Abstract
The study of micro RNA (miRNA) expression and function, a largely unexplored area of human muscle biology, may provide novel data regarding the development of targeted approaches that optimize skeletal muscle responses to exercise and amino acid manipulations. miRNAs are ubiquitously expressed, small noncoding RNAs that modulate posttranscriptional gene expression. Quantifying miRNA expression and predicting function as regulators of both single targets and complex networks is technically challenging and requires a combined approach of bioinformatics, molecular, and systems biology. Recent evidence suggests that the expression of muscle-specific miRNAs (myomirs), including miR-1, miR-133a/b, miR-206, and miR-499, is modulated by essential amino acid ingestion, endurance exercise, and endurance exercise training. The expression of miRNAs has also been implicated in the anabolic intracellular signaling and muscle hypertrophic response associated with resistance exercise training. Although these findings are intriguing, comprehensive human trials assessing functional outcomes associated with changes in miRNA expression in response to exercise and nutrition interventions have not been conducted. This article reviews the current understanding of miRNA biology and includes analytical techniques used to detect miRNA expression and methods to predict function. The intent is to provide the framework for future research studies that use miRNA analysis in an effort to elucidate optimal exercise and nutritional countermeasures for the prevention of muscle loss.
Introduction
Exercise and amino acids are independent regulators of human skeletal muscle protein synthesis (1). The protein synthetic response to exercise is differentially affected by the mode of exercise performed (2, 3), and the timing and components of amino acid intake may affect the protein synthetic response (1, 4). Nutrition and physical activity interventions that effectively integrate the metabolic effects of exercise and amino acids result in a more efficient, pronounced, and sustained anabolic response than either stimulus elicits alone, which may contribute to the long-term maintenance and accretion of muscle mass (5, 6).
In recent years, muscle biology research has focused on intracellular networks that regulate muscle protein synthesis, including the mammalian target of rapamycin complex 1 (7) and mitogen-activated protein kinase pathways (8). It is evident that exercise and amino acids, in particular, the branched-chain amino acid leucine, stimulate anabolic intracellular signaling through independent mechano- and nutrient-sensing mechanisms (5). However, exercise and amino acid consumption do not consistently translate into a clear mechanistic link between intracellular signaling and human muscle protein turnover (9). Most recently, new evidence suggests that exploring the expression and function of skeletal muscle micro RNAs (miRNAs)3, small RNA molecules that function in posttranscriptional gene regulation, may be critical to understanding the acute and long-term independent and integrative effects of exercise and amino acids on intracellular regulators of muscle protein turnover.
miRNAs were first discovered nearly 20 years ago in Caenorhabditis elegans (10, 11). Subsequently, the study of miRNA function in humans and other mammals has advanced understanding of the role of these molecules in the development of disease, as well as the identification of novel therapeutic targets (12). Exercise and nutrition may influence the expression of miRNAs in skeletal muscle (referred to as myomirs), suggesting that miRNAs are important components of the intracellular regulatory systems that modulate skeletal muscle protein turnover (13, 14). miRNA expression patterns may be used to assist with the interpretation and prediction of intracellular signaling, protein turnover, and phenotypic adaptations to an anabolic stimulus (15–17). As such, the analysis of miRNAs may prove to be an effective tool for the development of targeted exercise and nutrition interventions for muscle conservation. This article is a review of miRNA biology and discusses analytical techniques used to detect miRNA expression and methods to determine function. Studies investigating miRNA responses to exercise and exogenous amino acids are highlighted (13, 14, 18), and approaches to assess integrated exercise and nutritional countermeasures for the prevention of muscle loss using miRNA analysis coupled with conventional measures of cell signaling and protein turnover are described.
Understanding miRNA
The discovery of miRNA altered the long-standing principle that RNA serves as the sole messenger between genes and protein expression because these noncoding molecules confer critical structural and regulatory functions affecting gene expression. Inter- or intragenic genomic regions are transcribed as immature primary transcripts (pri-miRNAs), which are further transcribed to hairpin pre-miRNAs through the actions of Drosha, the RNAse enzyme, in the nucleus (19–21). The pre-miRNA is then exported to the cytoplasm, where it may be processed by Dicer, an RNA polymerase, and unwound to yield mature miRNA, or exported to other locations through the bloodstream (20). In the cytoplasm, mature miRNAs become incorporated into an RNA-induced silencing complex that coordinates binding between the miRNA and corresponding sequences within the 3′ untranslated region of target messenger RNAs (mRNAs) (22, 23). Complementary binding of miRNA to mRNA (in as few as 6 base pair matches) may then affect gene expression through a combination of mRNA degradation and translation inhibition (23, 24).
Analytical tools for the detection of miRNA
To date, >1000 miRNA sequences have been identified in humans, and it has been estimated that miRNA may affect the regulation of as much as 60% of the human genome (21). Each individual miRNA may affect the abundance or translation of multiple mRNAs. As such, the use of appropriate technologies for the detection of miRNAs in biological samples, as well as the study of miRNA function as a regulator of both single targets and complex networks, is a significant technical challenge in the fields of bioinformatics, molecular, and systems biology (16).
Detecting miRNA species in biological samples begins with the isolation of RNA. Given the small size of miRNAs (averaging ∼22 nucleotides), the use of commercial kits developed specifically for the isolation of small RNAs has become commonplace. Standard phenol-based extraction methods, such as the use of TRIzol/TRI Reagent (Invitrogen, Carlsbad, CA) isolation techniques, may also be used to isolate RNA. Once RNA has been isolated and assessed for RNA quality, a series of techniques may be used for the detection of miRNA species, to include the use of microarrays, real-time quantitative polymerase chain reaction (qPCR), and Northern blotting (15).
Microarray analysis is the best approach to assess large quantities of miRNA targets (genomes) simultaneously for the determination of relative changes in speciation in samples collected from a carefully controlled experiment or group of populations compared with the appropriate control (e.g., treated vs. untreated or diseased vs. healthy). The principles of microarray analysis for the detection of miRNAs are similar to those for standard RNA microarray analyses: RNA samples of interest containing endogenous miRNAs are hybridized to microarrays carrying probes for each miRNA identified in the species of interest, which results in quantifiable output (data). The degree of fold-difference in miRNA expression necessary to determine biological significance using microarray is dependent on the experimental context. Ideally, miRNA targets that yield the most robust responses to genomewide microarrays are then validated using real-time qPCR or Northern blotting, considering that microarrays have generally lower sensitivity than real-time qPCR and Northern blotting to predict biological significance and should be used primarily as discovery tools rather than as quantitative assays (16).
The use of real-time qPCR for the detection of miRNA includes 2 appropriate methodologies: miRNA-specific reverse transcription primers and universal reverse transcriptase primers. The first method uses stem-loop miRNA–specific primers that bind to the 3′ portion of miRNA molecules that are then reverse transcribed. After reverse transcription, the product is quantified using TaqMan (Invitrogen) or other commercially available assays that use miRNA-specific primers and a labeled probe. If using universal reverse transcriptase primers, a common sequence [poly(A) tail] is added to the 3′ end of all of the miRNAs in the sample. Reverse transcription is then conducted using the universal primer followed by real-time qPCR using SYBR Green fluorescent dye (Invitrogen). Appropriate housekeeping genes (typically small nucleolar RNAs) should be used to normalize miRNA levels after real-time qPCR. Housekeeping genes must have constant expression across all biological samples used in the experiment and should not be affected by the treatments or disease conditions under study (15–17).
Although a detailed description of the laboratory methodologies associated with Northern blotting or in situ hybridization techniques is beyond the scope of this review, these techniques may also be used to validate alterations in miRNA profiles detected using microarray analyses. Although Northern blotting typically requires more RNA than real-time qPCR, this technique can also be used for the detection of both miRNAs and pre-miRNAs [real-time qPCR does detect both miRNAs and pre-miRNAs (25, 26)]. In situ hybridization is a commonly used laboratory tool for the assessment of miRNA expression, although 1 limitation of its use is an inability to glean consistent results for miRNAs with lower levels of expression (15).
Analysis of miRNA function
Each miRNA may regulate hundreds of mRNAs, thereby affecting entire gene expression networks, which results in alterations in biological function (16, 23). A series of approaches for assessing the function of miRNAs has been used, beginning with the identification of miRNA target genes. Recently developed computational approaches have been used for the identification of miRNA target genes. These bioinformatic target prediction programs use algorithms based on defined criteria regarding the behavior of miRNAs to predict the formation and stability of mRNA:miRNA duplexes. Because the algorithms used within target prediction programs may be different due to the complexity of criteria for predicting miRNA behavior, many researchers combine the results of multiple target prediction programs before final identification of targets for further verification (15).
After the use of computational approaches to identify potential targets for verification, both mRNA expression and protein detection techniques (such as Western blotting) may be used as tools to both confirm the regulation of mRNA targets by miRNAs and explore functional outcomes associated with changes in protein content. Other techniques used for the elucidation of miRNA function include technologies that inhibit miRNAs in vivo. Although not the focus of this review, the use of both antimiRs and miRNA knockout and overexpressing mice have been used to study miRNA function. AntimiRs are modified antisense oligonucleotides that reduce the levels of a pathogenic or aberrantly expressed miRNA, which results in an increase in target mRNA and protein levels and may affect function (15). Knockout laboratory animal models lacking Drosha, Dicer, and a number of individual miRNAs were recently developed and have assisted in the understanding of each of these targets through lethality and changes in phenotype (27). A simplified flowchart describing miRNA detection and analysis of function is provided in Figure 1.
Figure 1.
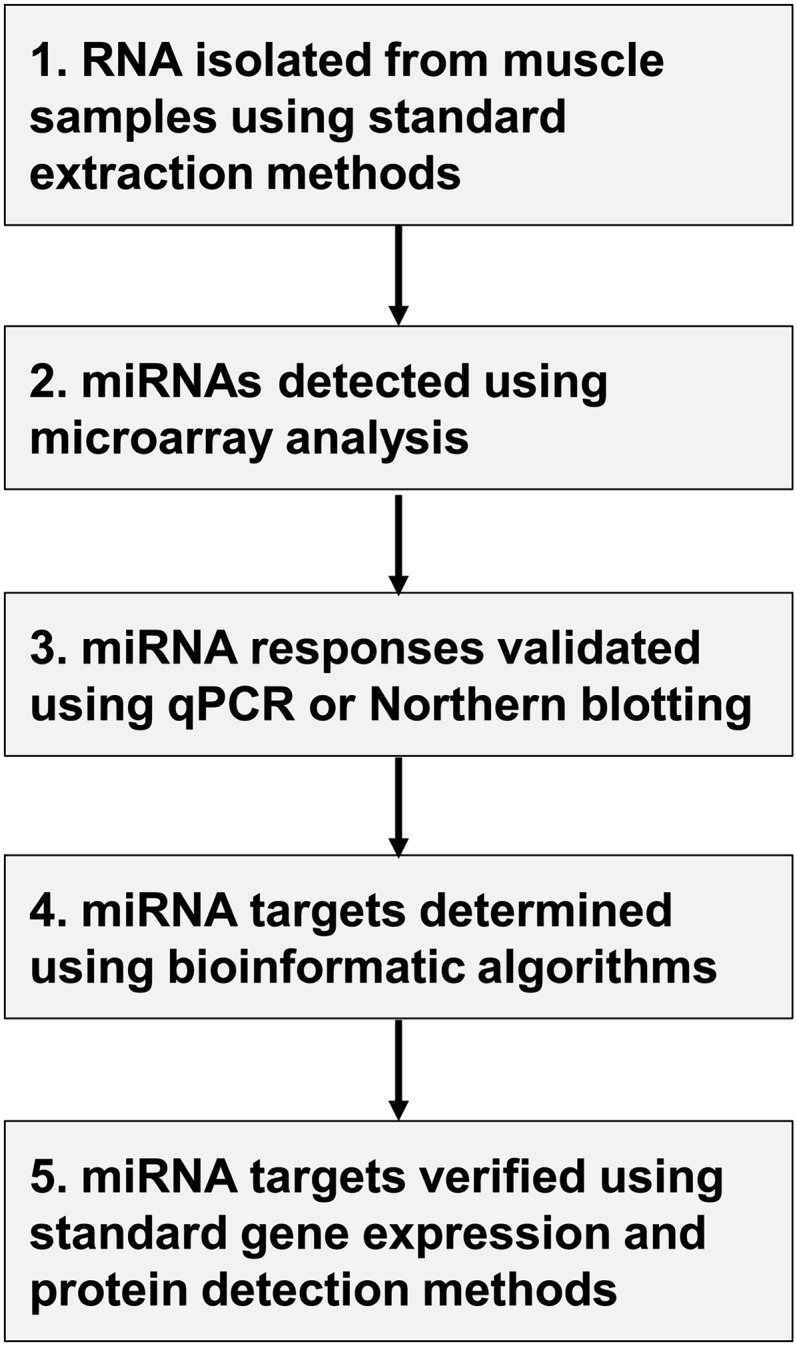
Micro RNA (miRNA) analysis and determination of function. Human skeletal muscle samples obtained during independent or combined amino acid and exercise interventions analyzed for miRNA expression using (1) standard RNA isolation extraction methods, (2) microarray, (3) real-time quantitative polymerase chain reaction (qPCR) and Northern blotting; function determined and verified using (4) bioinformatics prediction software and (5) qPCR and protein detection methodologies (e.g., Western blotting).
Exercise and amino acid interventions and miRNA assessment in human skeletal muscle
Cell and animal studies have identified a series of miRNAs specific to skeletal muscle (myomirs), including miR-1, miR-133a/b, and miR-206. Evidence using a developing muscle cell model (C2C12 myoblasts) suggests that myomirs are regulated during skeletal muscle development and may be associated with muscle growth and regeneration because miR-1 expression appears to promote myogenesis, whereas miR-133 expression may modulate myoblast proliferation, and miR-206 levels enhance satellite cell differentiation and fusion into muscle fibers (28, 29). Together, these data indicate a potential adaptive role for myomirs in skeletal muscle responses to acute anabolic stimuli.
Two recent studies assessed the expression of myomirs in human skeletal muscle after both resistance and endurance exercise (18, 30). The first, conducted in 10 healthy young males, assessed myomirs in muscle biopsy samples obtained from the vastus lateralis before and after a bout of endurance-type exercise (60 min of cycle ergometry at 65% of maximal power output) (18). Biopsy samples were collected again before and after an exercise bout performed 14 d after completing a 5 d per week supervised cycle ergometry training program for 12 wk. Myomirs were assessed in RNA isolated from the biopsy samples using TaqMan assays and real-time qPCR. After the quantification of myomirs in response to both the acute exercise activity and the training period, a Web-based computational tool [DIANA-mirPath (31)] was used to identify target molecular pathways that may have been affected by the coordinated changes in myomir expression. Western blotting was then used to confirm changes in the expression of proteins identified using the computational tool.
The major finding of this study was that the expression of both miR-1 and miR-133a were significantly increased after the exercise bout before, but not after, the 12-wk training period. Further, in resting biopsy samples, the basal expression of each myomir assessed in the experiment (miR-1, miR-133a, miR-133b, and miR-206) was diminished compared with samples collected before initiating endurance training. The expression of each of the myomirs returned to pretraining levels 14 d after terminating the exercise training program. The computational model predicted changes in the expression of critical proteins essential for skeletal muscle adaptations to endurance training such as the cell division control protein 42 and extracellular signal-regulated kinase 1/2, which function in the mitogen-activated protein kinase and transforming growth factor-β intracellular signaling pathways. As predicted using the model, the expression of these proteins decreased during the 14-d period after the exercise training program. However, the expression of other target proteins was not altered nor was myomir and target protein expression inversely related.
The data gleaned from this initial study to assess the regulatory effects of endurance exercise on human skeletal muscle miRNAs indicate a differential myomir response to exercise before and after a 12-wk training program and suggest an adaptive mechanism. Computational tools and subsequent protein detection partially supported the myomir findings. This was the first study to demonstrate that myomir expression in human skeletal muscle responds to acute exercise and that the exercise response of myomirs adapts to endurance training. Future studies are required to understand the lack of consistent changes in protein expression as predicted using computational analysis.
A second study assessed the regulation of myomirs and a series of other miRNA targets in human volunteers after resistance exercise (30). In this study, 56 healthy young men participated in a 5 d per week resistance training program for 12 wk. The program consisted of a series of intense pushing, pulling, and leg resistance-type exercises. Resting muscle biopsy samples were obtained from the vastus lateralis before and 48 h after the last training session of the 12-wk program. Volunteers were stratified into groups of low (n = 9) and high (n = 8) responders based on the accrual of lean body mass, gains in training-induced muscle fiber cross-sectional area, and improvements in muscle strength after the training period. After isolation of miRNA from the biopsy samples, a series of 21 miRNAs were detected using TaqMan assays, followed by the use of gene ontology and computational models for the prediction of mRNA targets [TargetScan 4.2 (17)].
Of the 21 miRNAs assessed in this study, 4 were differentially expressed between the low and high responders. In low responders, miR-26a, miR-29a, and miR-378 were downregulated and miR-451 was upregulated compared with baseline after the 12-wk training period. Perhaps most interestingly, changes in miR-378 expression were positively associated with gains in lean body mass, and miR-126 levels at baseline tended to positively correlate with a higher percentage of type 1 muscle fibers. Gene ontology analysis predicted changes in the mammalian target of rapamycin complex 1 signaling pathway, which is a central regulator of skeletal muscle hypertrophy (32). In a final step, the expression of predicted mRNA targets was assessed to include insulin-like growth factor 1, eukaryotic translation initiation factor 4e type 2, and vascular endothelial growth factor A. Of these potential mRNA targets, insulin-like growth factor 1 mRNA levels increased with training in high responders, but not low responders.
The novel contribution of this study included not only the first assessment of miRNA expression in human muscle tissue from individuals participating in resistance exercise training, but also evidence linking miRNA expression to changes in functional hypertrophy. Although the study did assess differences in a limited number of mRNA targets identified using predicted targets, future studies will be required to assess the relationship between miRNA, mRNA, and resulting changes in protein expression.
Studies assessing the impact of nutritional interventions on miRNA expression in human skeletal muscle are extremely limited. One study assessed the impact of performing resistance exercise and consuming supplemental essential amino acids in a study on aging and elucidated age-related differences in the expression of myomirs in response the combined anabolic stimulus (13). However, the contribution of essential amino acids on myomir expression was not assessed independently from exercise. In a second recent study, Drummond et al. (14) assessed the effects of a nutrition intervention on young volunteers (n = 7) from whom muscle biopsy samples were collected from the vastus lateralis before and 3 h after ingestion of a drink containing 10 g of essential amino acids. Muscle-related genes and miRNAs were assessed using real-time qPCR. As expected, consuming the essential amino acid drink resulted in increased plasma levels of insulin, isoleucine, leucine, and valine. A series of miRNAs were affected by the intervention, including significant increases in the expression of miR-1, miR-23a, miR-208b, and miR-499. The expression of muscle-related genes was affected as well as MyoD1 expression increased, and a series of other genes, including myostatin, decreased after the nutrition intervention. Because this study was the first to characterize miRNA responses to the ingestion of amino acids in human skeletal muscle, the findings are novel and indicate a robust miRNA response to essential amino acid supplementation. However, a series of future studies are needed to elucidate the impact of these responses on both changes in protein expression and the physiologic response to nutrition intervention.
Future directions: comprehensive approaches to human muscle biology research
Although the studies detailed in this review provide novel data and insight, the quantification and determination of muscle miRNA expression and biological function in response to exercise and amino acids was often a secondary objective of these human research studies. As such, these studies may not have been powered to assess miRNA expression and predict biological function. For example, volunteers characterized as low and high responders to resistance training (30) were down-selected from a larger pool of volunteers who participated in a study originally designed to assess long-term consequences of isonitrogenous and isocaloric postexercise protein supplementation (milk vs. soy vs. energy-matched placebo) on lean mass accretion (33). Despite originally concluding that milk protein supplementation (two 17.5-g doses within 1 h after each exercise bout) enhanced muscle hypertrophy to a greater extent than both the placebo and control supplement (33), the investigators chose not to conduct miRNA analysis on all 56 volunteers assigned to their original experimental groups. Instead, the volunteers were stratified into smaller groups of low and high responders based on the degree of lean mass accrual, which included a combination of volunteers from the milk [high responders (n = 4) and low responders (n = 1)], soy [high responders (n = 2) and low responders (n = 5)], and placebo control [high responders (n = 2) and low responders (n = 3)] groups. If the source of supplemental protein influenced the accrual of lean mass, a more comprehensive analysis of miRNA responses to the combined nutrition and resistance exercise intervention may have provided critical data regarding the impact of protein (amino acid) nutrition on muscle adaptations to resistance training. Other reports highlighted in this review (13) include the analysis of miRNAs in muscle samples obtained from volunteers who participated in experiments originally designed to assess intracellular signaling and protein synthetic responses to amino acids and resistance exercise (34). Only the follow-up study by Drummond et al. (14) appears to have been primarily designed to assess miRNA responses to essential amino acid ingestion, although the sample size (n = 7) may limit the interpretation and extension of the study findings.
To the best of our knowledge, comprehensive research studies that are adequately powered to address multiple levels of molecular regulation of muscle mass (e.g., miRNAs, anabolic and proteolytic cell signaling, and gene expression) in response to prolonged dietary interventions and physiological stressors have not yet been conducted. Because miRNAs appear to be critical for muscle development and adaptive responses to anabolic stimuli, well-designed research studies that use miRNA analysis with conventional anabolic and proteolytic gene expression, intracellular signaling, and kinetic measures of protein turnover may be necessary to elucidate targeted approaches that optimize muscle health. For example, if acute essential amino acid ingestion elicits robust increases in miR-1, miR-23a, miR-208b, and miR-499 expression, with a concomitant reduction in myostatin (a negative regulator of muscle mass) expression (14), then it may be possible that habitual consumption of a high protein diet would elicit a similar miRNA expression pattern and subsequent functional outcome. Such data may improve the current level of understanding regarding the intracellular mechanisms by which high protein diets confer lean mass protection in response to periods of sustained energy deficit (35–38). Future nutrition- and exercise-related muscle biology studies should include the design of systematic investigations that use miRNA analysis to identify nutrient and physical activity targets that predispose beneficial skeletal muscle functional outcomes (e.g., maintenance or muscle protein accretion) in populations susceptible to muscle loss.
Conclusions
Intracellular mediators of human skeletal muscle protein turnover have been studied extensively in recent years. It is evident that exercise and amino acid consumption elicit distinct anabolic intracellular signaling and protein turnover responses in human muscle. In recent years, miRNAs have emerged as critical regulators of posttranscriptional gene expression, and limited data suggest that the expression of skeletal muscle–specific miRNAs (myomirs) is sensitive to acute and long-term exercise (e.g., resistance and endurance) and amino acid interventions. Due to advances in bioinformatics, molecular, and systems biology, miRNA expression may now be used to predict biological function and assist in the interpretation of intracellular signaling, protein turnover, and muscle phenotypic responses to acute and sustained anabolic stimuli. Researchers should consider the efficacy of miRNA analysis in the design of future research studies designed to evaluate physical activity and nutritional countermeasures for the prevention of muscle loss.
Acknowledgments
The authors acknowledge Dr. Andrew J. Young for his critical review in the development of this manuscript. The authors have read and approved the final manuscript.
Footnotes
Abbreviations used: MAPK, mitogen-activated protein kinase; miRNA, micro RNA; qPCR, quantitative PCR.
Literature Cited
- 1.Drummond MJ, Dreyer HC, Fry CS, Glynn EL, Rasmussen BB. Nutritional and contractile regulation of human skeletal muscle protein synthesis and mTORC1 signaling. J Appl Physiol. 2009;106:1374–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burd NA, Mitchell CJ, Churchward-Venne TA, Phillips SM. Bigger weights may not beget bigger muscles: evidence from acute muscle protein synthetic responses after resistance exercise. Appl Physiol Nutr Metab. 2012;37:551–4 [DOI] [PubMed] [Google Scholar]
- 3.Kumar V, Atherton P, Smith K, Rennie MJ. Human muscle protein synthesis and breakdown during and after exercise. J Appl Physiol. 2009;106:2026–39 [DOI] [PubMed] [Google Scholar]
- 4.Atherton PJ, Etheridge T, Watt PW, Wilkinson D, Selby A, Rankin D, Smith K, Rennie MJ. Muscle full effect after oral protein: time-dependent concordance and discordance between human muscle protein synthesis and mTORC1 signaling. Am J Clin Nutr. 2010;92:1080–8 [DOI] [PubMed] [Google Scholar]
- 5.Pasiakos SM. Exercise and amino acid anabolic cell signaling and the regulation of skeletal muscle mass. Nutrients. 2012;4:740–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol. 2011;110:561–8 [DOI] [PubMed] [Google Scholar]
- 7.Proud CG. Cell signaling. mTOR, unleashed. Science. 2007;318:926–7 [DOI] [PubMed] [Google Scholar]
- 8.Kramer HF, Goodyear LJ. Exercise, MAPK, and NF-kappaB signaling in skeletal muscle. J Appl Physiol. 2007;103:388–95 [DOI] [PubMed] [Google Scholar]
- 9.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, et al. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Timmons JA, Good L. Does everything now make (anti)sense? Biochem Soc Trans. 2006;34:1148–50 [DOI] [PubMed] [Google Scholar]
- 11.Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75:843–54 [DOI] [PubMed] [Google Scholar]
- 12.Couzin J. MicroRNAs make big impression in disease after disease. Science. 2008;319:1782–4 [DOI] [PubMed] [Google Scholar]
- 13.Drummond MJ, McCarthy JJ, Fry CS, Esser KA, Rasmussen BB. Aging differentially affects human skeletal muscle microRNA expression at rest and after an anabolic stimulus of resistance exercise and essential amino acids. Am J Physiol Endocrinol Metab. 2008;295:E1333–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drummond MJ, Glynn EL, Fry CS, Dhanani S, Volpi E, Rasmussen BB. Essential amino acids increase microRNA-499, -208b, and -23a and downregulate myostatin and myocyte enhancer factor 2C mRNA expression in human skeletal muscle. J Nutr. 2009;139:2279–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernardo BC, Charchar FJ, Lin RC, McMullen JR. A microRNA guide for clinicians and basic scientists: background and experimental techniques. Heart Lung Circ. 2012;21:131–42 [DOI] [PubMed] [Google Scholar]
- 16.Pritchard CC, Cheng HH, Tewari M. MicroRNA profiling: approaches and considerations. Nat Rev Genet. 2012;13:358–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis BP, Shih IH, Jones-Rhoades MW, Bartel DP, Burge CB. Prediction of mammalian microRNA targets. Cell. 2003;115:787–98 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen S, Scheele C, Yfanti C, Akerstrom T, Nielsen AR, Pedersen BK, Laye MJ. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J Physiol. 2010;588:4029–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis CD, Ross SA. Evidence for dietary regulation of microRNA expression in cancer cells. Nutr Rev. 2008;66:477–82 [DOI] [PubMed] [Google Scholar]
- 20.Ross SA, Davis CD. MicroRNA, nutrition, and cancer prevention. Adv Nutr. 2011;2:472–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–87 [DOI] [PubMed] [Google Scholar]
- 22.Schwarz DS, Hutvagner G, Haley B, Zamore PD. Evidence that siRNAs function as guides, not primers, in the Drosophila and human RNAi pathways. Mol Cell. 2002;10:537–48 [DOI] [PubMed] [Google Scholar]
- 23.Thomson DW, Bracken CP, Goodall GJ. Experimental strategies for microRNA target identification. Nucleic Acids Res. 2011;39:6845–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doench JG, Sharp PA. Specificity of microRNA target selection in translational repression. Genes Dev. 2004;18:504–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chugh P, Tamburro K, Dittmer DP. Profiling of pre-micro RNAs and microRNAs using quantitative real-time PCR (qPCR) arrays. J Vis Exp. 2010;(46). pii: 2210. [DOI] [PMC free article] [PubMed]
- 26.Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C. Real-time PCR quantification of precursor and mature microRNA. Methods. 2008;44:31–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park CY, Choi YS, McManus MT. Analysis of microRNA knockouts in mice. Hum Mol Genet. 2010;19: R2:R169–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JF, Mandel EM, Thomson JM, Wu Q, Callis TE, Hammond SM, Conlon FL. The role of microRNA-1 and microRNA-133 in skeletal muscle proliferation and differentiation. Nat Genet. 2006;38:228–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu N, Williams AH, Maxeiner JM, Bezprozvannaya S, Shelton JM, Richardson JA, Basssel-Duby R, Olson EN. microRNA-206 promotes skeletal muscle regeneration and delays progression of Duchenne muscular dystrophy in mice. J Clin Invest. 2012;122:2054–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davidsen PK, Gallagher IJ, Hartman JW, Tarnopolsky MA, Dela F, Helge JW, Timmons JA, Phillips SM. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J Appl Physiol. 2011;110:309–17 [DOI] [PubMed] [Google Scholar]
- 31.Papadopoulos GL, Alexiou P, Maragkakis M, Reczko M, Hatzigeorgiou AG. DIANA-mirPath: integrating human and mouse microRNAs in pathways. Bioinformatics. 2009;25:1991–3 [DOI] [PubMed] [Google Scholar]
- 32.Miyazaki M, Esser KA. Cellular mechanisms regulating protein synthesis and skeletal muscle hypertrophy in animals. J Appl Physiol. 2009;106:1367–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hartman JW, Tang JE, Wilkinson SB, Tarnopolsky MA, Lawrence RL, Fullerton AV, Phillips SM. Consumption of fat-free fluid milk after resistance exercise promotes greater lean mass accretion than does consumption of soy or carbohydrate in young, novice, male weightlifters. Am J Clin Nutr. 2007;86:373–81 [DOI] [PubMed] [Google Scholar]
- 34.Drummond MJ, Dreyer HC, Pennings B, Fry CS, Dhanani S, Dillon EL, Sheffield-Moore M, Volpi E, Rasmussen BB. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mettler S, Mitchell N, Tipton KD. Increased protein intake reduces lean body mass loss during weight loss in athletes. Med Sci Sports Exerc. 2010;42:326–37 [DOI] [PubMed] [Google Scholar]
- 36.Phillips SM. Higher protein during an energy deficit: muscle's guardian and fat's enemy? Med Sci Sports Exerc. 2008;40:503–4 [DOI] [PubMed] [Google Scholar]
- 37.Layman DK, Boileau RA, Erickson DJ, Painter JE, Shiue H, Sather C, Christou DD. A reduced ratio of dietary carbohydrate to protein improves body composition and blood lipid profiles during weight loss in adult women. J Nutr. 2003;133:411–7 [DOI] [PubMed] [Google Scholar]
- 38.Layman DK, Evans E, Baum JI, Seyler J, Erickson DJ, Boileau RA. Dietary protein and exercise have additive effects on body composition during weight loss in adult women. J Nutr. 2005;135:1903–10 [DOI] [PubMed] [Google Scholar]


