Abstract
Background
Ulva genus, an edible seaweed, and an important food source in many south-east Asian countries is also recognized by its synonymous name as Enteromorpha.
Objectives
This study was carried out to evaluate antioxidant activity, contents of total phenolics, and flavonoids of methanolic extracts of edible green seaweeds including Ulva clathrata (Roth) C. Agardh and three samples of Ulva prolifera O.F.Müller grown at different parts of Bushehr Province along the northern coasts of the Persian Gulf.
Materials and Methods
The seaweeds were collected from Bordekhoun, Northern Ouli, Taheri and Kangan coasts in December 2011. Methanolic extracts of the seaweeds were assessed for their antioxidant activity using DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay and was performed in a microplate reader. Total phenolics were determined by Folin-Ciocalteu reagent and flavonoid content was evaluated by colorimetric method.
Results
All samples showed antioxidant activity to various degrees. Ulva clathrata exhibited a high DPPH radical scavenging activity with a low IC50 (the half-maximal inhibitory concentration) (0.715 ± 0.078 mg. mL-1). The highest phenolic content (4.468 ± 0.379 mg GAE g-1) (gallic acid equivalent) and flavonoid content (45.577 ± 0.949 mg RE g-1) (rutin equivalent) were also observed in U .clathrata. The phenolic and flavonoid contents showed positive correlations with the DPPH radical scavenging activity and negative correlations with IC50 (P < 0.01). Besides, Results showed that there was a positive correlation between total phenolics and flavonoid content of extracts (P < 0.01).
Conclusions
Strong positive and significant correlations between DPPH radical scavenging and phenolic and flavonoid contents showed that, phenolic compounds, including flavonoids are the main contributors of antioxidant activity in these Ulva species and variations in phenolics and flavonoid contents of the seaweed extracts may be due to the variation in physicochemical parameters such as salinity amongst the selected stations.
Keywords: Antioxidant Capacity, Total Phenolics, Flavonoid, DPPH
1. Background
Reactive oxygen species (ROS) have been implicated in pathogenesis of many diseases, including cancer, mutagenesis, Alzimer’s disease, AIDS, etc. Many synthetic antioxidants are currently in use, nevertheless, there is a growing evidence of consumer preference for natural antioxidants because of their potentially lower toxicity (1).
According to the previous studies, terrestrial plants are rich sources of phytochemicals possessing important properties such as antioxidant activity. Many investigators have found several types of antioxidants from different parts of various plant species such as oilseeds, cereal crops, vegetables and spices (2).
Recently, polyphenolic compounds including flavonoids are known as safe and non-toxic antioxidants. Many studies have shown that a high dietary intake of natural phenolics is strongly associated with longer life expectancy, reduced risk of developing some chronic diseases, various types of cancer, diabetes, obesity, improved endothelial function and reduced blood pressure (3-5).
Phenolic compounds are commonly found in plants and seaweeds. Seaweeds are known to contain a wide variety of bioactive compounds, many of which have commercial applications in pharmaceutical, medical, cosmetic, food industries and agriculture (6). It has been observed that ROS production in algae is stimulated by various environmental stresses, such as high light levels, heavy metals, high salt concentrations, UV radiation etc. Algae generally has higher antioxidant activity due to higher contents of nonenzymatic antioxidant components, such as ascorbic acid, reduced glutathione, phenols and flavonoids (7). As a result, many marine bio-sources in the last decades have attracted attention in the search for natural bioactive compounds to develop new drugs and healthy foods. Compounds with antioxidant, antiviral, antifungal, antimicrobial, antitumor and anti-inflammatory activities have been found in brown, red and green algae (8). The antioxidant activity of several seaweeds has been reported (9). Ulva genus is an important food source in many south-east Asian countries which is also recognized by its synonymous name as Enteromorpha. However, research on the use of this green seaweed genus for food or the treatment of various diseases has received less attention in Iran.
2. Objectives
The antioxidant properties of Ulva clathrata and U. prolifera from Iran have not been previously published. The present study aimed to investigate the antioxidant capacity, total phenolics and flavonoids of these edible seaweeds from the northern coasts of the Persian Gulf for future applications in medicine, dietary supplements, cosmetics or food industries.
3. Materials and Methods
3.1. Chemicals
Ascorbic acid, Folin-ciocalteu reagent, Gallic acid and Methanol were purchased from Merck Company (Darmstadt, Germany). DPPH (1, 1-diphenyl-2-picrylhydrazyl) and Rutin were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All the chemicals and reagents used were of analytical grade.
3.2. Collection and Preparing of Algal Extract
The seaweeds were collected at low tide time (according to the tide time table obtained from www.iranhydrography.org) along the northern coasts of the Persian Gulf from Bordekhoun, Northern Ouli, Taheri and Kangan in December 2011. The latitude and longitude of each sampling location was recorded by GPS tracking device. Once harvested, seaweeds were washed with fresh water to remove sands, salts and epiphytes, and then were air-dried at room temperature with good controlled air condition carefully. Voucher specimens were pressed and, stored in 5% formol for identification. Samples were observed under a light microscope for anatomical examination. The samples were identified according to the characteristics and identification keys in the taxonomic publications (10-14). Herbarium voucher specimens were kept in Jundishapur marine pharmaceutical research center (JMPRC) herbarium and samples were kept at -50ºC until experiments were processed and milled into powder before extraction.
Dried seaweed sample (200 mg) was extracted with 6 mL 80% methanol in an ultrasonic bath for 20 min, vortexed for 30 min then left to stand at room temperature for 48 h. The extract centrifuged at 1500g for 10 min and filtered through Watmann No.1 paper filter. The stock solutions of extracts were adjusted with 80% methanol to final concentration of 2 mg mL-1. Dilutions were made to obtain concentrations 1, 0.5 and 0.1 mg mL-1.
3.3. DPPH Free Radical Scavenging Activity
DPPH radical scavenging activity was determined according to the method of Zhang et al. (2007) with slight modifications (15). Briefly, 100 µl of each extract at various dilutions, were mixed with 100 µl of 0.16 mM DPPH methanolic solution. The mixture was vortexed for 1 min, kept for 30 min in dark and then, the absorbance was read at 517 nm in an automated microplate reader (Sunrise-Elisa Reader,Tecan ,Swiss). The antioxidant capacity was calculated using the following equation:
% Inhibition = (Acotrol - (Asample - Ablank) / Acotrol) × 100
Where the Acotrol is the absorbance of the control (DPPH without sample), the Asample is the absorbance of the test sample (the sample test and DPPH solution), and the Ablank is the absorbance of the sample blank (Sample without the DPPH solution). The half-maximal inhibitory concentration IC50 (the half-maximal inhibitory concentration) was calculated by linear regression analysis and expressed as mean of three determinations. Ascorbic acid was used as positive control.
3.4. Determination of Total Phenolic Compounds and Flavonoid Content
Total phenolic compounds (TPC) of algal extracts was determined by Folin-Ciocalteu reagent according to the method of Antolovich et al. (2002) (16) with minor modifications. In Brief, 20 µL of extracts were mixed with 100 µl of 1:10 Folin-Ciocalteu reagent followed by the addition of Na2CO3 (80 µL, 7.5%). The assay was carried out in microplate. After incubation at room temperature for 2 hours in dark, the absorbance at 600 nm was recorded. Gallic acid was used as the standard reference. TPC (total phenolic content) was expressed as mg gallic acid equivalents per gram of dried extract (mg GAE g-1) (gallic acid equivalent).
Flavonoid content (FC) (flavonoid content) of each extract was determined by following colorimetric method (17). Briefly, 20 µL of each extract was separately mixed with 20 µL of 10 % aluminium chloride, 20 µL of 1 M potassium acetate and 180 µL of distilled water, and left at room temperature for 30 min. The absorbance of the reaction was recorded at 415 nm. The calibration curve was prepared by rutin methanolic solutions at concentrations of 12.5 to 100 µg mL-1. FC was expressed as mg rutin equivalents per gram of dried extract (mg RE g-1) (rutin equivalent).
3.5. Statistics
Data were expressed as means ± standard errors of three replicate determinations. Statistical analyses were carried out using SPSS 16.0 for Windows. To determine whether there were any differences among the means, one way analysis of (ANOVA) and the Duncan’s new multiple range test were applied to the result at 0.05 level of significance (P < 0.05). The Pearson correlation analysis was performed between variables.
4. Results
4.1. DPPH Radical Scavenging Activity
During the study, Ulva clathrata and three samples of Ulva prolifera were collected from middle and lower intertidal zones of northern coasts of the Persian Gulf. The scientific names of seaweeds and their collection locations are listed in Table 1. All seaweed extracts showed antioxidant activity to various degrees ( Table 2 ). Lower IC50 value indicates higher antioxidant activity. As shown in Table 2, the IC50 of Ulva clathrata extract was significantly different compared with those of Ulva prolifera extracts (P < 0.05).The IC50 of Ulva prolifera extracts (S2-S4) were not significantly different and was higher in Ulva prolifera grown in Northern Ouli (S4). The IC50 of ascorbic acid as a standard antioxidant was evaluated 0.037 ± 0.018 mg mL-1 in this work and was significantly different in comparison to the seaweed extracts (P < 0.05). The scavenging effect of ascorbic acid ranged from 16.67 ± 2.98 % at concentration of 5 µg mL-1 to 90.34 ± .35 % at concentration of 100 µg mL-1.
Table 1. The Seaweeds and Their Collection Information.
| ID code | Sample | Scientific name | Locality | Latitude, Longitude |
|---|---|---|---|---|
| G111032 | S1 | Ulva clathrata (Roth) C. Agardh | Bordekhoun | N2800306,E05122437 |
| G110831 | S2 | Ulva prolifera O.F. Müller | Kangan | N2740024,E05219893 |
| G110731 | S3 | Ulva prolifera O.F. Müller | Taheri | N2740040,E05219711 |
| G110932 | S4 | Ulva prolifera O.F. Müller | Northern Ouli | N2750316,E0515308 |
Table 2. IC50, TPC and FC of the Seaweed Extracts.
| Sample | Species | IC50 (mg mL-1) | TPC(mg g-1) | FC(mg g-1) |
|---|---|---|---|---|
| S1 | Ulva clathrata | 0.715 ± 0.078 a | 4.468 ± 0.379 a | 45.577 ± 0.949 a |
| S2 | Ulva prolifera | 3.101 ± 0.107 b | 1.371 ± 0.148 b | 10.184 ± 1.044 b |
| S3 | Ulva prolifera | 3.026 ± 0.221 b | 1.465 ± 0.068 b | 9.370 ± 1.252 b |
| S4 | Ulva prolifera | 3.026 ± 0.221 c | 1.449 ± 0.138 b | 7.959 ± 0.769 b |
Each value is expressed as the mean ± SE (n = 3)
a, b, c Means with different letters are significantly different at P < 0.05
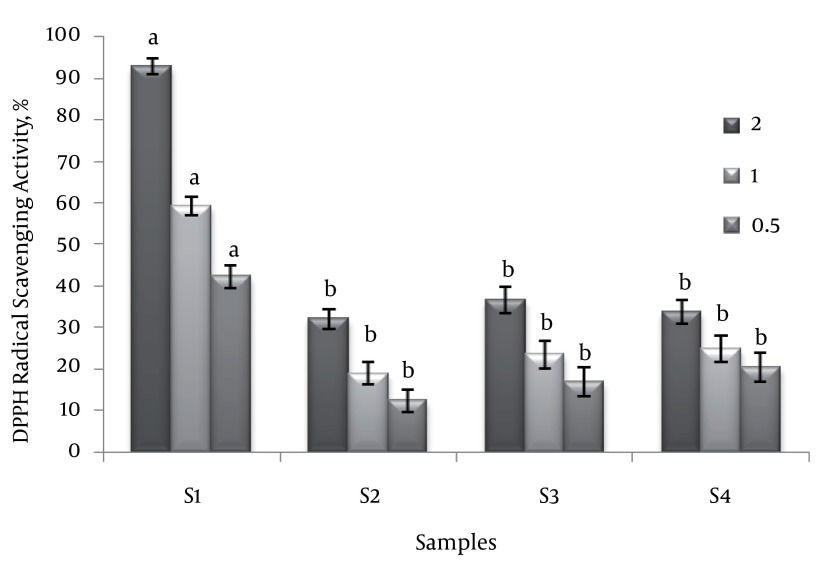
The scavenging effect of the tested extracts at concentration of 2 mg mL-1 on the DPPH radical decreased in the order of: S1 > S3 > S4 > S2, and were 92.7 ± 1.84, 36.59 ± 3.09, 33.82 ± 3.00 and 32.15 ± 2.45 % , respectively (Figure 1). It can be noticed that the DPPH radical scavenging values of all extracts were dose dependent in the range of the tested concentrations. However, the extract of U. clathrata was found to be the most potent scavenger in the tested algae. The DPPH radical scavenging activity of the U. clathrata extract in the range of the tested concentrations was significantly different in comparison to those of the U. prolifera extracts. The scavenging effect of the U. clathrata extract at concentration of 2 mg mL-1 was comparable to that of the positive control, ascorbic acid at concentration of 0.1 mg mL-1.
Figure 1. DPPH Radical Scavenging Activity of Extracts at Concentrations of 2, 1 and 0.5 mg mL-1.
Each value is expressed as the mean ± SE (n = 3). Means with different letters (For each concentration level) are significantly different at P < 0.05
4.2. Total Phenolic Compounds and Flavonoid Content
Total phenolic content (TPC) and flavonoid content (FC) of the algal extracts are also presented in Table 2. The phenolic content of U. clathrata extract was significantly different compared with those of the other species (P < 0.05). In general, the higher total phenolic content resulted in higher antioxidant capacity. As shown in Table 2, the flavonoid content of algal extracts varied from 45.577 ± 0.949 (Ulva clathrata) to 7.959 ± 0.769 (U. prolifera grown in Northern Ouli) mg RE g-1. The flavonoid content of U. clathrata extract was significantly different (P < 0.05) as compared with the other species. The flavonoid contents of the U. prolifera samples (S2-S4) were not significantly different and were higher in S2 which was collected from Kangan.
The Pearson’s correlation coefficients between the variables are presented in Table 3. As shown in this table, there were strong positive and significant correlations between DPPH radical scavenging and contents of phenolics and flavonoids, and high negative correlations between IC50 and the variables and these correlations were stronger for flavonoid contents than the phenolic contents. Also, the results revealed that there was a strong positive correlation between flavonoids and total phenolics of the seaweeds extracts (P < 0.01).
Table 3. Pearson’s Correlation Coefficients Between the Variables.
| TPC | FC | IC50 | |
|---|---|---|---|
| TPC | 0.957 a | ||
| IC50 | -0.898 a | -0.957 a | |
| DPPH radical scavenging activity | 0.959 a | 0.985 a | -0.935 a |
a Correlation is significant at the 0.01 level (2-tailed)
5. Discussion
Due to the presence of different bioactive components with antioxidative potential in the crude extracts of the samples, many different methods have been used to investigate various sample extracts in recent years. In the current study, the DPPH radical scavenging method was used to evaluate the antioxidant capacity of the seaweed extracts, because the use of DPPH radical provides an easy, rapid and convenient method to evaluate the antioxidants and radical scavengers (18).
Many studies have been done to determine antioxidant capacity in Ulva species and some researchers have stated high scavenging activity for Ulva species. For instance, higher scavenging capability of polysaccharides obtained from U. prolifera extract than that of butylated hydroytoluene(BHT) to DPPH radical has been reported (65.2 % at the concentration of 0.5 mg mL-1) (19). Also, three edible species of Ulva including U.compressa, U. linza and U. tubulosa were assessed for evaluating their antioxidative activities and all the tested seaweeds exhibited high antioxidant activity in linoleic acid system and the best DPPH radical scavenging was observed in methanolic extract of U. compressa (IC50 =1.89 mg mL-1) (20). It has been shown that, chronic consumption of polysaccharides supplied by Ulva species; prevent the fall of antioxidant defences and the development of atherosclerosis in hamsters (21). Besides, Polysaccharides obtained from U. lactuca extract with potent hypocholesterolemic and antioxidant effects in experimentally-induced hypercholesterolemic animal model have been reported (22). In addition, a high value of astaxanthin (a naturally occurring carotenoid pigment and a powerful antioxidant) has been reported in Ulva species (23). Moreover, some researchers have isolated natural Ulvan, a group of sulfated heteropolysaccharides and its derivatives (24) and sesquiterpenoids (25) from Ulva species with high free radical scavenging properties.
In the current study, the antioxidant activity of Ulva species was in accordance with their amount of phenolic and flavonoid contents. Several reports have indicated a close relationship between total phenolic content and high antioxidant activity, and many researchers demonstrated that phenolic compounds were one of the most effective antioxidants in marine algae (26, 27).
The best-described property of almost every group of flavonoids is their capacity to act as antioxidants (28). A positive correlation was documented between antioxidation capabilities and total polyphenol contents for Ulva prolifera, but not with the contents of flavonoids (29). On contrary, Cho et al (30). (2011) (30) reported little correlation between antioxidant activities and total phenolic contents of the extracts of Ulva prolifera. They suggested that the strong antioxidant activity of the extracts from U. prolifera was because of a chlorophyll compound, pheophorbide rather than phenolic compounds. In the current study strong, positive correlations was found between total phenol and flavonoid contents and the antioxidant capacity. Similar observation has been reported by Chai and Wong (2012) (31). The current research findings were in agreement with the results of Bouba et al. (2010) which reported a positive correlation between total phenolics and flavonoids in extracts of twenty Cameroonian spices (32).
Despite the fact that, species were from the same collection season, however, contents of their flavonoids and total phenolics were different. Previous studies had found marked changes in the chemical constituents with change of seasons and environmental conditions (33, 34) .These variations may be due to the variation in physicochemical parameters such as salinity amongst the selected stations.
In the current study, U. clathrata were collected from middle intertidal zone where the seaweeds are exposed to UV radiation for several hours in a day. U. prolifera samples were collected from lower intertidal zones. Prolonged seaweed exposure to solar UV radiation may result in producing bioactive compounds such as phenolics and flavonoids and may be an explanation of higher antioxidant capacity of Ulva clathrata in comparison with those of the other tested species.
Acknowledgments
The authors are grateful to the Department of Pharmacognosy, School of Pharmacy, Jundishapur University of Medical Sciences, Ahvaz, for laboratory facilities to carry out this study. The present work was supported by Natural Resources Research Center of Bushehr. Authors also thank B.M. Gharanjik for his help in confirmation of seaweeds identification.
Footnotes
Implication for health policy/practice/research/medical education: In our country research towards the use of marine algae for food or the treatment of various diseases has received less attention. To the best of our knowledge, it is the first project about antioxidative properties of green seaweeds in Iran.
Please cite this paper as: Farasat M, Khavari-Nejad RA, Nabavi SMB, Namjooyan F. Antioxidant Properties of two Edible Green Seaweeds From Northern Coasts of the Persian Gulf. Jundishapur J Nat Pharm Prod. 2013:8(1): 47-52.
Authors’ Contribution None Declared.
Financial Disclosure None Declared.
Funding/Support None Declared.
References
- 1.Orcic DZ, Mimica-Dukic NM, Franciskovic MM, Petrovic SS, Jovin ED. Antioxidant activity relationship of phenolic compounds in Hypericum perforatum L. Chem Cent J. 2011;5(34) doi: 10.1186/1752-153X-5-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moraes-de-Souza RA, Oldoni TLC, Regitano-d'Acre MAB, Alencar SM. Antioxidant activity and phenolic composition of herbal infusions consumed in Brazil. Cienc Technol Aliment. 2008;6(1):41–7. [Google Scholar]
- 3.Halliwell B. Oxidative stress and cancer: have we moved forward? Biochem J. 2007;401(1):1–2007;401(1):1-11. doi: 10.1042/BJ20061131. [DOI] [PubMed] [Google Scholar]
- 4.Yan S, Asmah R. Comparison of total phenolic contents and antioxidant activities of turmeric leaf, pandan leaf and torch ginger flower. Interl Food Res J. 2010;17(2):417–23. [Google Scholar]
- 5.Hodgson JM, Croft KD. Dietary flavonoids: effects on endothelial function and blood pressure. J. Sci. Food Agr. 2006;86(15):2492–8. doi: 10.1002/jsfa.2675. [DOI] [Google Scholar]
- 6.Kelman D, Posner EK, McDermid KJ, Tabandera NK, Wright PR, Wright AD. Antioxidant activity of Hawaiian marine algae. Mar Drugs. 2012;10(2):403–16. doi: 10.3390/md10020403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu SC, Wang FJ, Pan CL. The comparison of antioxidative properties of seaweed oligosaccharides fermented by two lactic acid bacteria. J Mar Scie Tech. 2010;18(4):537–45. [Google Scholar]
- 8.Cox S, Abu-Ghannam N, Gupta S. An assessment of the antioxidant and antimicrobial activity of six edible Irish seaweeds. Interl Food Res J. 2012;17(1):205–20. [Google Scholar]
- 9.Boonchum W, Peerapornpisal Y, Kanjanapothi D, Pekkoh J, Pumas C, Jamjai U, et al. Antioxidant activity of some seaweeds from the Gulf of Thailand. Int J AgricBiol. 2011;13(1):95–9. [Google Scholar]
- 10.BǾrgesen F. Marine algae from the Iranian Gulf, In: Danish Scientific Investigation in Iran, part 1. Ejnar Munksgaard. Copenhagen: 1939. [Google Scholar]
- 11.Lawson GW, John DM. The marine algae and coastal environment of tropical West Africa. Cramer. 1987. [Google Scholar]
- 12.Tseng CKP. Common seaweeds of China, Beijing and Amsterdam,Berkeley. Science Press; Kugler; 1984. [Google Scholar]
- 13.Lewis JE. History and Annotated Account of the Benthic Marine Algae of Taiwan, Cr1-2030681-00069. Proquest Information. 1998 [Google Scholar]
- 14.Coppejans E, Leliaert F, Dargent O, De Clerck O. Marine algae (Chlorophyta) from the north coast of Papua New Guinea. Cryptogamie Algol. 2001;22(4):1–69. doi: 10.1016/S0181-1568(01)01070-4. [DOI] [Google Scholar]
- 15.Zhang W.W, Duan X.J, Huang H.L, Zhang Y, Wang B.G. Evaluation of 28 marine algae from the Qingdao coast for antioxidative capacity and determination of antioxidant efficiency and total phenolic content of fractions and subfractions derived from Symphyocladia latiuscula (Rhodomelaceae). J Applied Phycology. 2007;19(2):97–108. doi: 10.1007/s10811-006-9115-x. [DOI] [Google Scholar]
- 16.Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K. Methods for testing antioxidant activity. Analyst. 2002;127(1):183–98. doi: 10.1039/b009171p. [DOI] [PubMed] [Google Scholar]
- 17.Chang C, Yang M, Wen H, Chern J. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J Food Drug Anal. 2002;10(3):178–82. [Google Scholar]
- 18.Nickavar B, Kamalinejad M, Izadpanah H. In vitro free radical scavenging activity of five Salvia species. Pak J Pharm Sci. 2007;20(4):291–4. [PubMed] [Google Scholar]
- 19.Wang B, Tong GZ, Y.L Q, Li L. Microwave-assisted extraction and in vitro antioxidant evaluation of polysaccharides from Enteromorpha prolifera. Appl Mech Mater. 2011;79(1):204–9. doi: 10.4028/www.scientific.net/AMM.79.204. [DOI] [Google Scholar]
- 20.Ganesan K, Kumar KS, Rao PVS. Comparative assessment of antioxidant activity in three edible species of green seaweed, Enteromorpha from Okha, Northwest coast of India. Innovative Food Science. 2011;12:73–8. doi: 10.1016/j.ifset.2010.11.005. [DOI] [Google Scholar]
- 21.Godard M, Décordé K, Ventura E, Soteras G, Baccou J.C, Cristol J.P, et al. Polysaccharides from the green alga Ulva rigida improve the antioxidant status and prevent fatty streak lesions in the high cholesterol fed hamster, an animal model of nutritionally-induced atherosclerosis. Food Chemistry. 2009;115(1):176–80. doi: 10.1016/j.foodchem.2008.11.084. [DOI] [Google Scholar]
- 22.Hassan S, El-Twab SA, Hetta M, Mahmoud B. Improvement of lipid profile and antioxidant of hypercholesterolemic albino rats by polysaccharides extracted from the green alga Ulva lactuca Linnaeus. Saudi J Bio Sci. 2011;18(4):333–40. doi: 10.1016/j.sjbs.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Banerjee K, Ghosh R, Homechaudhuri S, Abhijit M. Biochemical composition of marine macroalgae from Gangetic Delta at the apex of Bay of Bengal. Afr J Basic Appl Sci. 2009;1(5):96–104. [Google Scholar]
- 24.Qi H, Zhang Q, Zhao T, Hu R, Zhang K, Li Z. In vitro antioxidant activity of acetylated and benzoylated derivatives of polysaccharide extracted from Ulva pertusa (Chlorophyta). Bioorg Med Chem Lett. 2006;16(9):2441–5. doi: 10.1016/j.bmcl.2006.01.076. [DOI] [PubMed] [Google Scholar]
- 25.Chakraborty K, Paulraj R. Sesquiterpenoids with free-radical-scavenging properties from marine macroalga Ulva fasciata Delile. Food Chemistry. 2010;122(1):31–41. doi: 10.1016/j.foodchem.2010.02.012. [DOI] [Google Scholar]
- 26.Namjooyan F, Azemi M, Rahmanian V. Investigation of antioxidant activity and total phenolic content of various fractions of aerial parts of pimpinella barbata (dc.) Boiss. Jundishapur J Nat Pharm Prod. 2007;2(1):1–5. [Google Scholar]
- 27.Luo HY, Wang B, Yu CG, Qu YL, Su CL. Evaluation of antioxidant activities of five selected brown seaweeds from China. J Med Plants Res. 2010;4(18):2557–65. [Google Scholar]
- 28.Zakaria NA, Ibrahim D, Sulaiman SF, Supardy A. Assessment of antioxidant activity, total phenolic content and in vitro toxicity of Malaysian red seaweed, Acanthophora spicifera. J Chem Pharm Res. 2011;3(3):182–91. [Google Scholar]
- 29.Cho M, Lee H.S, Kang I.J, Won M.H, You S. Antioxidant properties of extract and fractions from Enteromorpha prolifera, a type of green seaweed. Food Chemistry. 2011;127(3):999–1006. doi: 10.1016/j.foodchem.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 30.Liu CC, Zhao Gl, Li YN, Ding ZP, Liu QG, Li JL. Contribution of phenolics and flavonoids to antioxidant activity of ethanol extract from Eichhornia crassipes. Adv Mater Res. 2010;8:1372–7. [Google Scholar]
- 31.Nijveldt RJ, Van Nood E, Van Hoorn DE, Boelens PG, Van Norren K, Van Leeuwen PA. Flavonoids: a review of probable mechanisms of action and potential applications. Am J Clin Nut. 2001;74(4):418–25. doi: 10.1093/ajcn/74.4.418. [DOI] [PubMed] [Google Scholar]
- 32.Chai TT, Wong FC. Whole-plant profiling of total phenolic and flavonoid contents, antioxidant capacity and nitric oxide scavenging capacity of Turnera subulata. J Med Plants Res. 2012;6(9):1730–5. [Google Scholar]
- 33.Bouba A, Njintang YN, Scher J, Mbofung CMF. Phenolic compounds and radical scavenging potential of twenty Cameroonian spices. Agric Biol J N Am. 2010;1(3):213–24. [Google Scholar]
- 34.Yoshie Y, Wang W, Hsieh Y, Suzuki T. Compositional difference of phenolic compounds between two seaweeds, Halimeda spp. J Tokyo Univ of Fish. 2002;88(1):21–4. [Google Scholar]