Abstract
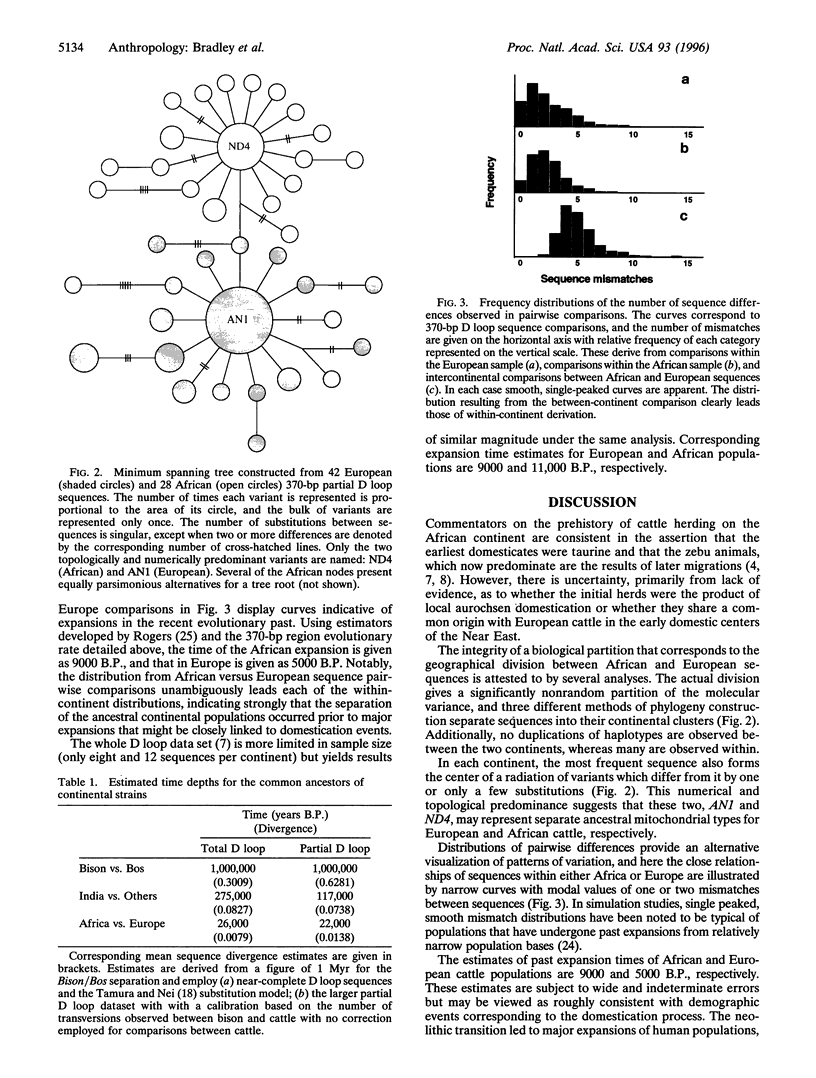
The nature of domestic cattle origins in Africa are unclear as archaeological data are relatively sparse. The earliest domesticates were humpless, or Bos taurus, in morphology and may have shared a common origin with the ancestors of European cattle in the Near East. Alternatively, local strains of the wild ox, the aurochs, may have been adopted by peoples in either continent either before or after cultural influence from the Levant. This study examines mitochondrial DNA displacement loop sequence variation in 90 extant bovines drawn from Africa, Europe, and India. Phylogeny estimation and analysis of molecular variance verify that sequences cluster significantly into continental groups. The Indian Bos indicus samples are most markedly distinct from the others, which is indicative of a B. taurus nature for both European and African ancestors. When a calibration of sequence divergence is performed using comparisons with bison sequences and an estimate of 1 Myr since the Bison/Bos Leptobos common ancestor, estimates of 117-275,000 B.P. and 22-26,000 B.P. are obtained for the separation between Indians and others and between African and European ancestors, respectively. As cattle domestication is thought to have occurred approximately 10,000 B.P., these estimates suggest the domestication of genetically discrete aurochsen strains as the origins of each continental population. Additionally, patterns of variation that are indicative of population expansions (probably associated with the domestication process) are discernible in Africa and Europe. Notably, the genetic signatures of these expansions are clearly younger than the corresponding signature of African/European divergence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., de Bruijn M. H., Coulson A. R., Eperon I. C., Sanger F., Young I. G. Complete sequence of bovine mitochondrial DNA. Conserved features of the mammalian mitochondrial genome. J Mol Biol. 1982 Apr 25;156(4):683–717. doi: 10.1016/0022-2836(82)90137-1. [DOI] [PubMed] [Google Scholar]
- Bradley D. G., MacHugh D. E., Loftus R. T., Sow R. S., Hoste C. H., Cunningham E. P. Zebu-taurine variation in Y chromosomal DNA: a sensitive assay for genetic introgression in west African trypanotolerant cattle populations. Anim Genet. 1994 Feb;25(1):7–12. [PubMed] [Google Scholar]
- Excoffier L., Smouse P. E., Quattro J. M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics. 1992 Jun;131(2):479–491. doi: 10.1093/genetics/131.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Excoffier L., Smouse P. E. Using allele frequencies and geographic subdivision to reconstruct gene trees within a species: molecular variance parsimony. Genetics. 1994 Jan;136(1):343–359. doi: 10.1093/genetics/136.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai S., Kondo R., Murayama K., Hayashi S., Koike H., Nakai N. Phylogenetic affiliation of ancient and contemporary humans inferred from mitochondrial DNA. Philos Trans R Soc Lond B Biol Sci. 1991 Sep 30;333(1268):409–417. doi: 10.1098/rstb.1991.0091. [DOI] [PubMed] [Google Scholar]
- Isaac E. On the Domestication of Cattle: Zoology and cultural history both illuminate the view that the original motive was religious, not economic. Science. 1962 Jul 20;137(3525):195–204. doi: 10.1126/science.137.3525.195. [DOI] [PubMed] [Google Scholar]
- Kolman C. J., Bermingham E., Cooke R., Ward R. H., Arias T. D., Guionneau-Sinclair F. Reduced mtDNA diversity in the Ngöbé Amerinds of Panamá. Genetics. 1995 May;140(1):275–283. doi: 10.1093/genetics/140.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg G. L., Koehler C. M., Mayfield J. E., Myers A. M., Beitz D. C. Recovery of mitochondrial DNA from blood leukocytes using detergent lysis. Biochem Genet. 1992 Feb;30(1-2):27–33. doi: 10.1007/BF00554425. [DOI] [PubMed] [Google Scholar]
- Loftus R. T., MacHugh D. E., Bradley D. G., Sharp P. M., Cunningham P. Evidence for two independent domestications of cattle. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2757–2761. doi: 10.1073/pnas.91.7.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundstrom R., Tavaré S., Ward R. H. Estimating substitution rates from molecular data using the coalescent. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5961–5965. doi: 10.1073/pnas.89.13.5961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M., Morrison W. I., Whitelaw D. D. Host susceptibility to African trypanosomiasis: trypanotolerance. Adv Parasitol. 1982;21:1–68. doi: 10.1016/s0065-308x(08)60274-2. [DOI] [PubMed] [Google Scholar]
- Perkins D., Jr Fauna of Catal Hüyük: evidence for early cattle domestication in Anatolia. Science. 1969 Apr 11;164(3876):177–179. doi: 10.1126/science.164.3876.177. [DOI] [PubMed] [Google Scholar]
- Rogers A. R., Harpending H. Population growth makes waves in the distribution of pairwise genetic differences. Mol Biol Evol. 1992 May;9(3):552–569. doi: 10.1093/oxfordjournals.molbev.a040727. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Tamura K., Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993 May;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- Thompson J. D., Higgins D. G., Gibson T. J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994 Nov 11;22(22):4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigilant L., Stoneking M., Harpending H., Hawkes K., Wilson A. C. African populations and the evolution of human mitochondrial DNA. Science. 1991 Sep 27;253(5027):1503–1507. doi: 10.1126/science.1840702. [DOI] [PubMed] [Google Scholar]