Abstract
Vitamin K exists in the food supply as phylloquinone, a plant-based form and as menaquinones (MKs), a collection of isoprenologues mostly originating from bacterial synthesis. Although multiple bacterial species used as starter cultures for food fermentations synthesize MK, relatively little is known about the presence and distribution of MK in the food supply and the relative contribution of MK to total dietary vitamin K intake. Dairy products may be a predominant source of dietary MK in many regions of the world, and there is recent interest in enhancing the MK content of dairy products through identification and selection of MK-producing bacteria in dairy fermentations. This interest is increased by emerging evidence that current dietary recommendations based on the classic role of vitamin K as an enzyme cofactor for coagulation proteins may not be optimal for supporting vitamin K requirements in extrahepatic tissues and that MK may have unique bioactivity beyond that as an enzyme cofactor. Observational studies have reported favorable associations between MK intake and bone and cardiovascular health. Although randomized trials have provided some evidence to support the beneficial effects of MK on bone, the evidence to date is not definitive, and randomized trials have not yet examined MK intake in relation to cardiovascular outcomes. Food production practices provide a means to enhance dietary MK availability and intake. However, parallel research is needed to optimize these production practices, develop comprehensive food MK content databases, and test hypotheses of unique beneficial physiological roles of MK beyond that achieved by phylloquinone.
Introduction
Vitamin K is an essential fat-soluble vitamin existing in multiple dietary forms. Phylloquinone (PK)7, also known as vitamin K-1, is the predominant dietary form, and is primarily found in green leafy vegetables and their oils (1). Menaquinones (MKs), also known as vitamin K-2, are primarily synthesized by bacteria and are found in much lower amounts in the food supply in meat, dairy, and fermented food products. The classic role of vitamin K as an enzyme cofactor for γ-carboxylation of peptide-bound glutamate residues established the vitamin as essential to normal coagulation; however, emerging roles for vitamin K in bone, cardiovascular, and metabolic health are purported (2). Although existing knowledge of vitamin K’s health benefits are primarily based on studies examining PK, MK may have similar bioactivity in addition to postulated exclusive physiological roles (3).
MKs exist in multiple forms; however, the tendency in the literature to group all MKs under the term vitamin K-2 has erroneously led many to assume that all MKs are similar in origin and function. As is described in this review, this is not a valid assumption. Moreover, despite the knowledge that MKs are present in the food supply, the relevance to human vitamin K nutriture has received little attention. Expanding the knowledge base regarding the health effects of MKs and the distribution of MKs in the food supply is essential for guiding the development of dietary intake recommendations for vitamin K. This review discusses the relevance of MK biosynthesis to industrial food production, the presence of MKs in foods, and potential effects on human health.
MK structure and biosynthesis
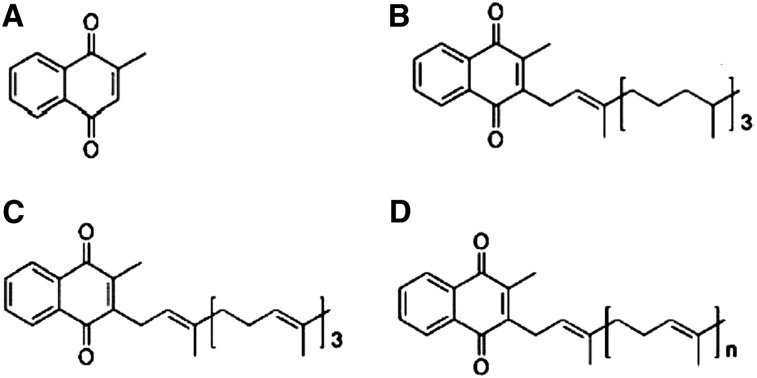
Bacterial synthesis of MKs was discovered in a series of studies conducted in the 1930s, demonstrating that an antihemorrhagic factor present in dried chick feed was produced by Bacillus cereus (4). Most bacteria are now known to synthesize a limited set of naphthoquinones, which share the same 2-methyl-1,4-naphthoquinone ring but differ in the length of an isoprenoid side chain attached at the 3-position, which generally ranges from 5 to 13 prenyl units (each having 5 carbons) in length (Fig. 1). These compounds are known as long-chain MKs or MK-n; n representing the number of prenyl units (e.g., MK-7 is the term for 2-methyl-3-heptaprenyl-1,4-naphthoquinone). The 2-methyl-1,4-naphthoquinone ring, also known as menadione or vitamin K-3, is common to all forms of vitamin K. However, PK differs from MKs by virtue of a phytyl side chain located at the 3-position and is produced primarily in plants (3). Notably, bacteria do not synthesize MK-4. Instead MK-4 is produced in humans and animals by tissue-specific conversion of PK and/or menadione (5).
Figure 1.
Forms of vitamin K: menadione (A), phylloquinone (B), menaquinone-4 (C), and menaquinone-n+1 (D). Reproduced from reference (2) with permission.
Bacteria use 2 distinct biochemical pathways for MK synthesis (Supplemental Figure 1), both of which have been described in detail elsewhere (6–8). In the pathway used by lactic acid bacteria (LAB) that are commonly used in industrial food fermentations, the napthoquinone ring is synthesized from chorismate derived from the shikimate pathway by a series of enzymes encoded by men genes. The isoprenoid side chain is synthesized separately and joined to the napthoquinone ring to form demethylmenaquinone (DMK). Subsequent methylation of DMK completes MK biosynthesis.
Depending on the organism and growing conditions, the basic MK structure can be altered through chemical modification of the napthoquinone ring or side chain. Common modifications include demethylation of the napthoquinone ring to reform DMK and, to varying degrees, saturation of the isoprenoid side chain, which is usually fully unsaturated. In general, gram-positive bacteria primarily produce MK, whereas gram-negative bacteria produce MK, DMK, and ubiquinones (9). Several bacterial species lack the required methylase and exclusively produce DMK (9).
Before the emergence of the more discriminating sequencing tools now available, the unique distribution of specific MK production among bacterial species was used as a chemotaxonomic marker (9). As such, methods for extracting and analyzing MK isoprenologues as major and minor components in the cells of numerous bacteria were developed and a compendium of species-specific bacterial MK production compiled (9).
Function of MKs in bacteria
MKs play a key role in prokaryotic respiratory electron transport chains by functioning as electron carriers in the cytoplasmic membrane (8, 10). In addition to a role in microbial respiration, reduced MK forms exhibit antioxidant properties and can play a role in protecting cellular membranes from lipid oxidation (7). MKs have also been shown to be involved in the active transport of molecules across the cell membrane and in sporulation in Bacillus subtilis (11, 12).
The function of MK in LAB is of particular interest due to the importance of this class of bacteria to the food industry. LAB are the principal organisms used in starter cultures required to produce fermented dairy, meat, and vegetable food products. Considerable interest is currently being paid to developing more efficient means of growing LAB to increase bacterial yield and decrease production costs (13). Many LAB lack a heme biosynthesis pathway, which results in an incomplete electron transport chain (13). As such, LAB were historically considered to be obligate fermentative and were industrially produced under microaerophilic conditions. However, a link was established between the presence of heme in the growth media and the use of oxygen among some strains of LAB, specifically Lactococcus lactis and Leuconostoc mesenteroides (14, 15). Subsequent studies demonstrated that these bacteria were able to use extracellular molecules in growth media to complement their respiratory chains (16). In several species, MKs were required in addition to heme and oxygen for successful respiration (17). These findings were of paramount importance for industrial applications as respiration is a more efficient means of energy production than fermentation. Consequently, the addition of heme and oxygen, and in some cases MK, to the media of LAB facilitates aerobic growth leading to greater biomass, lower acid production, greater amounts of desirable but normally minor end products, and a higher tolerance to different stressors encountered during processing, preservation, and other industrial processes (16, 18).
MKs in the food supply
Whereas PK is widely distributed in the food supply, predominantly concentrated in green leafy vegetables and certain plant oils, MKs are primarily found in dairy products, meats, and fermented foods (1, 3). However, the MK and PK contents of many food products are unknown. Of 30 national food composition databases reviewed, only 7 included the vitamin K content of individual food items. Of these 7, 2 (United Kingdom and Germany) list PK concentrations only, 1 country (Finland) reports total vitamin K, and 3 (Denmark, Sweden, and Canada) do not specify whether MKs are included in the values for vitamin K. To the best of our knowledge, the only database that provides MK data is from the United States. The MK-4 content of 273 food items in the USDA National Nutrient Database for Standard Reference, Release 25 (19) are published as part of an ongoing collaboration between Tufts University and the USDA in which foods obtained from the National Food and Nutrient Analysis Program are analyzed for PK, dihydrophylloquinone, and MK-4 (20). These values include MK-4 content in milk, dairy products, and various meats; however, the data are not comprehensive and do not include other MK forms. The absence of comprehensive data on PK and MK food content in national databases severely limits the ability of scientists to reliably establish associations between vitamin K intake and health. Moreover, regional differences in food production practices and dietary consumption patterns underlie the necessity of developing national databases for food MK content.
In the United States, menadione is used in poultry feed and some swine feeds as a source of vitamin K (1, 21). As such, MK-4 formed from menadione is present in poultry and pork products in the U.S. food supply and is the primary dietary source of MK-4 (20). Although MK-4 is also formed from tissue-specific conversion of PK (5), the impact on dietary intake from this conversion is likely negligible as animal organs containing high MK-4 concentrations including kidney, brain, and pancreas, are not commonly consumed in most regions of the world. MK-4 is also found in modest amounts in milk, butter, and cheeses, which may make a small contribution to total vitamin K intake (Tables 1 and 2) The high consumption of poultry, pork, and dairy products in the United States (22), however, suggests that MK-4 may make a relevant contribution to total vitamin K intake. In regions where food systems do not use menadione in animal feed or consumption of dairy products is low, MK-4 is most likely not an important dietary source of vitamin K. For example, MK-4 has been estimated to account for ∼3% of total vitamin K intake in the Netherlands (23, 24) and is found in animal products in relatively lower amounts compared with the United States and Japan (Table 1).
Table 1.
Regional variability in the menaquinone-4 concentration in commonly consumed animal products
Table 2.
Representative ranges of measured menaquinone concentration in dairy foods and fermented food products1
| Menaquinones2 |
||||||||
| Food | MK-4 | MK-5 | MK-6 | MK-7 | MK-8 | MK-9 | MK-10 | Reference |
| Milk3 | ||||||||
| Whole | 0.8 | 0.1 | ND | ND–2.04 | ND | ND | ND | (28, 29) |
| Buttermilk | 0.2 | 0.1 | 0.1 | 0.1 | 0.6 | 1.4 | ND | (28) |
| Yogurt4 | ||||||||
| Whole | 0.6–1.0 | 0.1–0.3 | ND–0.2 | ND–0.4 | 0.2–2.0 | ND–4.7 | ND | (27–29) |
| Skimmed | ND | ND | ND | ND | ND–0.1 | ND | ND | (28) |
| Cheese4 | ||||||||
| Curd | 0.4 | 0.1 | 0.2 | 0.3 | 5.1 | 18.7 | ND | (28) |
| Hard | 4.7–10.2 | 1.5 | ND–3.0 | ND–2.3 | ND–16.9 | ND–51.1 | ND–6.5 | (20, 28, 32) |
| Semihard | NR | NR | 1.0–3.5 | ND–2.1 | 2.5–7.3 | 10.0–32.1 | ND–13.8 | (32) |
| Soft | 3.7 | 0.3 | 0.4–2.6 | ND–1.7 | 2.1–14.0 | 6.6–94.0 | ND–5.7 | (28, 32) |
| Other4 | ||||||||
| Salami | 9.0 | ND | ND | ND | ND | ND | ND | (28) |
| Sauerkraut | 0.4 | 0.8 | 1.5 | 0.2 | 0.8 | 1.1 | ND | (28) |
| Natto | ND–2.0 | 7.5 | 13.8 | 939–998 | 84.1 | ND | ND | (28, 29) |
MK, menaquinone; ND, not detectable; NR, not reported.
Values represent the mean concentration (if 1 study), or lowest and highest mean concentrations (if ≥2 studies or foods within the same category) reported in representative studies from the United States, Europe and Japan. Studies reported values as a range or mean of multiple samples for each food type. MK 11–13 concentrations were not reported in any study.
In μg/100 mL.
In μg/100 g.
The specific bacterial strains used and production conditions during fermentation (i.e., pH, temperature, duration) likely determine the concentrations and forms of MK found in fermented food products. For example, tetrahydromenaquinone [MK-9(4H)], produced by propionibacteria and formed from partial hydrogenation of the isoprenoid chain in MK-9 (25, 26), has been measured in different varieties of cheese in which propionibacteria are used in starter cultures (12). High concentrations of MK-8 and MK-9 measured in Edam type cheese (27) is consistent with the use of the MK-8– and MK-9–producing LAB strains Lactococcus lactis ssp. lactis and L. lactis ssp. cremoris as starter cultures for these cheeses. However, few studies have used validated HPLC methods to quantify MK in fermented foods (12, 20, 27–32). The available literature shows that MK-8 and MK-9 are the most common bacterially synthesized MKs found in fermented dairy products, with the presence of other MKs being more limited (Table 2). In a recent report, total long-chain MK concentrations ranged from nondetectable to 118 μg/100 g, with a median concentration of 15 μg/100 g in 62 European fermented dairy products (32). Long-chain MKs have also been measured in fermented plant-based foods such as sauerkraut and natto, a fermented soybean product popular in certain regions of Japan but not widely consumed elsewhere (28, 29). The long-chain MK contents of meat and fish products are generally low (28, 29) and likely have little public health importance. As such, the evidence available to date suggests that dairy products are likely the predominant dietary sources of long-chain MKs. In support of this claim, cheese and milk products were estimated to contribute to 54% and 22% of total MK intake, respectively, in a cohort of Dutch women in whom long-chain MKs were estimated to account for 9% of total vitamin K intake (24, 33). However, the absence of comprehensive data on food MK contents and regional differences in dairy consumption patterns indicate that much more research is needed to accurately quantify MK intake at the individual and population levels.
Enhancing food MK content
The diversity of bacterial species used in food fermentations has doubled in the past decade, increasing from 82 different species in 2002 to 195 in 2012 (34). Strains are selected based on their capacity to derive energy from organic compounds in foodstuffs (e.g., lactose), to form desired metabolites aiding food preservation (e.g., lactic acid), to inhibit pathogens, to remove toxins, and to improve organoleptic properties (34). Fermentation can also be used to improve the nutritional qualities of food products, which can include increasing MK content.
Emerging evidence purporting multiple health benefits of MKs (discussed below) has led to substantial industry interest in increasing MKs in the food supply, particularly through fermented foods. As discussed above, food production practices, bacterial strain selection in particular, are likely to provide a relevant means of altering the MK content of fermented foods. As such, selection of the most efficient bacterial producers of MKs granted generally recognized as safe (GRAS) status by the FDA is an active area of research within the food industry. The focus of this research is on bacteria used as starters in food fermentations and as catalysts in industrial production of MKs intended for use in dietary supplements. Although the MK forms are ubiquitous in bacteria, it should be noted that some genera considered to be mainly obligate fermentative have lost the functional ability to produce MKs. Among these, Lactobacillus and Streptococcus are the most relevant genera for this review based on their widespread use as starters in the dairy and meat fermentation industries and their presence in the food supply. Accordingly, foods using these bacteria as starters do not contain detectable amounts of long-chain MKs (32). However, a number of MK-producing species are commonly used in current industrial food fermentation applications (Table 3). The potential relevance to dietary vitamin K intake is exemplified in 1 study in which L. lactis subsp. cremoris, L. lactis subsp. lactis, and Leuconostoc lactis strains demonstrated the capacity to synthesize >230 nmol of MK-7 to MK-10/g of dried cells (35). Growing these strains in reconstituted nonfat dry milk or soy milk medium produced long-chain MK concentrations ranging from 29 to 123 μg/L, leading the authors to conclude that fermented foods could serve as an important dietary source of vitamin K (35). In addition to strain selection, manipulation of growth conditions is also known to alter bacterial MK production, although many of these techniques remain proprietary.
Table 3.
| Species/subspecies | Food use | MK-5 | MK-6 | MK-7 | MK-8 | MK-9 | MK-10 |
| Lactococcus lactis subsp. lactis | Cheese, buttermilk, sour cream, cottage cheese, cream cheese, kefir | √ | √ | √ | √√ | ||
| Lactococcus lactis subsp. cremoris | Cheese, buttermilk, sour cream, cottage cheese, cream cheese, kefir | √ | √ | √√ | |||
| Leuconostoc lactis | Cheese | √ | √ | √√ | |||
| Brevibacterium linens | Cheese | √ | |||||
| Brochontrix thermosphacta | Meat | √ | √ | √√ | |||
| Hafnia alvei | Cheese | √ | |||||
| Staphylococcus xylosus | Dairy, sausage | √ | √√ | √ | |||
| Staphylococcus equorum | Dairy, meat | √ | √√ | √ | |||
| Arthrobacter nicotinae | Cheese | √ | √√ | √ | |||
| Bacillus subtilis “natto” | Natto | √√ | |||||
| Propionibacterium shermanii | Cheese | √ |
MK, menaquinone; √, minor form; √√, major form.
Danisco internal data. Note that most species within the genera Lactobacillus, Bifidobacterium, and Streptococcus commonly used in fermentation or added to foods as probiotics are not known to produce MK.
In addition to the bacterial production of MK in dairy products, there has been considerable industrial interest in MK found in the traditional Japanese food, natto. Natto is a popular preparation of soybeans fermented with Bacillus “natto,” a Bacillus subtilis species. This species produces very high amounts of MK-7, reportedly ∼900–1000 μg/100 g natto (28, 29). Natto also contains modest amounts of MK-8 and PK (84 μg/100 g and 35 μg/100 g, respectively) (28). The genome of a natto production strain, Bacillus subtilis natto, was recently sequenced and annotated (36). Use of this genome database in the future will allow for a more comprehensive in silico investigation of MK production by this bacterial species, with an emphasis on ways to improve the bacterial productivity. Several methods have already been used, including classic mutagenesis and conferring resistance to analogs like menadione or diphenylamine, which have reportedly increased MK-7 production resulting in concentrations as high as 1720 μg MK-7/100 g natto (37). In the future, similar techniques may prove fruitful for increasing MK production among other bacterial strains.
Bioavailability
Intestinal absorption of all dietary forms of vitamin K appears to occur through the pathway common to most dietary lipids (3). Bile acids and pancreatic enzymes facilitate solubilization, emulsification, and incorporation of vitamin K into mixed micelles. After micelles are taken into enterocytes, vitamin K is repackaged into chylomicrons and enters the lymphatic circulation. The bioavailability of PK varies with the integrity of the food matrix and presence of dietary lipid (38, 39). Although the same is likely true of MK, few studies have addressed this issue, and there is little information regarding the relative absorption efficiency and subsequent transport, distribution, and cellular uptake of different MK isoprenologues (3, 40).
The limited available evidence suggests that among the different long-chain MKs, isoprenoid side-chain length may alter cellular uptake, transport, and storage. Differences in absorption and transport of vitamin K forms were demonstrated in a study comparing plasma PK, MK-4, and MK-9 concentrations after consumption of equivalent doses of each respective form (41). Postprandial plasma PK concentrations peaked at more than twice the relative concentration of that of MK-4 or MK-9, suggesting reduced absorption of MK relative to PK, faster uptake of MK into tissues, or both. In contrast to PK, which is predominantly concentrated in triglyceride-rich lipoproteins during the postprandial and fasting states (3), MK-4 and MK-9 were redistributed from triglyceride-rich lipoproteins to low-density lipoproteins during and after the postprandial period (41). Relative to MK-9, this redistribution was observed earlier with MK-4, and although MK-4 also incorporated into high-density lipoproteins, MK-9 did not (41). In addition, MK-9 was detectable in plasma for up to 48 h, whereas PK and MK-4 clearance was more rapid (41). The plasma kinetics of MK-9 were similar to those of MK-7, which has been shown to have a plasma half-life of several days, much longer than that of PK and MK-4 (28, 41–44). Plasma kinetics for other long-chain MK have not been investigated, and whether these kinetics extend long-chain MK availability for uptake by extrahepatic tissues or the increased hydrophobicity resulting from longer side-chain length decreases bioavailability is undetermined. Moreover, the relative absorption and transport of MK from different food sources are unknown as most MK bioavailability studies have used purified MK sources.
Within tissues, long-chain MKs appear to be abundant in the liver, comprising ∼90% of liver vitamin K content, but are present at much lower concentrations in extrahepatic tissues (45). In contrast, MK-4 appears to concentrate in the brain, kidney, and pancreas, which may reflect selective uptake of MK-4 or tissue-specific conversion from PK (46). Although animal models have suggested tissue-specific uptake of PK and MK-4 (47, 48), whether selective uptake of long-chain MK isoprenologues occurs is undetermined.
Dietary Recommendations
As reviewed elsewhere (40), dietary vitamin K recommendations are based on our current knowledge of PK. Recommended intakes currently range from 50 to 120 μg/d for adults 19 y and older (40). Depending on the country, these recommendations are generally presented as adequate intakes or estimated values, reflecting the uncertainty of which biochemical criteria should be used for determining dietary vitamin K requirements (40, 49). For example, maintaining carboxylation of extrahepatic vitamin K–dependent proteins requires higher dietary vitamin K intake than that which is sufficient for the classic function of maintaining carboxylation of hepatic vitamin K–dependent coagulation proteins (40). In other words, this hypothesis of tissue-specific vitamin K requirements suggests that dietary intakes sufficient to maintain coagulation may be insufficient to optimize vitamin K nutriture (2, 3). With respect to MK, contributions beyond that obtained in the diet (i.e., through intestinal bacterial synthesis and/or through conversion from PK) present additional challenges when defining dietary requirements (50).
Although there are differences in substrate affinity, all forms of vitamin K act as an enzyme cofactor and support carboxylation of vitamin K–dependent proteins. Recently, evidence suggesting potential exclusive physiological roles for MK-4 has generated considerable speculation (3). It is unlikely that MK-4 is formed from PK if its only function is as an enzyme cofactor. Instead, tissue-specific conversion suggests that MK-4 has unique function among the vitamin K forms. Recently, UBIAD1 was identified as the enzyme catalyzing prenylation of menadione to form MK-4 (51), leading to the speculation of a role for MK-4 in cholesterol metabolism (52). Several other functions unique to MK-4 have been proposed, including inhibition of oxidative cell death in primary cultures of oligodendrocyte precursors and immature neurons (53), apoptosis induction in leukemia and other malignant cell lines (54, 55), and as a ligand for the steroid xenobiotic receptor in bone cells (56). However, verification of these potential roles in mouse models has been complicated by the common use of standard rodent chow, which is rich in menadione (57). The menadione is converted to MK-4 (58), which remains in the tissues even after replacement of standard chow with menadione-free chow (59). Further, none of these potential roles for MK-4 have been sufficiently substantiated with empirical evidence in humans to be used to generate dietary requirements.
The capacity of intestinal bacteria to produce long-chain MKs that could become available for the host has been established for decades (8, 10, 60). However, despite recent interest in the human microbiome, very little progress has been made since the literature was last reviewed nearly 2 decades ago in understanding the role of MK synthesized by intestinal bacteria in human nutrition (60). Not all intestinal bacteria produce MK, and the contribution to human vitamin K nutriture is unknown with estimates of the total vitamin K requirement being met by endogenous MK production varying from 10% to 50% (10, 60). Long-chain MKs have been measured in the human ileum where bile salts are available to facilitate absorption (61). However, the majority of long chain MKs are found in the colon where bile salt concentrations are low (61). Thus, if MKs synthesized by intestinal bacteria substantially contribute to human vitamin K requirements, absorption likely takes place through a bile salt–independent route. Whether this occurs remains unclear, although separate studies have demonstrated poor absorption of MK-9 in animal models (62, 63). Moreover, the majority of bacterially synthesized MKs remains bound in bacterial membranes and are therefore not available for absorption. Although long-chain MKs are the primary form of vitamin K stored in the human liver (45), hepatic uptake of long-chain MKs is not well understood (3), and the relative contributions of dietary MKs and MKs synthesized by intestinal bacteria to total liver MK content are not known. The low turnover of hepatic long-chain MKs relative to PK (45) was once thought to be important in maintaining normal coagulation under conditions of chronic PK inadequacy (64), and antibiotic-induced hypoprothrombinemias were assumed to result from decreased MK synthesis by intestinal bacteria (65). However, these assumptions have since been consistently challenged by data from human studies demonstrating that subclinical vitamin K deficiency can be induced solely by limiting PK intake (66–68).
At present, no dietary intake recommendation for vitamin K differentiates between PK and MK. A recent review on the need for specific dietary reference values for MK commissioned by the International Life Sciences Institute of Europe concluded that at present significant knowledge gaps prevent establishing a reference value for MK intake, although future recommendations for vitamin K intake should consider both MK and PK (50). Some groups have suggested a recommended daily MK intake of 45 μg, comparable to the amount present in 100 g of some cheeses or 4 g of natto (47). However, in the absence of robust physiological endpoints that can be used to differentiate the contribution of MKs to human health from that of PK, it is unlikely that specific dietary recommendations such as these will be widely adopted in the near future. Instead, it may be preferable to recommend consumption of a wide variety of foods containing a combination of PK and MKs.
Currently, no tolerable upper intake level has been established for any form of vitamin K because no known toxicity is associated with high doses of PK or MK (49). However, menadione is not used clinically and is prohibited as a human food supplement in the United States due to concerns of liver toxicity (49).
MKs and health
Bone health
As reviewed elsewhere (2, 69), considerable attention has been given to the role of vitamin K in bone health, with an emphasis on MK. The biological basis for this has been the presence of vitamin K in bone and the dependence on vitamin K for carboxylation of multiple Gla-containing proteins synthesized in bone. The most notable of these proteins is osteocalcin (OC), a vitamin K–dependent protein synthesized by osteoblasts during bone formation and the predominant noncollagenous protein in bone (70). Chronically low vitamin K intake results in suboptimal carboxylation of OC, which is thought to lead to decreased bone mineralization and increased risk of fracture and osteoporosis (71). However, the relationship of OC carboxylation status to bone health is unclear (69). More recently, some have proposed mechanisms that are independent of vitamin K’s role as an enzyme cofactor and that may be exclusive to MKs. Numerous in vitro and in vivo studies have been conducted, but overall, there is a lack of consensus regarding differential effects of PK and MK on bone health (69).
In 2009, the European Food Safety Authority issued a scientific opinion concluding that “a cause and effect relationship has been established between the dietary intake of vitamin K and the maintenance of normal bone” (72). However, although both MK-4 and MK-7 supplementation consistently reduce the proportion of OC that is undercarboxylated (43), whether this effect favorably influences bone health is less clear (69). The first meta-analysis of MK-4 supplementation and bone health outcomes reported an overall benefit of MK-4 supplementation on reducing fracture risk (73). However, the authors articulated many caveats regarding their analysis, including geographic homogeneity, varied supplementation with other nutrients and medications, and heterogeneous study designs. In addition, the majority of these studies focused on MK-4 given therapeutically in daily doses of 45 mg, an amount unattainable through diet alone. A more recent meta-analysis combined findings of studies that used either PK or MK supplementation and examined bone mineral density (74). The authors concluded that there were modest improvements in bone mineral density with vitamin K treatment, but that the results should be interpreted with caution, especially as PK, MK-4, and MK-7 were combined in the analysis, which assumes equivalent bioavailability. Findings from the 2 included studies in which MK-7 supplementation alone was examined were inconsistent with bone health benefits documented at MK-7 doses of 180 μg/d over 1 y in 1 study (75), but not at 360 μg/d over 1 y in another (76).
Several relevant studies have been published since these meta-analyses. In a large clinical trial of >4000 postmenopausal women, supplementation with MK-4 and calcium compared with calcium supplementation alone provided no additional protection against bone fracture over 3 y (77). Kanellakis et al. (78) examined bone markers during a 1-y regimen in which postmenopausal women were randomized to daily consumption of dairy products fortified with calcium, vitamin D-3, and either no vitamin K, 100 μg PK, or 100 μg MK-7. After 1 y, all 3 interventions improved total bone mineral density relative to a group receiving no intervention. Although no additional benefit attributable to PK or MK-7 consumption was observed on total bone mineral density, both PK and MK-7 were shown to have favorable effects on lumbar spine bone mineral density relative to the control and calcium + vitamin D groups. In the longest clinical trial of MK-7 supplementation conducted to date, Knapen et al. (79) assessed bone health and strength in >200 postpmenopausal women randomized to receive a supplement containing 180 μg/d MK-7 or a placebo over 3 y. Site-specific effects of MK-7 were reported, with marginal attenuation of reductions in bone mineral content and bone mineral density observed at the lumbar spine and femoral neck. No effects of MK-7 supplementation were documented for the total hip, and the effects of MK-7 on bone strength indices were inconsistent. Limited evidence of favorable bone health effects when lower amounts of MK-7 are consumed from dietary sources also containing calcium and/or vitamin D (78, 80) suggests that the combination of nutrients rather than MK-7 alone may have greater efficacy in improving bone health. In this regard, it is also important to note that, to date, there is no indication of greater efficacy of dietary MK relative to PK (78, 81, 82). Further, as is discussed later, supplemental MK-7 has adverse effects on coagulation parameters in individuals receiving oral anticoagulant therapy (83, 84). Therefore, any putative benefit of MK-7 supplementation on bone health will need to be balanced with potential safety concerns regarding stability of oral anticoagulant therapy among patients on vitamin K–antagonists.
Cancer
Although the mechanisms are still being elucidated, in vitro studies suggest that MK may arrest cell growth and induce apoptosis (3). A small number of in vivo studies have investigated the efficacy of MK-4 supplementation in doses far in excess of what could be achieved in the diet for preventing hepatocellular carcinoma in high-risk patients. In a secondary analysis of a trial investigating MK-4 supplementation and bone loss, 45 mg/d of MK-4 was shown to reduce hepatocellular carcinoma risk in women with viral cirrhosis (85). However, results from subsequent studies investigating the efficacy of MK-4 supplementation for preventing hepatocellular carcinoma recurrence have been equivocal (86–89). MKs have not been tested as an adjunct cancer treatment. The most likely explanation for this is that vitamin K supplementation is contraindicated in cancer patients who are at increased risk of venous thromboembolism and are often prescribed vitamin K antagonists (90, 91). Limited evidence is available to suggest that vitamin K intake reduces cancer risk in healthy adults. In 1 large prospective cohort, inverse relationships between dietary MK intake with incidence of prostate cancer, overall cancer incidence, and cancer mortality were reported (92, 93). However, replication of these findings is needed.
Cardiovascular health
Calcification of vessel walls reduces their elasticity, increasing cardiovascular disease risk (94). There is growing recognition that oral anticoagulants such as warfarin may be associated with increased arterial calcification (95). As recently reviewed in detail elsewhere (96), anticoagulants inhibit the vitamin K–dependent carboxylation of matrix-Gla protein (MGP) in vascular smooth-muscle cells. In its carboxylated form, MGP functions as a calcification inhibitor. As such, anticoagulants are thought to impair the calcium-regulating activity of MGP, thereby leading to increased calcium deposition in the vessel walls. The functional dependence of MGP on vitamin K and associations between anticoagulant use and calcification underpin current speculation that vitamin K may be involved in the progression of cardiovascular disease.
Only 1 randomized clinical trial examining vitamin K supplementation and coronary artery calcification (CAC) has been published to date. In this trial, PK supplementation reduced progression of existing CAC, although there was no effect on incidence of CAC (97). These findings are consistent with the proposed biological mechanism of MGP, which inhibits further calcification (98). In another study, postmenopausal women receiving a supplement containing PK demonstrated better carotid artery compliance and elasticity after 3 y than those receiving a similar supplement with no PK (99). However, although suggestive, no direct measures of calcification were reported.
One would predict that the impact of PK and MK supplementation on reducing progression of vascular calcification should be similar given that both forms support the γ-carboxylation of MGP. However, a series of observational studies suggest possible differential effects of MK and PK on arterial calcification and coronary heart disease (CHD) risk. For example, inverse associations between MK intake and severe aortic calcification (33, 100), the risk of CHD (24), and the risk of CHD mortality and all-cause mortality (100) have been reported. In these studies, estimated MK intakes were up to 10 times lower than PK intakes, and PK intakes were not associated with disease risk. In the Dutch PROSPECT study cohort, the reduction in CHD risk was mainly driven by MK-7, MK-8, and MK-9 consumption (21).
Although these findings are intriguing and have stimulated considerable interest in a potential therapeutic role for MK in the progression of CAC and CHD, these observational data should be interpreted with caution. Unlike PK, long-chain MKs are not detectable in circulation unless provided in high doses, either in the form of a supplement (42) or a concentrated food source, such as natto (28). Interestingly, MK-4 is often not detectable in the circulation, even after administration with doses as high as 420 μg (42). This is problematic for validation of self-reported intakes of MK as there is no comparable biomarker. Use of vitamin K biomarkers that measure the proportion of carboxylation of vitamin K–dependent proteins reflect the dietary contribution of all forms of vitamin K and cannot be used to validate intakes of a single MK. Because food composition databases for long-chain MKs are unfortunately not available, it is unlikely that the epidemiological data can be replicated in other countries in the near future. Alternatively, the findings may reflect the use of MKs as a marker of another nutrient or food constituent that has heart-healthy properties (101). For example, dairy products, which were the primary dietary source of MKs in the Dutch PROSPECT study cohort, contain specific fatty acids that may confer protective effects on CHD risk (102). Notably an inverse association between CHD risk and the consumption of meat, milk, and cheese, among the richest sources for MKs in the Dutch food supply, was also observed (24, 28). Given the challenges in interpreting the results of these studies, there is clearly a need for randomized clinical trials that isolate any putative effect of individual MKs on CAC. There have been recent media reports that nutritional doses of MK-7 (180 μg/d) taken over a 3-y period prevented age-related arterial stiffening in postmenopausal women (103). However, the results of the clinical trial were not available in the peer-reviewed literature at the time of this review, and the unique protective role of MK in heart health remains speculative.
MKs and oral anticoagulation therapy
The γ-carboxylation reaction is critical to the calcium-binding function of vitamin K–dependent proteins involved in coagulation (2). Vitamin K antagonists such as warfarin and acenocoumarol are widely prescribed oral anticoagulants for the prevention and treatment of thrombosis (104, 105). These drugs inhibit enzymes involved in vitamin K recycling resulting in insufficient γ-carboxylation of vitamin K–dependent coagulation proteins (4). Oral anticoagulant therapy is challenging as both insufficient or excessive doses can increase the risk of thrombotic or bleeding events (106). Variable vitamin K intake has been identified as a risk factor for instability of oral anticoagulant therapy, and high vitamin K intake can result in suboptimal anticoagulation (107).
The contribution of dietary MKs to oral anticoagulant therapy stability is not well understood. Using healthy adults maintained on acenocoumarol, Schurgers et al. (84) demonstrated that, on a molar basis, MK-7 appears to be 3.5 times more potent than PK when administered as a dietary supplement. In that study, the lowest dose of MK-7 administered (95 μg/d) was sufficient to significantly reduce the efficacy of anticoagulation therapy. More recently, the same group investigated the effects of lower daily doses of supplemental MK-7 on oral anticoagulation therapy. In healthy adults stabilized at the lower standard therapeutic range, Theuwissen et al. (83) reported that 45 μg/d of supplemental MK-7 significantly reduced measures of clotting function at the group level. At the individual level, the authors concluded that in 40% of cases as little as 10 μg/d of MK-7 was sufficient to produce increases in clotting activity requiring oral anticoagulant dose adjustments in a clinical setting. However, it is important to note that this study was conducted in healthy adults maintained for ethical reasons at the lower end of the therapeutic range, which may have magnified the relevance of the small changes documented. Nonetheless, the results do suggest that 45 μg/d of supplemental MK-7 may interfere with oral anticoagulant therapy. The amount of MK that would need to be consumed from dietary sources to have a similar effect is unknown. Until robust relative bioavailability studies are conducted to compare absorption and uptake of individual MKs relative to PK, it is unlikely that patients on oral anticoagulants who obtain modest amounts of MK in their diets through dairy and meat consumption will be counseled to change their dietary habits in the near future.
Conclusions
Much of our understanding of the health effects of vitamin K remains focused on PK, the major dietary form. Major strides have recently been made in our understanding of MK-4 as an active form of vitamin K that may have functions extending beyond that of vitamin K’s classic role as an enzyme cofactor. However, MK-4 is unique among the MKs in that it is a metabolite of PK and not of bacterial origin. Although there is speculation that bacterially synthesized MK may also convert to MK-4, the empirical evidence is currently lacking. Similarly, our understanding of the absorption and metabolism of bacterially synthesized MK has not progressed much in the past few decades. As reviewed elsewhere (40), the use of stable isotope technology has been paramount to understanding PK metabolism. Similar studies are needed for individual MKs to determine their bioavailability and relative contribution to human health and to appropriately test current claims in the media that certain MKs are superior forms of vitamin K (50). In parallel, studies are needed to generate systematic and comprehensive food composition data for MKs, which, to date, are largely absent from national food composition databases. Current knowledge indicates that dairy products are poised to be primary contributors to dietary MK intake, and industrial application of MK-producing bacteria in fermented dairy products may provide an effective means of increasing MK in the food supply. However, the importance of MK in the food supply requires additional empirical evidence to substantiate whether putative health benefits are conferred at intakes that can be achieved through dietary means alone.
Acknowledgments
The authors acknowledge the support of the International Dairy Federation in preparation of this review. All authors have read and approved the final version of this manuscript.
Footnotes
Abbreviations used: CAC, coronary artery calcification; CHD, coronary heart disease; DMK, demethylmenaquinone; LAB, lactic acid bacteria; MGP, matrix-Gla protein; MK, menaquinone; OC, osteocalcin; PK, phylloquinone.
Literature Cited
- 1.Booth SL. Vitamin K: food composition and dietary intakes. Food Nutr Res. 2012;56. [DOI] [PMC free article] [PubMed]
- 2.Booth SL. Roles for vitamin K beyond coagulation. Annu Rev Nutr. 2009;29:89–110 [DOI] [PubMed] [Google Scholar]
- 3.Shearer MJ, Newman P. Metabolism and cell biology of vitamin K. Thromb Haemost. 2008;100:530–47 [PubMed] [Google Scholar]
- 4.Suttie JW. Vitamin K in health and disease. Boca Raton, FL: Taylor & Francis Group, LLC; 2009.
- 5.Okano T, Shimomura Y, Yamane M, Suhara Y, Kamao M, Sugiura M, Nakagawa K. Conversion of phylloquinone (vitamin K1) into menaquinone-4 (vitamin K2) in mice: two possible routes for menaquinone-4 accumulation in cerebra of mice. J Biol Chem. 2008;283:11270–9 [DOI] [PubMed] [Google Scholar]
- 6.Hiratsuka T, Furihata K, Ishikawa J, Yamashita H, Itoh N, Seto H, Dairi T. An alternative menaquinone biosynthetic pathway operating in microorganisms. Science. 2008;321:1670–3 [DOI] [PubMed] [Google Scholar]
- 7.Nowicka B, Kruk J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim Biophys Acta. 2010;1797:1587–605. [DOI] [PubMed]
- 8.Bentley R, Meganathan R. Biosynthesis of vitamin K (menaquinone) in bacteria. Microbiol Rev. 1982;46:241–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins MD, Jones D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol Rev. 1981;45:316–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conly JM, Stein K. The production of menaquinones (vitamin K-2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog Food Nutr Sci. 1992;16:307–43 [PubMed] [Google Scholar]
- 11.Farrand SK, Taber HW. Changes in menaquinone concentration during growth and early sporulation in bacillus subtilis. J Bacteriol. 1974;117:324–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hojo K, Watanabe R, Mori T, Taketomo N. Quantitative measurement of tetrahydromenaquinone-9 in cheese fermented by propionibacteria. J Dairy Sci. 2007;90:4078–83 [DOI] [PubMed] [Google Scholar]
- 13.Pedersen MB, Gaudu P, Lechardeur D, Petit MA, Gruss A. Aerobic respiration metabolism in lactic acid bacteria and uses in biotechnology. Annu Rev Food Sci Technol. 2012;3:37–58 [DOI] [PubMed] [Google Scholar]
- 14.Sijpesteijn AK. Induction of cytochrome formation and stimulation of oxidative dissimilation by hemin in streptococcus lactis and leuconostoc mesenteroides. Antonie van Leeuwenhoek. 1970;36:335–48 [DOI] [PubMed] [Google Scholar]
- 15.Whittenbury R. Biochemical characteristics of streptococcus species. Soc Appl Bacteriol Symp Ser. 1978;7:51–69 [PubMed] [Google Scholar]
- 16.Duwat P, Sourice S, Cesselin B, Lamberet G, Vido K, Gaudu P, Le Loir Y, Violet F, Loubiere P, Gruss A. Respiration capacity of the fermenting bacterium lactococcus lactis and its positive effects on growth and survival. J Bacteriol. 2001;183:4509–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooijmans R, Smit B, Santos F, van Riel J, de Vos WM, Hugenholtz J. Heme and menaquinone induced electron transport in lactic acid bacteria. Microb Cell Fact. 2009;8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaudu P, Vido K, Cesselin B, Kulakauskas S, Tremblay J, Rezaiki L, Lamberret G, Sourice S, Duwat P, Gruss A. Respiration capacity and consequences in lactococcus lactis. Antonie van Leeuwenhoek. 2002;82:263–9 [PubMed] [Google Scholar]
- 19. Open database: USDA National Nutrient Database for Standard Reference, Release 25 [Internet]. [updated 2012 Dec 14; cited 2012 Dec 30]. Available from: http://www.ars.usda.gov/Services/docs.htm?docid=8964.
- 20.Elder SJ, Haytowitz DB, Howe J, Peterson JW, Booth SL. Vitamin /k contents of meat, dairy, and fast food in the U.S. diet. J Agric Food Chem. 2006;54:463–7 [DOI] [PubMed] [Google Scholar]
- 21.Pillai PB, Alewynse MG, Benz SA. Vitamin K substances and animal feed. FDA Veterinarian Newsletter [Internet]. 2008 [cited 2013 Feb 4];23(5). Available from: http://www.fda.gov/AnimalVeterinary/NewsEvents/FDAVeterinarianNewsletter/ucm149087.htm.
- 22.Wells HF, Buzby JC. Dietary assessment of major trends in U.S. food consumption, 1970–2005. Economic Information Bulletin No (EIB-33) p 27, March 2008.
- 23.Schurgers LJ, Geleijnse JM, Grobbee DE, Hofman A, Witteman JC, Vermeer C. Nutritional intake of vitamins K1 (phylloquinone) and K2 (menaquinone) in the Netherlands. J Nutr Environ Med. 1999;9:115–22 [Google Scholar]
- 24.Gast GC, de Roos NM, Sluijs I, Bots ML, Beulens JW, Geleijnse JM, Witteman JC, Grobbee DE, Peeters PH, van der Schouw YT. A high menaquinone intake reduces the incidence of coronary heart disease. Nutr Metab Cardiovasc Dis. 2009;19:504–10 [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AC. Terpenoid quinones of the anaerobic propionibacterium shermanii. I. (ii,3)-tetrahydromenaquinone-9. Arch Mikrobiol. 1973;91:273–9 [DOI] [PubMed] [Google Scholar]
- 26.Furuichi K, Hojo K, Katakura Y, Ninomiya K, Shioya S. Aerobic culture of propionibacterium freudenreichii et-3 can increase production ratio of 1,4-dihydroxy-2-naphthoic acid to menaquinone. J Biosci Bioeng. 2006;101:464–70 [DOI] [PubMed] [Google Scholar]
- 27.Koivu-Tikkanen TJ, Ollilainen V, Piironen VI. Determination of phylloquinone and menaquinones in animal products with fluorescence detection after postcolumn reduction with metallic zinc. J Agric Food Chem. 2000;48:6325–31 [DOI] [PubMed] [Google Scholar]
- 28.Schurgers LJ, Vermeer C. Determination of phylloquinone and menaquinones in food. Effect of food matrix on circulating vitamin K concentrations. Haemostasis. 2000;30:298–307 [DOI] [PubMed] [Google Scholar]
- 29.Kamao M, Suhara Y, Tsugawa N, Uwano M, Yamaguchi N, Uenishi K, Ishida H, Sasaki S, Okano T. Vitamin K content of foods and dietary vitamin K intake in Japanese young women. J Nutr Sci Vitaminol (Tokyo). 2007;53:464–70 [DOI] [PubMed] [Google Scholar]
- 30.Indyk HE, Woollard DC. Vitamin K in milk and infant formulas: determination and distribution of phylloquinone and menaquinone-4. Analyst. 1997;122:465–9 [DOI] [PubMed] [Google Scholar]
- 31.Vermeer C, Knapen MH, Schurgers LJ. Vitamin K and metabolic bone disease. J Clin Pathol. 1998;51:424–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manoury E, Jourdon K, Boyaval P, Fourcassie P. Quantitative measurement of vitamin K(2) (menaquinones) in various fermented dairy products using a reliable high-performance liquid chromatography method. J Dairy Sci. 2013;96:1335–46 [DOI] [PubMed] [Google Scholar]
- 33.Beulens JW, Bots ML, Atsma F, Bartelink ML, Prokop M, Geleijnse JM, Witteman JC, Grobbee DE, van der Schouw YT. High dietary menaquinone intake is associated with reduced coronary calcification. Atherosclerosis. 2009;203:489–93 [DOI] [PubMed] [Google Scholar]
- 34.Bourdichon F, Casaregola S, Farrokh C, Frisvad JC, Gerds ML, Hammes WP, Harnett J, Huys G, Laulund S, Ouwehand A, et al. Food fermentations: microorganisms with technological beneficial use. Int J Food Microbiol. 2012;154:87–97 [DOI] [PubMed] [Google Scholar]
- 35.Morishita T, Tamura N, Makino T, Kudo S. Production of menaquinones by lactic acid bacteria. J Dairy Sci. 1999;82:1897–903 [DOI] [PubMed] [Google Scholar]
- 36.Nishito Y, Osana Y, Hachiya T, Popendorf K, Toyoda A, Fujiyama A, Itaya M, Sakakibara Y. Whole genome assembly of a natto production strain bacillus subtilis natto from very short read data. BMC Genomics. 2010;11:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukamoto Y, Kasai M, Kakuda H. Construction of a bacillus subtilis (natto) with high productivity of vitamin K2 (menaquinone-7) by analog resistance. Biosci Biotechnol Biochem. 2001;65:2007–15 [DOI] [PubMed] [Google Scholar]
- 38.Booth SL, Lichtenstein AH, Dallal GE. Phylloquinone absorption from phylloquinone-fortified oil is greater than from a vegetable in younger and older men and women. J Nutr. 2002;132:2609–12 [DOI] [PubMed] [Google Scholar]
- 39.Jones KS, Bluck LJ, Wang LY, Stephen AM, Prynne CJ, Coward WA. The effect of different meals on the absorption of stable isotope-labelled phylloquinone. Br J Nutr. 2009;102:1195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shearer MJ, Fu X, Booth SL. Vitamin K nutrition, metabolism, and requirements: current concepts and future research. Adv Nutr. 2012;3:182–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schurgers LJ, Vermeer C. Differential lipoprotein transport pathways of K-vitamins in healthy subjects. Biochim Biophys Acta. 2002;1570:27–32 [DOI] [PubMed] [Google Scholar]
- 42.Sato T, Schurgers LJ, Uenishi K. Comparison of menaquinone-4 and menaquinone-7 bioavailability in healthy women. Nutr J. 2012;11:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C. Vitamin K-containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood. 2007;109:3279–83 [DOI] [PubMed] [Google Scholar]
- 44.Novotny JA, Kurilich AC, Britz SJ, Baer DJ, Clevidence BA. Vitamin K absorption and kinetics in human subjects after consumption of 13c-labelled phylloquinone from kale. Br J Nutr. 2010;104:858–62 [DOI] [PubMed] [Google Scholar]
- 45.Usui Y, Tanimura H, Nishimura N, Kobayashi N, Okanoue T, Ozawa K. Vitamin K concentrations in the plasma and liver of surgical patients. Am J Clin Nutr. 1990;51:846–52 [DOI] [PubMed] [Google Scholar]
- 46.Thijssen HH, Drittij-Reijnders MJ. Vitamin K status in human tissues: Tissue-specific accumulation of phylloquinone and menaquinone-4. Br J Nutr. 1996;75:121–7 [DOI] [PubMed] [Google Scholar]
- 47.Schurgers LJ, Dissel PE, Spronk HM, Soute BA, Dhore CR, Cleutjens JP, Vermeer C. Role of vitamin K and vitamin K-dependent proteins in vascular calcification. Z Kardiol. 2001;90: Suppl 3:57–63 [DOI] [PubMed] [Google Scholar]
- 48.Ronden JE, Thijssen HH, Vermeer C. Tissue distribution of K-vitamers under different nutritional regimens in the rat. Biochim Biophys Acta. 1998;1379:16–22 [DOI] [PubMed] [Google Scholar]
- 49.Institute of Medicine. Dietary reference intakes for vitamin A, vitamin K, arsenic boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. Washington, DC: National Academies Press, 2001. [PubMed]
- 50.Beulens JW, Booth SL, van den Heuvel EG, Stoecklin E, Baka A, Vermeer C. The role of menaquinones (vitamin K2) in human health. Br J Nutr. 2013 April 16; 1–12 Epub [DOI] [PubMed] [Google Scholar]
- 51.Nakagawa K, Hirota Y, Sawada N, Yuge N, Watanabe M, Uchino Y, Okuda N, Shimomura Y, Suhara Y, Okano T. Identification of UBIAD1 as a novel human menaquinone-4 biosynthetic enzyme. Nature. 2010;468:117–21 [DOI] [PubMed] [Google Scholar]
- 52.Nickerson ML, Bosley AD, Weiss JS, Kostiha BN, Hirota Y, Brandt W, Esposito D, Kinoshita S, Wessjohann L, Morham SG, et al. The UBIAD1 prenyltransferase links menaquione-4 synthesis to cholesterol metabolic enzymes. Hum Mutat. 2013;34:317–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li J, Lin JC, Wang H, Peterson JW, Furie BC, Furie B, Booth SL, Volpe JJ, Rosenberg PA. Novel role of vitamin K in preventing oxidative injury to developing oligodendrocytes and neurons. J Neurosci. 2003;23:5816–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoshida T, Miyazawa K, Kasuga I, Yokoyama T, Minemura K, Ustumi K, Aoshima M, Ohyashiki K. Apoptosis induction of vitamin K2 in lung carcinoma cell lines: the possibility of vitamin K2 therapy for lung cancer. Int J Oncol. 2003;23:627–32 [PubMed] [Google Scholar]
- 55.Yaguchi M, Miyazawa K, Katagiri T, Nishimaki J, Kizaki M, Tohyama K, Toyama K. Vitamin K2 and its derivatives induce apoptosis in leukemia cells and enhance the effect of all-trans retinoic acid. Leukemia. 1997;11:779–87 [DOI] [PubMed] [Google Scholar]
- 56.Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, et al. Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem. 2003;278:43919–27 [DOI] [PubMed] [Google Scholar]
- 57.Fu X, Booth SL, Smith DE. Vitamin K contents of rodent diets: a review. J Am Assoc Lab Anim Sci. 2007;46:8–12 [PubMed] [Google Scholar]
- 58.Davidson RT, Foley AL, Engelke JA, Suttie JW. Conversion of dietary phylloquinone to tissue menaquinone-4 in rats is not dependent on gut bacteria. J Nutr. 1998;128:220–3 [DOI] [PubMed] [Google Scholar]
- 59.Al Rajabi A, Booth SL, Peterson JW, Choi SW, Suttie JW, Shea MK, Miao B, Grusak MA, Fu X. Deuterium-labeled phylloquinone has tissue-specific conversion to menaquinone-4 among fischer 344 male rats. J Nutr. 2012;142:841–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suttie JW. The importance of menaquinones in human nutrition. Annu Rev Nutr. 1995;15:399–417 [DOI] [PubMed] [Google Scholar]
- 61.Conly JM, Stein K. Quantitative and qualitative measurements of K vitamins in human intestinal contents. Am J Gastroenterol. 1992;87:311–6 [PubMed] [Google Scholar]
- 62.Ichihashi T, Takagishi Y, Uchida K, Yamada H. Colonic absorption of menaquinone-4 and menaquinone-9 in rats. J Nutr. 1992;122:506. [DOI] [PubMed] [Google Scholar]
- 63.Groenen-van Dooren MM, Ronden JE, Soute BA, Vermeer C. Bioavailability of phylloquinone and menaquinones after oral and colorectal administration in vitamin K-deficient rats. Biochem Pharmacol. 1995;50:797–801 [DOI] [PubMed] [Google Scholar]
- 64.Ramotar K, Conly J, Chubb H, Louie T. Production of menaquinones by intestinal anaerobes. J Infect Dis. 1984;150:213–8 [DOI] [PubMed] [Google Scholar]
- 65.Frick PG, Riedler G, Brogli H. Dose response and minimal daily requirement for vitamin k in man. J Appl Physiol. 1967;23:387–9 [DOI] [PubMed] [Google Scholar]
- 66.Booth SL, Lichtenstein AH, O'Brien-Morse M, McKeown NM, Wood RJ, Saltzman E, Gundberg CM. Effects of a hydrogenated form of vitamin K on bone formation and resorption. Am J Clin Nutr. 2001;74:783–90 [DOI] [PubMed] [Google Scholar]
- 67.Booth SL, Martini L, Peterson JW, Saltzman E, Dallal GE, Wood RJ. Dietary phylloquinone depletion and repletion in older women. J Nutr. 2003;133:2565–9 [DOI] [PubMed] [Google Scholar]
- 68.Ferland G, Sadowski JA, O'Brien ME. Dietary induced subclinical vitamin K deficiency in normal human subjects. J Clin Invest. 1993;91:1761–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gundberg CM, Lian JB, Booth SL. Vitamin K-dependent carboxylation of osteocalcin: friend or foe? Adv Nutr. 2012;3:149–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047 [DOI] [PubMed] [Google Scholar]
- 71.Vermeer C, Theuwissen E. Vitamin K, osteoporosis and degenerative diseases of ageing. Menopause Int. 2011;17:19–23 [DOI] [PubMed] [Google Scholar]
- 72. EFSA Panel on Dietetic Products Nutrition, and Allergies (NDA). Scientific opinion on the substantiation of health claims related to vitamin K and maintenance of bones (ID 123, 127, 128 and 2879), blood coagulation (ID 124 and 126), and function of the heart and blood vessels (ID 124, 125 and 2880) pursuant to Article 13(1) of Regulation (EC) No 1924/2006 on request from the European Commission. EFSA J. 2009;7:1228.
- 73.Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ. Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med. 2006;166:1256–61 [DOI] [PubMed] [Google Scholar]
- 74.Fang Y, Hu C, Tao X, Wan Y, Tao F. Effect of vitamin K on bone mineral density: a meta-analysis of randomized controlled trials. J Bone Miner Metab. 2012;30:60–8 [DOI] [PubMed] [Google Scholar]
- 75.Forli L, Bollerslev J, Simonsen S, Isaksen GA, Kvamsdal KE, Godang K, Gadeholt G, Pripp AH, Bjortuft O. Dietary vitamin K2 supplement improves bone status after lung and heart transplantation. Transplantation. 2010;89:458–64 [DOI] [PubMed] [Google Scholar]
- 76.Emaus N, Gjesdal CG, Almas B, Christensen M, Grimsgaard AS, Berntsen GK, Salomonsen L, Fonnebo V. Vitamin K2 supplementation does not influence bone loss in early menopausal women: A randomised double-blind placebo-controlled trial. Osteoporos Int. 2010;21:1731–40 [DOI] [PubMed] [Google Scholar]
- 77.Inoue T, Fujita T, Kishimoto H, Makino T, Nakamura T, Sato T, Yamazaki K. Randomized controlled study on the prevention of osteoporotic fractures (OF study): a phase iv clinical study of 15-mg menatetrenone capsules. J Bone Miner Metab. 2009;27:66–75 [DOI] [PubMed] [Google Scholar]
- 78.Kanellakis S, Moschonis G, Tenta R, Schaafsma A, van den Heuvel EG, Papaioannou N, Lyritis G, Manios Y. Changes in parameters of bone metabolism in postmenopausal women following a 12-month intervention period using dairy products enriched with calcium, vitamin D, and phylloquinone (vitamin K(1)) or menaquinone-7 (vitamin K(2)): The postmenopausal health study II. Calcif Tissue Int. 2012;90:251–62 [DOI] [PubMed] [Google Scholar]
- 79.Knapen MH, Drummen NE, Smit E, Vermeer C, Theuwissen E. Three-year low-dose menaquinone-7 supplementation helps decrease bone loss in healthy postmenopausal women. Osteoporos Int. Epub 2013 Mar 23 [DOI] [PubMed] [Google Scholar]
- 80.Katsuyama H, Ideguchi S, Fukunaga M, Fukunaga T, Saijoh K, Sunami S. Promotion of bone formation by fermented soybean (natto) intake in premenopausal women. J Nutr Sci Vitaminol (Tokyo). 2004;50:114–20 [DOI] [PubMed] [Google Scholar]
- 81.Tsugawa N, Shiraki M, Suhara Y, Kamao M, Ozaki R, Tanaka K, Okano T. Low plasma phylloquinone concentration is associated with high incidence of vertebral fracture in Japanese women. J Bone Miner Metab. 2008;26:79–85 [DOI] [PubMed] [Google Scholar]
- 82.Apalset EM, Gjesdal CG, Eide GE, Tell GS. Intake of vitamin K1 and K2 and risk of hip fractures: The Hordaland Health Study. Bone. 2011;49:990–5 [DOI] [PubMed] [Google Scholar]
- 83.Theuwissen E, Teunissen K, Spronk H, Hamulyak K, Cate HT, Shearer M, Vermeer C, Schurgers L. Effect of low-dose supplements of menaquinone-7 [vitamin K2(35)] on the stability of oral anticoagulant treatment: dose-response relationship in healthy volunteers. J Thromb Haemost. Epub Mar 26 2013 [DOI] [PubMed] [Google Scholar]
- 84.Schurgers LJ, Shearer MJ, Hamulyak K, Stocklin E, Vermeer C. Effect of vitamin K intake on the stability of oral anticoagulant treatment: dose-response relationships in healthy subjects. Blood. 2004;104:2682–9 [DOI] [PubMed] [Google Scholar]
- 85.Habu D, Shiomi S, Tamori A, Takeda T, Tanaka T, Kubo S, Nishiguchi S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA. 2004;292:358–61 [DOI] [PubMed] [Google Scholar]
- 86.Kakizaki S, Sohara N, Sato K, Suzuki H, Yanagisawa M, Nakajima H, Takagi H, Naganuma A, Otsuka T, Takahashi H, et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J Gastroenterol Hepatol. 2007;22:518–22 [DOI] [PubMed] [Google Scholar]
- 87.Kojima K, Tamano M, Akima T, Hashimoto T, Kuniyoshi T, Maeda C, Majima Y, Kusano K, Murohisa T, Iijima M, et al. Effect of vitamin K2 on the development of hepatocellular carcinoma in type c cirrhosis. Hepatogastroenterology. 2010;57:1264–7 [PubMed] [Google Scholar]
- 88.Mizuta T, Ozaki I, Eguchi Y, Yasutake T, Kawazoe S, Fujimoto K, Yamamoto K. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: a pilot study. Cancer. 2006;106:867–72 [DOI] [PubMed] [Google Scholar]
- 89.Yoshida H, Shiratori Y, Kudo M, Shiina S, Mizuta T, Kojiro M, Yamamoto K, Koike Y, Saito K, Koyanagi N, et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology. 2011;54:532–40 [DOI] [PubMed] [Google Scholar]
- 90.Pasierski T. Vitamin K antagonists in anticoagulant therapy of patients with cancer. Pol Arch Med Wewn. 2012;122:60–4 [PubMed] [Google Scholar]
- 91.Verso M, Agnelli G. New and old anticoagulants in cancer. Thromb Res. 2012;129: Suppl 1:S101–5 [DOI] [PubMed] [Google Scholar]
- 92.Nimptsch K, Rohrmann S, Linseisen J. Dietary intake of vitamin K and risk of prostate cancer in the heidelberg cohort of the European Prospective Investigation Into Cancer and nutrition (EPIC-Heidelberg). Am J Clin Nutr. 2008;87:985–92 [DOI] [PubMed] [Google Scholar]
- 93.Nimptsch K, Rohrmann S, Kaaks R, Linseisen J. Dietary vitamin K intake in relation to cancer incidence and mortality: results from the Heidelberg cohort of the European Prospective Investigation Into Cancer and Nutrition (EPIC-Heidelberg). Am J Clin Nutr. 2010;91:1348–58 [DOI] [PubMed] [Google Scholar]
- 94.Karwowski W, Naumnik B, Szczepanski M, Mysliwiec M. The mechanism of vascular calcification - a systematic review. Med Sci Monit. 2012;18:RA1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chatrou ML, Winckers K, Hackeng TM, Reutelingsperger CP, Schurgers LJ. Vascular calcification: the price to pay for anticoagulation therapy with vitamin K-antagonists. Blood Rev. 2012;26:155–66 [DOI] [PubMed] [Google Scholar]
- 96.Theuwissen E, Smit E, Vermeer C. The role of vitamin K in soft-tissue calcification. Adv Nutr. 2012;3:166–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shea MK, O'Donnell CJ, Hoffmann U, Dallal GE, Dawson-Hughes B, Ordovas JM, Price PA, Williamson MK, Booth SL. Vitamin K supplementation and progression of coronary artery calcium in older men and women. Am J Clin Nutr. 2009;89:1799–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shanahan CM, Proudfoot D, Farzaneh-Far A, Weissberg PL. The role of Gla proteins in vascular calcification. Crit Rev Eukaryot Gene Expr. 1998;8:357–75 [DOI] [PubMed] [Google Scholar]
- 99.Braam L, McKeown N, Jacques P, Lichtenstein A, Vermeer C, Wilson P, Booth S. Dietary phylloquinone intake as a potential marker for a heart-healthy dietary pattern in the Framingham Offspring cohort. J Am Diet Assoc. 2004;104:1410–4 [DOI] [PubMed] [Google Scholar]
- 100.Geleijnse JM, Vermeer C, Grobbee DE, Schurgers LJ, Knapen MH, van der Meer IM, Hofman A, Witteman JC. Dietary intake of menaquinone is associated with a reduced risk of coronary heart disease: the Rotterdam study. J Nutr. 2004;134:3100–5 [DOI] [PubMed] [Google Scholar]
- 101.Erkkilä AT, Booth SL. Vitamin K intake and atherosclerosis. Curr Opin Lipidol. 2008;19:39–42 [DOI] [PubMed] [Google Scholar]
- 102.German JB, Dillard CJ. Composition, structure and absorption of milk lipids: a source of energy, fat-soluble nutrients and bioactive molecules. Crit Rev Food Sci Nutr. 2006;46:57–92 [DOI] [PubMed] [Google Scholar]
- 103.NattoPharma.com[Internet] MENAQ7®: 3 year human study with vitamin K2 demonstrates that MenaQ7® significantly improves bone strength, and prevents cardiovascular aging. [updated 2012 May 22; cited 2013 Feb 4]. Available from: http://www.cisionwire.com/nattopharma-asa/r/menaq7–3-year-human-study-with-vitamin-k2-demonstrates-that-menaq7–significantly-improves-bone-str,c9262978.
- 104.Mega JL. A new era for anticoagulation in atrial fibrillation. N Engl J Med. 2011;365:1052–4 [DOI] [PubMed] [Google Scholar]
- 105.Cropp JS, Bussey HI. A review of enzyme induction of warfarin metabolism with recommendations for patient management. Pharmacotherapy. 1997;17:917–28 [PubMed] [Google Scholar]
- 106.Oake N, Jennings A, Forster AJ, Fergusson D, Doucette S, van Walraven C. Anticoagulation intensity and outcomes among patients prescribed oral anticoagulant therapy: a systematic review and meta-analysis. CMAJ. 2008;179:235–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Palareti G, Cosmi B. Bleeding with anticoagulation therapy - who is at risk, and how best to identify such patients. Thromb Haemost. 2009;102:268–78 [DOI] [PubMed] [Google Scholar]