Abstract
Background
Treatment of infectious diseases is becoming more challenging with each passing year. This is especially true for infections caused by Pseudomonas aeruginosa, an opportunistic pathogen with the ability to rapidly develop resistance to multiple classes of antibiotics.
Objectives
This study was conducted to determine the prevalence of metallo-β-lactamase (MBL)–producing strains among multidrug-resistant P. aeruginosa strains isolated from burn patients.
Materials and Methods
The isolates were identified, tested for susceptibility to various antimicrobial agents, and screened for the presence of MβLs by using the double-disk synergy test. The minimal inhibitory concentration of imipenem was determined by microplate broth dilution method on Mueller-Hinton agar. To detect VIM, SIM, and GIM MBLs, the isolates were subjected to polymerase chain reaction.
Results
In this study, we identified 100 P. aeruginosa isolates from 176 clinical specimens obtained from burn patients. The isolates showed maximum resistance to ampicillin (100%), ceftazidime (94%), and ceftriaxone (89%). The CLSI-MBL phenotypic test showed that of the 100 P. aeruginosa isolates, 22 (22%) were positive for MBL production in the double-disk synergy test. Of the 22 MBL-positive P. aeruginosa isolates, 8 were resistant to imipenem. PCR analysis showed that 8 isolates were positive for blaVIM1. The other genes blaSIM1 and blaGIM1 were not detected.
Conclusions
The study results demonstrate the serious therapeutic threat of the spread of MBL producers among P. aeruginosa populations. Metallo-β-lactamases were detected in 22% of imipenem-resistant P. aeruginosa isolates. Early detection and infection-control practices are the best antimicrobial strategies for this organism; therefore, systematic surveillance to detect MBL producers is necessary.
Keywords: Burn Patients, P. aeruginosa, Metallo-β-lactamase, Imipenem Resistance
1. Background
Pseudomonas aeruginosa, a versatile pathogen associated with a broad spectrum of infections in humans and widely known as an opportunistic organism, is frequently involved in infections among susceptible populations, especially patients with burns (1). Furthermore, this organism possesses several virulence factors and is intrinsically resistant to most antimicrobials, a feature that is also responsible for the difficulty in treating infected patients (2). In the past decade, P. aeruginosa strains showing resistance to multiple β-lactam antibiotics, in particular, metallo-β-lactamase (MBL)–producing P. aeruginosa, have become an increasing public health problem worldwide (3-5). Furthermore, infection with MBL-producing organisms is associated with higher rates of mortality, morbidity, and health care costs (6-8).
In P. aeruginosa, resistance to extended-spectrum β-lactams is mediated by lack of drug penetration, which may occur due to porin mutations, efflux pumps, or hydrolysis by β-lactamases (9). On the basis of molecular studies, we can classify carbapenem-hydrolyzing enzymes into 4 groups: A, B, C, and D. The MBLs, which belong to group B, are enzymes that require divalent cations as cofactors for optimal enzyme activity and are inhibited by the action of a metal ion chelator. Hospital infections caused by P. aeruginosa are often difficult to eradicate because the organisms are resistant to drugs. Therefore, detection of MBL-producing P. aeruginosa is crucial to control the spread of resistance and for optimal treatment of patients, especially burn patients (10). There is no information concerning the prevalence of MBL-producing P. aeruginosa strains in Kurdistan province.
2. Objectives
We conducted this study to detect MBL-producing strains among P. aeruginosa isolates obtained from burn patients at Tohid hospital, which is affiliated to Kurdistan University of Medical Sciences and has a burn unit with a heavy patient turnover and extensive antibiotic use.
3. Materials and Methods
Between April 2009 and April 2010, we isolated and identified 100 strains of P. aeruginosa in a clinical laboratory at Tohid hospital, Sanandaj, Iran. The strains were identified as P. aeruginosa on the basis of colony morphology, gram staining results, motility, oxidase reaction, production of the pyocyanin pigment, nitrate reduction, the use of citrate and malonate as carbon sources, and the ability to grow at 5˚C and 42˚C.
3-1. Antimicrobial Susceptibility Testing
Antimicrobial susceptibility testing was done by the disk-diffusion method on Muller-Hinton agar (Merck, Germany) (11). The following antibiotics were used: amikacin, gentamicin, carbencillin, ciprofloxacin, ofloxacin, cefepime, ceftazidime, cefotaxime, ampicillin, ceftriaxone, imipenem, and meropenem.
3-2. Detection of MBL
Detection of MBL-producing P. aeruginosa strains was performed according to Clinical and Laboratory Standards Institute (CLSI) guidelines (11). P. aeruginosa ATCC 27853 was used as the negative control. For the double-disk synergy test, the inoculum was prepared after emulsifying 5–6 colonies of the suspected isolate in Mueller–Hinton broth, and the turbidity was adjusted to 0.5 McFarland opacity standard. A lawn culture was obtained on Mueller–Hinton agar, and double-disk synergy test was performed.
3-3. Determination of Minimal Inhibitory Concentration
Imipenem minimal inhibitory concentration (MIC) was determined by microplate (NUNC, Denmark) broth dilution method on Mueller–Hinton agar. Zone diameter and MIC interpretive standards for breakpoints for P. aeruginosa published by CLSI were used (11).
3-4 Genotypic Detection of VIM, SIM, and GIM MBL Genes by PCR
To detect VIM, SIM, and GIM MBL genes, PCR was performed for P. aeruginosa isolates (12)
4. Results
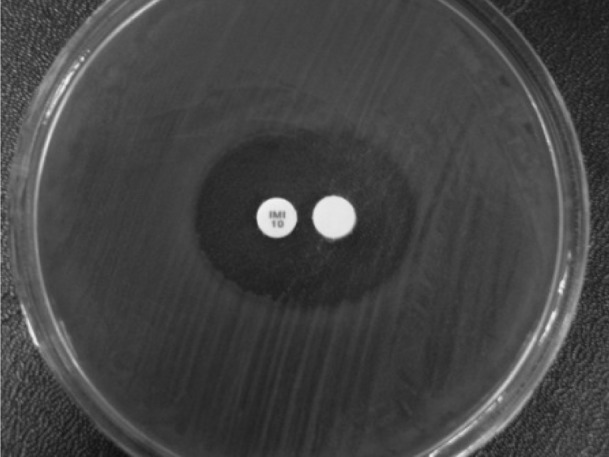
In this study, we identified 100 P. aeruginosa isolates among 176 clinical specimens obtained from patients. Table 1 shows the antibiotic-resistance pattern of P. aeruginosa isolates. The isolates showed maximum resistance to ampicillin (100%), ceftazidime (94%), and ceftriaxone (89%). The next step in testing was designed to identify the MBL-producing P. aeruginosa strains. In the CLSI-MBL phenotypic test, of the 100 P. aeruginosa isolates, 22 (22%) were positive for MBL production by the double-disk synergy test (Figure 1).
Table 1. In Vitro Antibiotic Resistance Pattern of Infection-Associated P. aeruginosa.
| Antibiotic | Resistance, % |
|---|---|
| Amikacin | 52 |
| Gentamicin | 54 |
| Carbencillin | 76 |
| Ciprofloxacin | 31 |
| Ofloxacin | 82 |
| Cefepime | 72 |
| Ceftazidime | 96 |
| Cefotaxime | 88 |
| Ampicillin | 100 |
| ceftriaxone | 89 |
| Imipenem | 21 |
| Meropenem | 14 |
Figure 1. Phenotypic Detection of MBLs by DDST Among P. aeruginosa Isolates in Burn Patients at Tohid Hospital, Sanandaj, Iran.
4-1 Determination of MIC
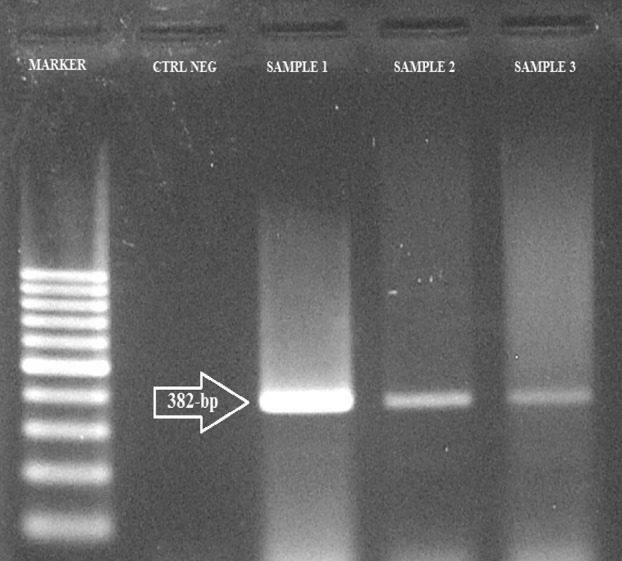
The MIC of MBL-positive isolates for imipenem is shown in Table 2; among 22 MBL-positive strains, 8 were resistant. PCR detection for MBL showed that 8 strains were positive for blaVIM1. The other genes, blaSIM1 and blaGIM1, were not detected (Figure 2).
Table 2. MIC of MBL positive isolates for Imipenem.
| Sensitive | Intermediate | Resistant |
|---|---|---|
| MICa, μg / ml | ||
| ≤ 0.125 | 08 | 16 |
| 0.25 | 32 | |
| 0.5 | 64 | |
| 01 | 128 ≤ | |
| 02 | ||
| 04 | ||
| No. of P. aeruginosa Isolates, % | ||
| 02 | 01 | 02 |
| 02 | 01 | |
| 04 | 03 | |
| 02 | 02 | |
| 03 | ||
| 00 | ||
| Total of P. aeruginosa Isolates | ||
| 13 | 01 | 08 |
aAbbreviation: MIC: Minimum Inhitory Concentration
Figure 2. PCR for the Detection of blaVIM Gene.
5. Discussion
Because of its broad antimicrobial spectrum and stability against most common β-lactamases, imipenem generally represents one of the last alternatives for the treatment of nosocomial infections caused by multidrug-resistant gram-negative bacteria, particularly P. aeruginosa (13). However, the rapid spread of MBLs among major gram-negative pathogens, particularly P. aeruginosa, is an emerging threat and a matter of concern worldwide (14-16); further, MBL-carrying bacteria are known to cause recalcitrant nosocomial infections (17). MBLs of the IMP, VIM, and SIM families are frequently detected in imipenem-resistant P. aeruginosa in Iran (18-20). In our study, a total of 100 P. aeruginosa strains were isolated from hospitalized burn patients in Sanandaj, Iran, in 2010, and 22% of these strains were found to be MBL producers, which is much lower than the findings from the study conducted by Mihani and Khosravi in Ahvaz (18). In this study, 22% of the imipenem-resistant P. aeruginosa isolates were MBL-positive, with 8% positive for VIM1, which is by far the most prevalent MBL in Iran20. To our knowledge, MBLs of other families like SIM have not been detected in P. aeruginosa. The prevalence of MBL-producing P. aeruginosa differs across Iranian studies, which may be because of differences in geographic regions. Further investigation is required to gain a better understanding of the epidemiology and genetic background of MBL-producing P. aeruginosa. The uncontrolled spread of MBL producers in hospitals may hamper treatment procedures and lead to increased morbidity and mortality. Systems for regular screening should be established to control the spread of MBL producers, and effective infection-control programs in hospitals should be developed and implemented thoroughly. The study results demonstrate the serious therapeutic threat of the spread of MBL-producing P. aeruginosa. This number (22% of imipenem-resistant P. aeruginosa) might have been higher if other genes were included. Early detection and infection control practices are the best defense against this organism; therefore, systematic surveillance to detect MBL producers is necessary.
Acknowledgments
None declared.
Footnotes
Implication for health policy/practice/research/medical education:Pseudomonas aeruginosa, has showed up as a significant nosocomial pathogen particularly among burn patients. We have very scant information about the current state of Pseudomonas aeruginosa infections and their resistance pattern particularly ESBL. Moreover, with the increased use of prophylactic antibiotics to burn patients, careful surveillance of the changing trend of bacterial organisms and their antibiotic resistance pattern among neonates is warranted.
Please cite this paper as: Kalantari E, Torabi V, Salimizand H, Soheili F, Beiranvand S, Soltan Dallal MM. First Survey of Metallo-β–Lactamase Producers in Clinical Isolates of Pseudomonas aeruginosa From a Referral Burn Center in Kurdistan Province. Jundishapur J Nat Pharm Prod. 2012;7(1):23-6.
Financial Disclosure: None declared.
Funding/Support: Funding for this project was provided by Vice-chancellor in Research Affairs of Kurdistan University of Medical Sciences, Sanandaj, Iran.
References
- 1.Sader HS, Gales AC, Pfaller MA, Mendes RE, Zoccoli C, Barth A, et al. Pathogen frequency and resistance patterns in Brazilian hospitals: summary of results from three years of the SENTRY Antimicrobial Surveillance Program. Braz J Infect Dis. 2001;5(4):200–14. doi: 10.1590/S1413-86702001000400006. [DOI] [PubMed] [Google Scholar]
- 2.Kerr KG, Snelling AM. Pseudomonas aeruginosa: a formidable and ever-present adversary. J Hosp Infect. 2009;73(4):338–44. doi: 10.1016/j.jhin.2009.04.020. [DOI] [PubMed] [Google Scholar]
- 3.Khosravi Y, Tee Tay S, Vadivelu J. Metallo-β-lactamase–producing imipenem-resistant Pseudomonas aeruginosa clinical isolates in a university teaching hospital in Malaysia: detection of IMP-7 and first identification of IMP-4, VIM-2, and VIM-11. Diagnos Microbiol Infect Dis. 2010;67(3):294–6. doi: 10.1016/j.diagmicrobio.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 4.Wirth FW, Picoli SU, Cantarelli VV, Goncalves AL, Brust FR, Santos LM, et al. Metallo-beta-lactamase-producing Pseudomonas aeruginosa in two hospitals from southern Brazil. Braz J Infect Dis. 2009;13(3):170–2. doi: 10.1590/S1413-86702009000300003. [DOI] [PubMed] [Google Scholar]
- 5.Yan JJ, Hsueh PR, Ko WC, Luh KT, Tsai SH, Wu HM, et al. Metallobeta-lactamases in clinical Pseudomonas isolates in Taiwan and identification of VIM-3, a novel variant of the VIM-2 enzyme. Antimicrob Agents Chemother. 2001;45(8):2224–8. doi: 10.1128/AAC.45.8.2224-2228.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee K, Ha GY, Shin BM, Kim JJ, Kang JO, Jang SJ, et al. Metallo-[beta]-lactamase-producing Gram-negative bacilli in Korean Nationwide Surveillance of Antimicrobial Resistance group hospitals in 2003: Continued prevalence of VIM-producing pseudomonas spp. and increase of IMP-producing Acinetobacter spp. Diagnos Microbiol Infect Dis. 2004;50(1):51–8. doi: 10.1016/j.diagmicrobio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 7.Libisch B, Gacs M, Csiszar K, Muzslay M, Rokusz L, Fuzi M. Isolation of an integron-borne blaVIM-4 type metallo-beta-lactamase gene from a carbapenem-resistant Pseudomonas aeruginosa clinical isolate in Hungary. Antimicrob Agents Chemother. 2004;48(9):3576–8. doi: 10.1128/AAC.48.9.3576-3578.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peleg AY, Franklin C, Bell J, Spelman DW. Emergence of IMP-4 metallo-beta-lactamase in a clinical isolate from Australia. J Antimicrob Chemother. 2004;54(3):699–700. doi: 10.1093/jac/dkh398. [DOI] [PubMed] [Google Scholar]
- 9.Hooper DC. Emerging mechanisms of fluoroquinolone resistance. Emerg Infect Dis. 2001;7(2):337–41. doi: 10.3201/eid0702.010239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richet HM, Mohammed J, McDonald LC, Jarvis WR. Building communication networks: international network for the study and prevention of emerging antimicrobial resistance. Emerg Infect Dis. 2001;7(2):319–22. doi: 10.3201/eid0702.010235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Performance Standards for Antimicrobial Susceptibiliti Testing; Twentienth Informational Supplement. Clin Lab Stand Inst. 2010;30(15):10–7. [Google Scholar]
- 12.Pitout JD, Gregson DB, Poirel L, McClure JA, Le P, Church DL. Detection of Pseudomonas aeruginosa producing metallo-betalactamases in a large centralized laboratory. J Clin Microbiol. 2005;43(7):3129–35. doi: 10.1128/JCM.43.7.3129-3135.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomson JM, Bonomo RA. The threat of antibiotic resistance in Gram-negative pathogenic bacteria: beta-lactams in peril! Curr Opin Microbiol. 2005;8(5):518–24. doi: 10.1016/j.mib.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Cornaglia G, Akova M, Amicosante G, Canton R, Cauda R, Docquier JD, et al. Metallo-beta-lactamases as emerging resistance determinants in Gram-negative pathogens: open issues. Int J Antimicrob Agents. 2007;29(4):380–8. doi: 10.1016/j.ijantimicag.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Nam Hee R, Jung Sook H, Dong Seok J, Jae Ryong K. Prevalence of Metallo-β-lactamases in Pseudomonas aeruginosa and Acinetobacter baumannii. Korean. Korean J Clin Microbiol. 2010;13(4):169–72. doi: 10.5145/KJCM.2010.13.4.169. [DOI] [Google Scholar]
- 16.Saderi H, Karimi Z, Owlia P, Bahar MA, Rad SMBA. Phenotypic Detection of Metallo-beta-lactamase Producing Pseudomonas aeruginosa Strains Isolated from Burned Patients. Iran J Patho. 2008;3(1):20–4. [Google Scholar]
- 17.Sarkar B, Biswas D, Prasad R, Sharma JP. A clinicomicrobiological study on the importance of pseudomonas in nosocomially infected ICU patients, with special reference to metallo beta1-lactamase production. Indian J Pathol Microbiol. 2006;49(1):44–8. [PubMed] [Google Scholar]
- 18.Mihani F, Khosravi A. Isolation Of Pseudomonas Aeruginosa Strains Producing Metallo Beta Lactamases From Infections In Burned Patients And Identification Of Bla Imp And Bla Vim Genes By Pcr. Iran J Med Microbiol. 2007;1(1):23–31. [Google Scholar]
- 19.Rezaei H, Behzadian G, Najar S, Mostafaei M. Prevalence and detection of metallo-ββ-lactamase (MBL)-producing Pseudomonas aeruginosa strains from clinical isolates in Iran. Annals of Microbiology. 2007;57(2):293–5. doi: 10.1007/BF03175223. [DOI] [Google Scholar]
- 20.Yousefi S, Farajnia S, Nahaei MR, Akhi MT, Ghotaslou R, Soroush MH, et al. Detection of metallo-beta-lactamase-encoding genes among clinical isolates of Pseudomonas aeruginosa in northwest of Iran. Diagn Microbiol Infect Dis. 2010;68(3):322–5. doi: 10.1016/j.diagmicrobio.2010.06.018. [DOI] [PubMed] [Google Scholar]


