMed] [Google Scholar]
- Hartman J. R., Nayak D. P., Fareed G. C. Human influenza virus hemagglutinin is expressed in monkey cells using simian virus 40 vectors. Proc Natl Acad Sci U S A. 1982 Jan;79(2):233–237. doi: 10.1073/pnas.79.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M., Halegoua S. Secretion and membrane localization of proteins in Escherichia coli. CRC Crit Rev Biochem. 1980;7(4):339–371. doi: 10.3109/10409238009105465. [DOI] [PubMed] [Google Scholar]
- Jay G., Nomura S., Anderson C. W., Khoury G. Identification of the SV40 agnogene product: a DNA binding protein. Nature. 1981 May 28;291(5813):346–349. doi: 10.1038/291346a0. [DOI] [PubMed] [Google Scholar]
- Klenk H. D., Rott R. Cotranslational and posttranslational processing of viral glycoproteins. Curr Top Microbiol Immunol. 1980;90:19–48. doi: 10.1007/978-3-642-67717-5_2. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Haslam E. A., White D. O. The polypeptides of influenza virus. VI. Composition of the neuraminidase. Virology. 1972 Sep;49(3):758–765. doi: 10.1016/0042-6822(72)90532-6. [DOI] [PubMed] [Google Scholar]
- Lowden J. A., O'Brien J. S. Sialidosis: a review of human neuraminidase deficiency. Am J Hum Genet. 1979 Jan;31(1):1–18. [PMC free article] [PubMed] [Google Scholar]
- Markoff L., Lai C. J. Sequence of the influenza A/Udorn/72 (H3N2) virus neuraminidase gene as determined from cloned full-length DNA. Virology. 1982 Jun;119(2):288–297. doi: 10.1016/0042-6822(82)90089-7. [DOI] [PubMed] [Google Scholar]
- Maroux S., Louvard D. On the hydrophobic part of aminopeptidase and maltases which bind the enzyme to the intestinal brush border membrane. Biochim Biophys Acta. 1976 Jan 21;419(2):189–195. doi: 10.1016/0005-2736(76)90345-x. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Mertz J. E., Berg P. Defective simian virus 40 genomes: isolation and growth of individual clones. Virology. 1974 Nov;62(1):112–124. doi: 10.1016/0042-6822(74)90307-9. [DOI] [PubMed] [Google Scholar]
- Nayak D. P., Tobita K., Janda J. M., Davis A. R., De B. K. Homologous interference mediated by defective interfering influenza virus derived from a temperature-sensitive mutant of influenza virus. J Virol. 1978 Oct;28(1):375–386. doi: 10.1128/jvi.28.1.375-386.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palese P., Tobita K., Ueda M., Compans R. W. Characterization of temperature sensitive influenza virus mutants defective in neuraminidase. Virology. 1974 Oct;61(2):397–410. doi: 10.1016/0042-6822(74)90276-1. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Expression from cloned cDNA of cell-surface secreted forms of the glycoprotein of vesicular stomatitis virus in eucaryotic cells. Cell. 1982 Oct;30(3):753–762. doi: 10.1016/0092-8674(82)90280-x. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman J. L., Palese P. Virulence factors of influenza A viruses: WSN virus neuraminidase required for plaque production in MDBK cells. J Virol. 1977 Oct;24(1):170–176. doi: 10.1128/jvi.24.1.170-176.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw M. W., Lamb R. A., Erickson B. W., Briedis D. J., Choppin P. W. Complete nucleotide sequence of the neuraminidase gene of influenza B virus. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6817–6821. doi: 10.1073/pnas.79.22.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura A., Ueda M. Neurovirulence of influenza virus in mice. I. Neurovirulence of recombinants between virulent and avirulent virus strains. Virology. 1980 Mar;101(2):440–449. doi: 10.1016/0042-6822(80)90457-2. [DOI] [PubMed] [Google Scholar]
- Walter G., Roblin R., Dulbecco R. Protein synthesis in Simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1972 Apr;69(4):921–924. doi: 10.1073/pnas.69.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Laver W. G., Air G. M., Schild G. C. Molecular mechanisms of variation in influenza viruses. Nature. 1982 Mar 11;296(5853):115–121. doi: 10.1038/296115a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wilson I. A., Skehel J. J., Wiley D. C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981 Jan 29;289(5796):366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- Wrigley N. G., Skehel J. J., Charlwood P. A., Brand C. M. The size and shape of influenza virus neuraminidase. Virology. 1973 Feb;51(2):525–529. doi: 10.1016/0042-6822(73)90457-1. [DOI] [PubMed] [Google Scholar]