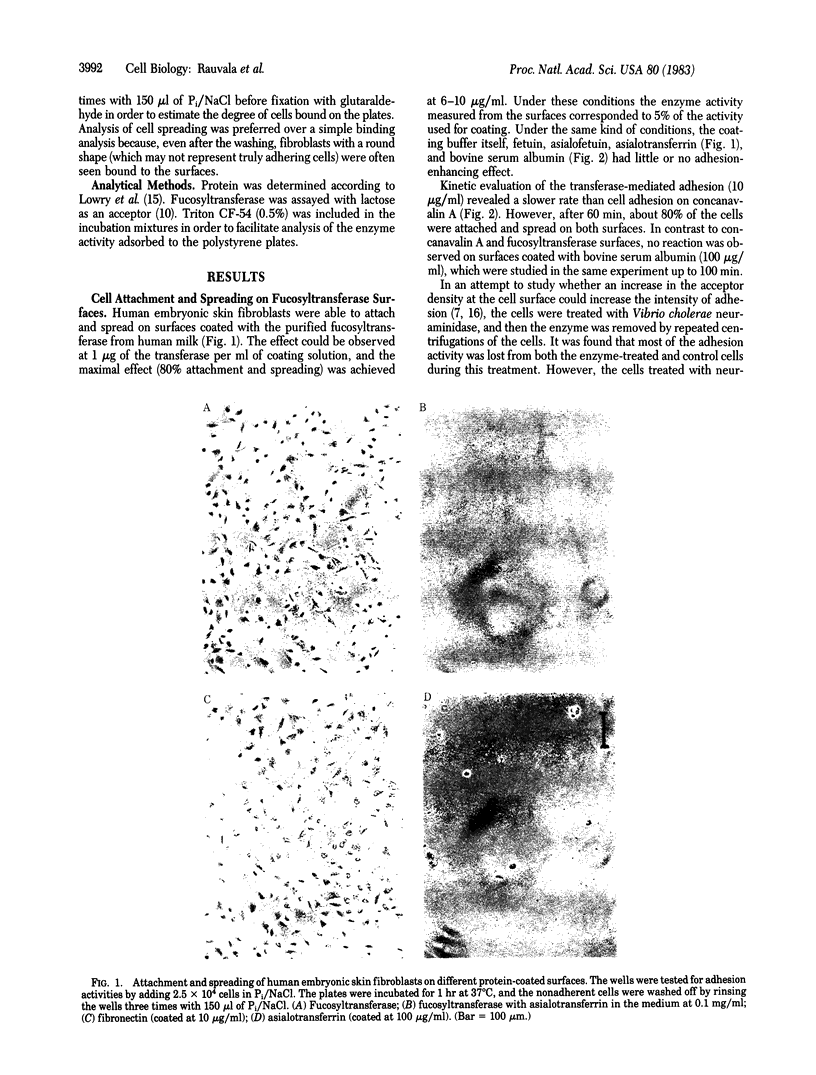
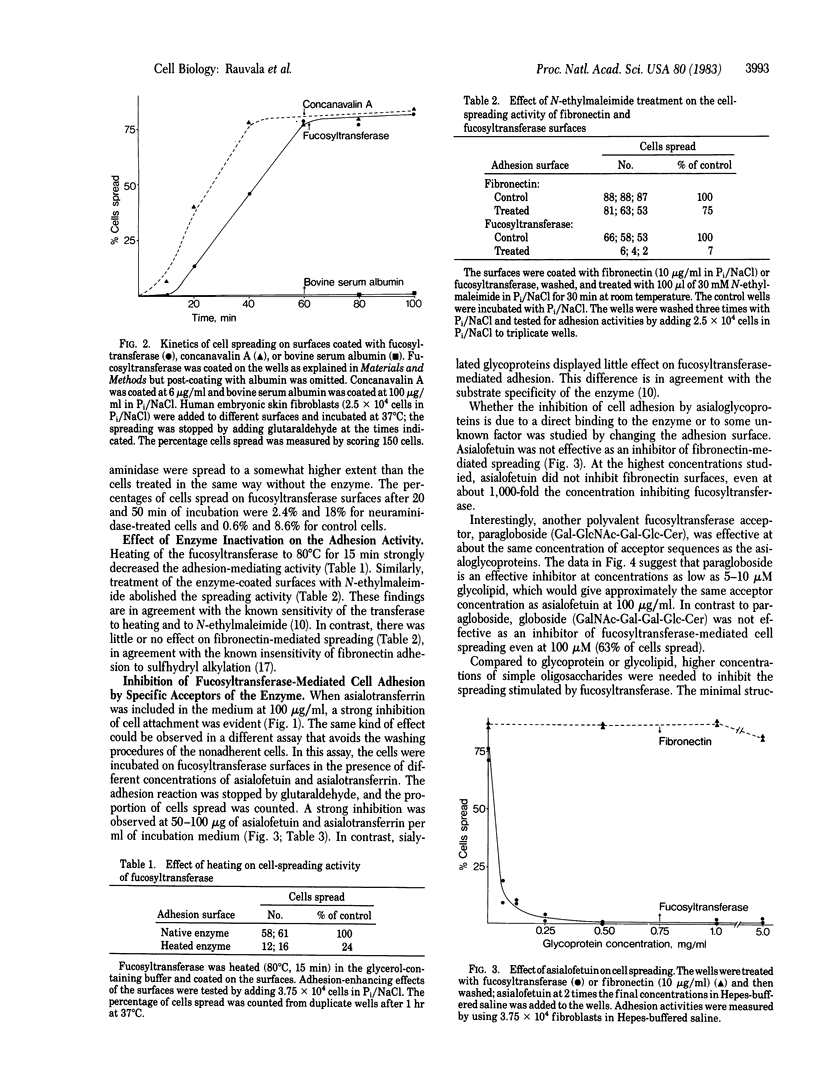
Abstract
Human embryonic skin fibroblasts attach and spread on surfaces on which a fucosyltransferase purified from human milk has been immobilized. The adhesion-enhancing effect of the transferase involves specific interactions of the enzyme surface with the cell surface carbohydrate acceptors, as suggested by the following findings. About 80% of human embryonic skin fibroblasts attach and spread in 1 hr on fucosyltransferase surfaces; in contrast, bovine serum albumin, fetuin, asialofetuin, and asialotransferrin surfaces fail to enhance adhesion. The adhesion-mediating activity of the transferase is destroyed by alkylation of the sulfhydryl groups or by heating. The adhesion on fucosyltransferase surfaces is inhibited by glycoprotein, glycolipid, and oligosaccharide acceptors containing the sugar sequence galactosyl-(beta 1 leads to 4)-N-acetylglucosamine, in agreement with the substrate specificity of the enzyme. The results suggest that glycosyltransferases are able to stimulate cell adhesion in a manner similar to that proposed for lectins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beyer T. A., Sadler J. E., Rearick J. I., Paulson J. C., Hill R. L. Glycosyltransferases and their use in assessing oligosaccharide structure and structure-function relationships. Adv Enzymol Relat Areas Mol Biol. 1981;52:23–175. doi: 10.1002/9780470122976.ch2. [DOI] [PubMed] [Google Scholar]
- Bretscher M. S., Raff M. C. Mammalian plasma membranes. Nature. 1975 Nov 6;258(5530):43–49. doi: 10.1038/258043a0. [DOI] [PubMed] [Google Scholar]
- Carter W. G., Rauvala H., Hakomori S. I. Studies on cell adhesion and recognition. II. The kinetics of cell adhesion and cell spreading on surfaces coated with carbohydrate-reactive proteins (glycosidases and lectins) and fibronectin. J Cell Biol. 1981 Jan;88(1):138–148. doi: 10.1083/jcb.88.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finne J., Burger M. M., Prieels J. P. Enzymatic basis for a lectin-resistant phenotype: increase in a fucosyltransferase in mouse melanoma cells. J Cell Biol. 1982 Feb;92(2):277–282. doi: 10.1083/jcb.92.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier W., Glaser L. Surface components and cell recognition. Annu Rev Biochem. 1979;48:491–523. doi: 10.1146/annurev.bi.48.070179.002423. [DOI] [PubMed] [Google Scholar]
- Gold L. I., Pearlstein E. Stability of fibronectin biological activity following chemical modification. Biochim Biophys Acta. 1979 Dec 14;581(2):237–251. doi: 10.1016/0005-2795(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Hoflack B., Cacan R., Montreuil J., Verbert A. Detection of ectosiallyltransferase activity using whole cells. Correction of misleading results due to the release of intracellular CMP-N-acetylneuraminic acid. Biochim Biophys Acta. 1979 Jun 6;568(2):348–356. doi: 10.1016/0005-2744(79)90302-4. [DOI] [PubMed] [Google Scholar]
- Hoflack B., Cacan R., Verbert A. Occurrence of two fucosyltransferase activities at the outer surface of rat lymphocytes. Eur J Biochem. 1978 Jul 17;88(1):1–6. doi: 10.1111/j.1432-1033.1978.tb12416.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Muramatsu T., Gachelin G., Damonneville M., Delarbre C., Jacob F. Cell surface carbohydrates of embryonal carcinoma cells: polysaccharidic side chains of F9 antigens and of receptors to two lectins, FBP and PNA. Cell. 1979 Sep;18(1):183–191. doi: 10.1016/0092-8674(79)90367-2. [DOI] [PubMed] [Google Scholar]
- Prieels J. P., Monnom D., Dolmans M., Beyer T. A., Hill R. L. Co-purification of the Lewis blood group N-acetylglucosaminide alpha 1 goes to 4 fucosyltransferase and an N-acetylglucosaminide alpha 1 goes to 3 fucosyltransferase from human milk. J Biol Chem. 1981 Oct 25;256(20):10456–10463. [PubMed] [Google Scholar]
- Rauvala H., Carter W. G., Hakomori S. I. Studies on cell adhesion and recognition. I. Extent and specificity of cell adhesion triggered by carbohydrate-reactive proteins (glycosidases and lectins) and by fibronectin. J Cell Biol. 1981 Jan;88(1):127–137. doi: 10.1083/jcb.88.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauvala H., Finne J. Structural similarity of the terminal carbohydrate sequences of glycoproteins and glycolipids. FEBS Lett. 1979 Jan 1;97(1):1–8. doi: 10.1016/0014-5793(79)80039-3. [DOI] [PubMed] [Google Scholar]
- Rauvala H. Gangliosides of human kidney. J Biol Chem. 1976 Dec 10;251(23):7517–7520. [PubMed] [Google Scholar]
- Rauvala H., Hakomori S. I. Studies on cell adhesion and recognition. III. The occurrence of alpha-mannosidase at the fibroblast cell surface, and its possible role in cell recognition. J Cell Biol. 1981 Jan;88(1):149–159. doi: 10.1083/jcb.88.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Shur B. D. Evidence that galactosyltransferase is a surface receptor for poly(N)-acetyllactosamine glycoconjugates on embryonal carcinoma cells. J Biol Chem. 1982 Jun 25;257(12):6871–6878. [PubMed] [Google Scholar]
- Shur B. D., Roth S. Cell surface glycosyltransferases. Biochim Biophys Acta. 1975 Dec 29;415(4):473–512. doi: 10.1016/0304-4157(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Umbreit J., Roseman S. A requirement for reversible binding between aggregating embryonic cells before stable adhesion. J Biol Chem. 1975 Dec 25;250(24):9360–9368. [PubMed] [Google Scholar]
- Veh R. W., Michalski J. C., Corfield A. P., Sander-Wewer M., Gies D., Schauer R. New chromatographic system for the rapid analysis and preparation of colostrum sialyloligosaccharides. J Chromatogr. 1981 Aug 7;212(3):313–322. doi: 10.1016/s0021-9673(01)84044-9. [DOI] [PubMed] [Google Scholar]
- Vuento M., Vaheri A. Purification of fibronectin from human plasma by affinity chromatography under non-denaturing conditions. Biochem J. 1979 Nov 1;183(2):331–337. doi: 10.1042/bj1830331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigel P. H., Schnaar R. L., Kuhlenschmidt M. S., Schmell E., Lee R. T., Lee Y. C., Roseman S. Adhesion of hepatocytes to immobilized sugars. A threshold phenomenon. J Biol Chem. 1979 Nov 10;254(21):10830–10838. [PubMed] [Google Scholar]