Abstract
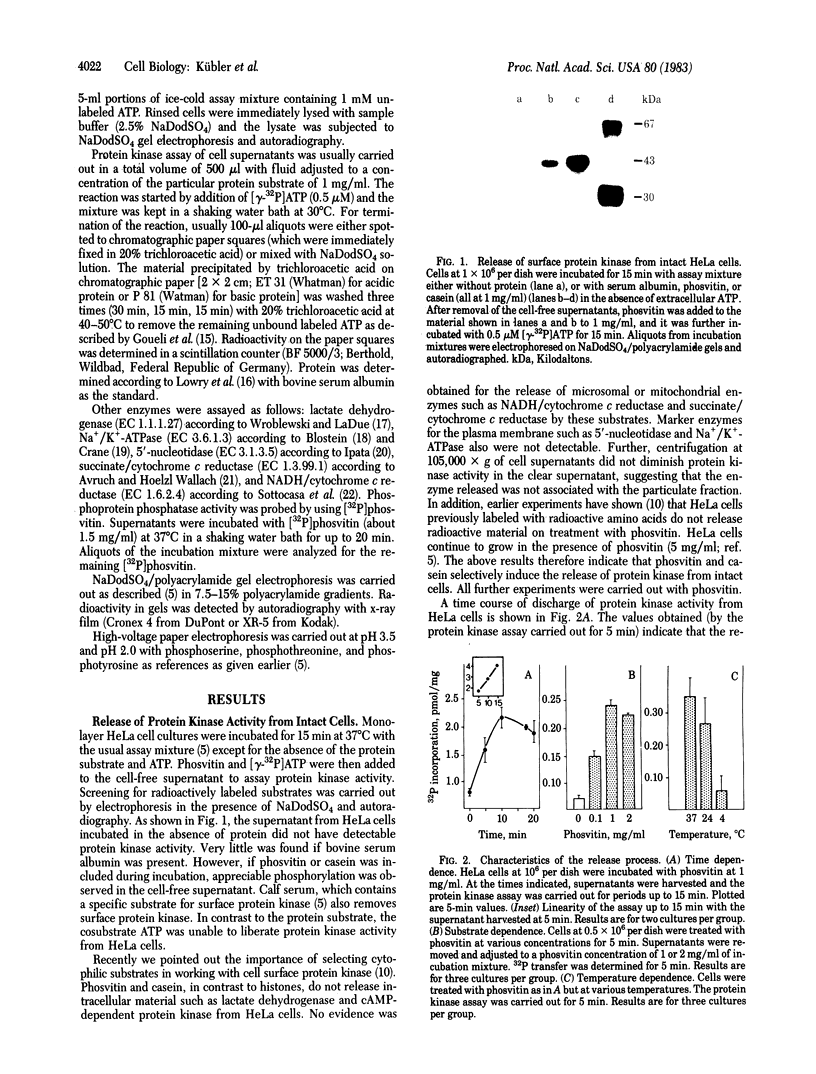
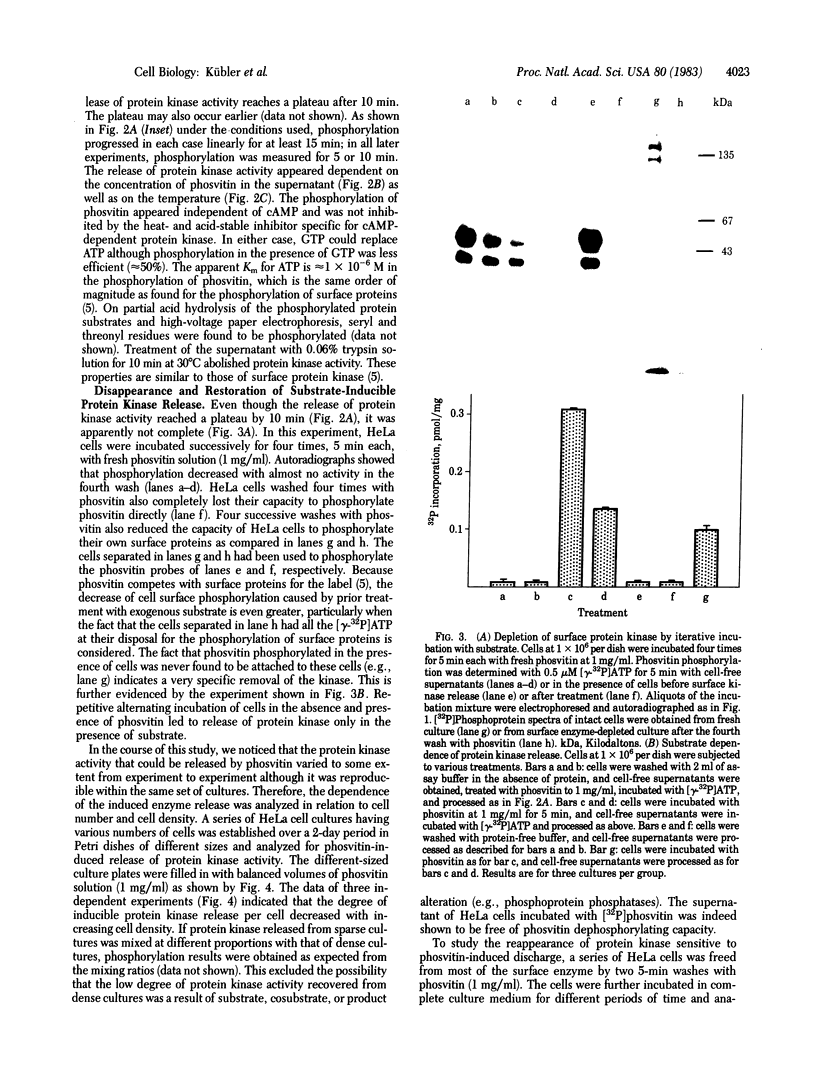
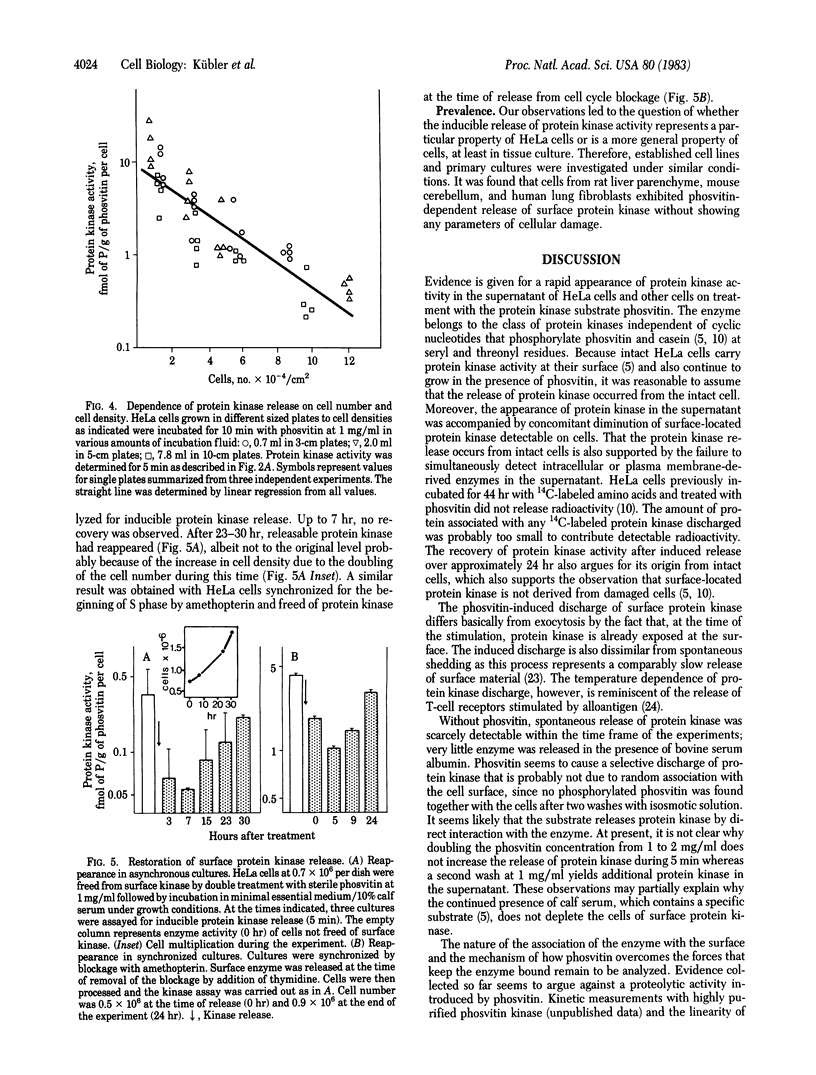
Protein kinase activity that is independent of cAMP has been reported to exist on the surface of intact HeLa cells. Here we report that the protein kinase activity can be released by the use of casein or phosvitin within a short period of time. The discharge of the enzyme occurs from intact cells since (i) the cells do not release intracellular material and (ii) the cultures continue to grow within any morphological alteration. As shown with phosvitin, the release of protein kinase depends on substrate concentration, incubation time, and temperature. The degree of inducible release or surface protein kinase is inversely related to cell density. Four incubations with phosvitin (1 mg/ml) are sufficient to liberate most of the enzyme, thus greatly reducing the capacity of the cells to phosphorylate cellular substrates at the surface. Within approximately 24 hr after protein kinase removal, cultures have restored their surface protein kinase. Cultured cells of different origin (rat liver, mouse cerebellum, and human lung) exhibited phosvitin-induced protein kinase release from intact cells. The possible significance of these observations with respect to extracellular protein phosphorylation is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agren G., Ronquist G. Formation of extracellular adenosine triphosphate by tumour cells. Acta Physiol Scand. 1969 Jan-Feb;75(1):124–128. doi: 10.1111/j.1748-1716.1969.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Avruch J., Wallach D. F. Preparation and properties of plasma membrane and endoplasmic reticulum fragments from isolated rat fat cells. Biochim Biophys Acta. 1971 Apr 13;233(2):334–347. doi: 10.1016/0005-2736(71)90331-2. [DOI] [PubMed] [Google Scholar]
- BISHOP C., RANKINE D. M., TALBOTT J. H. The nucleotides in normal human blood. J Biol Chem. 1959 May;234(5):1233–1237. [PubMed] [Google Scholar]
- Black P. H. Shedding from the cell surface of normal and cancer cells. Adv Cancer Res. 1980;32:75–199. doi: 10.1016/s0065-230x(08)60361-9. [DOI] [PubMed] [Google Scholar]
- Blostein R. Relationships between erythrocyte membrane phosphorylation and adenosine triphosphate hydrolysis. J Biol Chem. 1968 Apr 25;243(8):1957–1965. [PubMed] [Google Scholar]
- Bosmann H. B. Cell surface enzymes: effects on mitotic activity and cell adhesion. Int Rev Cytol. 1977;50:1–23. doi: 10.1016/s0074-7696(08)60097-2. [DOI] [PubMed] [Google Scholar]
- CRANE R. K., LIPMANN F. The effect of arsenate on aerobic phosphorylation. J Biol Chem. 1953 Mar;201(1):235–243. [PubMed] [Google Scholar]
- Carpenter G., King L., Jr, Cohen S. Rapid enhancement of protein phosphorylation in A-431 cell membrane preparations by epidermal growth factor. J Biol Chem. 1979 Jun 10;254(11):4884–4891. [PubMed] [Google Scholar]
- Chiang T. M., Kang E. S., Kang A. H. Ecto-protein kinase activity of fibroblasts. Arch Biochem Biophys. 1979 Jul;195(2):518–525. doi: 10.1016/0003-9861(79)90378-3. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Plasma membranes of mammalian cells: a review of methods for their characterization and isolation. J Cell Biol. 1973 Feb;56(2):275–303. doi: 10.1083/jcb.56.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goueli S. A., Slungaard R., Wilson M. J., Ahmed K. A modified paper-binding procedure for the assay of nucleus-associated protein phosphokinases. J Pharmacol Methods. 1980 May;3(3):235–242. doi: 10.1016/0160-5402(80)90004-2. [DOI] [PubMed] [Google Scholar]
- Ipata P. L. A coupled optical enzyme assay for 5'-nucleotidase. Anal Biochem. 1967 Jul;20(1):30–36. doi: 10.1016/0003-2697(67)90261-8. [DOI] [PubMed] [Google Scholar]
- Kang E. S., Gates R. E., Chiang T. M., Kang A. H. Ectoprotein kinase activity of the isolated rat adipocyte. Biochem Biophys Res Commun. 1979 Feb 14;86(3):769–778. doi: 10.1016/0006-291x(79)91779-0. [DOI] [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Kinzel V. Assays of cell surface protein kinase: importance of selecting cytophilic substrates. Eur J Cell Biol. 1982 Feb;26(2):306–309. [PubMed] [Google Scholar]
- Kübler D., Pyerin W., Kinzel V. Protein kinase activity and substrates at the surface of intact HeLa cells. J Biol Chem. 1982 Jan 10;257(1):322–329. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mastro A. M., Rozengurt E. Endgoenous protein kinase in outer plasma membrane of cultured 3T3 cells. Nature of the membrane-bound substrate and effect of cell density, serum addition, and oncogenic transformation. J Biol Chem. 1976 Dec 25;251(24):7899–7906. [PubMed] [Google Scholar]
- Ramseier H. Spontaneous release of T-cell receptors for alloantigens. I. Recognition of alloantigens and receptor release dynamics. J Exp Med. 1974 Sep 1;140(3):603–618. doi: 10.1084/jem.140.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shur B. D., Roth S. Cell surface glycosyltransferases. Biochim Biophys Acta. 1975 Dec 29;415(4):473–512. doi: 10.1016/0304-4157(75)90007-6. [DOI] [PubMed] [Google Scholar]
- Sommarin M., Henriksson T., Jergil B. Cyclic AMP-dependent protein phosphorylation on the surface of rat hepatocytes. FEBS Lett. 1981 May 18;127(2):285–289. doi: 10.1016/0014-5793(81)80225-6. [DOI] [PubMed] [Google Scholar]
- Sottocasa G. L., Kuylenstierna B., Ernster L., Bergstrand A. An electron-transport system associated with the outer membrane of liver mitochondria. A biochemical and morphological study. J Cell Biol. 1967 Feb;32(2):415–438. doi: 10.1083/jcb.32.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trams E. G. A proposal for the role of ecto-enzymes and adenylates in traumatic shock. J Theor Biol. 1980 Dec 7;87(3):609–621. doi: 10.1016/0022-5193(80)90239-8. [DOI] [PubMed] [Google Scholar]
- Trams E. G. Evidence for ATP action on the cell surface. Nature. 1974 Dec 6;252(5483):480–482. doi: 10.1038/252480a0. [DOI] [PubMed] [Google Scholar]
- WROBLEWSKI F., LADUE J. S. Lactic dehydrogenase activity in blood. Proc Soc Exp Biol Med. 1955 Oct;90(1):210–213. doi: 10.3181/00379727-90-21985. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Ashby C. D., Gonzalez C., Calkins D., Fischer E. H. Krebs EG: Purification and characterization of a protein inhibitor of adenosine 3',5'-monophosphate-dependent protein kinases. J Biol Chem. 1971 Apr 10;246(7):1977–1985. [PubMed] [Google Scholar]