Abstract
The majority of people who sustain a traumatic brain injury (TBI) have an injury that can be classified as mild (often referred to as concussion). Although head CT scans for most subjects who have sustained a mild TBI (mTBI) are negative, these persons may still suffer from neurocognitive and neurobehavioral deficits. In order to expedite pre-clinical research and develop therapies, there is a need for well-characterized animal models of mTBI that reflect the neurological, neurocognitive, and pathological changes seen in human patients. In the present study, we examined the motor, cognitive, and histopathological changes resulting from 1.0 and 1.5 atmosphere (atm) overpressure fluid percussion injury (FPI). Both 1.0 and 1.5 atm FPI injury caused transient suppression of acute neurological functions, but did not result in visible brain contusion. Animals injured with 1.0 atm FPI did not show significant motor, vestibulomotor, or learning and memory deficits. In contrast, 1.5 atm injury caused transient motor disturbances, and resulted in a significant impairment of spatial learning and short-term memory. In addition, 1.5 atm FPI caused a marked reduction in cerebral perfusion at the site of injury that lasted for several hours. Consistent with previous studies, 1.5 atm FPI did not cause visible neuronal loss in the hippocampus or in the neocortex. However, a robust inflammatory response (as indicated by enhanced GFAP and Iba1 immunoreactivity) in the corpus callosum and the thalamus was observed. Examination of fractional anisotropy color maps after diffusion tensor imaging (DTI) revealed a significant decrease of FA values in the cingulum, an area found to have increased silver impregnation, suggesting axonal injury. Increased silver impregnation was also observed in the corpus callosum, and internal and external capsules. These findings are consistent with the deficits and pathologies associated with mild TBI in humans, and support the use of mild FPI as a model to evaluate putative therapeutic options.
Key words: axonal injury, concussion, DTI, mild traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major health problem, both for the military and the general civilian population. It has been estimated that approximately 80% of all TBI cases are mild, translating into over a million new cases of mild TBI (mTBI) in the United States each year. mTBI patients exhibit a number of neuropsychiatric symptoms, including vestibular problems, cognitive deficits, and behavioral disorders. In 80%–90% of these individuals, the neuropsychological problems resolve within 10 days to 2 weeks after their injury.1–3 However, in 10%–20% of mTBI patients, some or all of these neuropsychological problems will persist for months.4 While standard head computed tomography (CT) does not typically indicate overt brain injury in mTBI patients, recent studies employing diffusion tensor imaging (DTI) have reported damage to major fiber tracts, including the corpus callosum, in some individuals with mTBI.5–7 Although these imaging methods have improved the ability to diagnose persons with mTBI accurately, effective pharmacological treatments to lessen mTBI-associated symptoms have not been developed. The availability of well-characterized rodent models of mTBI that reflect the neurological, neurocognitive, and pathological changes seen in persons with mTBI would therefore facilitate the pre-clinical screening of therapeutic agents for suitability in clinical testing.
Three rodent models: fluid percussion injury (FPI), controlled cortical impact injury, and weight drop, have been used to examine the behavioral and pathological changes associated with TBI. Although moderate-to-severe forms of injury have been extensively studied using these models, there have been relatively fewer investigations into the consequences of mild TBI.8–11 In humans, mild TBI is generally defined as an opening Glasgow Coma Score of 13–15, transient loss of memory for events immediately before or after the injury, loss or alterations of consciousness, and alterations in mental status at the time of injury. However, within this definition exists a range of injuries that can be classified as either uncomplicated or complicated, depending on neuroimaging abnormalities. Although most mTBI patients do not have gross structural changes that can be detected by head computerized tomography (CT), few animal studies have been carried out using magnitudes of TBI that do not result in brain contusion.12–16 For example, Shultz et al.16 reported that 1.2 atm FPI did not cause overt brain damage but resulted in significant short-term memory impairments when tested 24 h after injury. Employing a 1.5 atm FPI injury, Wu et al. observed that animals pre-trained in the Morris water maze task had poor memory recall when tested over the first 3 days after injury.17 As experimental treatments often need to be administered over a period of days after TBI, it is desirable that models give rise to cognitive deficits that persist beyond the acute stage of injury. With this in mind, we aimed to identify the magnitudes of mild FPI that can produce a significant cognitive deficit on day 5 post-injury that occurs in the absence of visible injury.
In this study, we examined the vestibulomotor, motor, and cognitive functions in rats injured using either 1.0 atm or 1.5 atm FPI. After the completion of behavioral testing, we performed ex vivo diffusion tensor imaging (DTI) to assess axonal integrity, and histopathological analysis to examine inflammation, neuronal loss, and morphological changes. Our results show that while a 1.0 atm injury did not cause a significant neurocognitive deficit, 1.5 atm injury caused a reproducible learning and short-term memory impairment that occurred in absence of visible contusion or neuronal loss, but that was associated with axonal damage and neuroinflammation. Our results indicate this level of injury would be suitable for pre-clinical drug screening studies to improve the outcome in this subset of mild TBI patients.
Methods
Materials
Male Sprague-Dawley rats (275–300 g) were purchased from Charles River Laboratories (Wilmington, MA). Antibodies to NeuN and GFAP (Millipore, Billerica, MA), IBA-1 (WAKO, Richmond, VA), amyloid precursor protein (APP, Invitrogen, Grand Island, NY), and myelin basic protein (Covance, Princeton, NJ) were obtained for use in these studies. A silver staining kit to identify degenerating neurons was purchased from FD Neurotechnologies (Columbia, MD).
Lateral fluid percussion injury
All experimental procedures were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with the recommendations provided in the Guide for the Care and Use of Laboratory Animals. Protocols were designed to minimize pain and discomfort during the injury procedure and recovery. Fluid percussion injury was carried out as described previously.18–20 Briefly, rats were initially anesthetized using 5% isoflurane with a 1:1 N2O/O2 mixture and then maintained with a 2.5% isoflurane with 1:1 N2O/O2 mixture via a face mask. Animals were mounted on the stereotaxic frame, a midline 4.8 mm-diameter craniectomy was carefully made midway between bregma and lambda. A hub (modified from 20-gauge needle) was planted into the burr hole and affixed to the skull by contact adhesive and dental cement. Once the assembly was secured, the rat was removed from anesthesia and allowed to regain its tail pinch reflex. Immediately upon regaining this reflex, the rat was injured using a FPI device and a pressure of 1.0 or 1.5 atmosphere (atm) over base room pressure. After injury, the hub and surrounding dental cement were immediately removed, and the incision closed by wound clips. Sham-operated animals received all the aforementioned surgical procedures except hub implantation and the injury. Animals' body temperature was maintained at 37°C during the surgery, using a rectal thermometer coupled to a heating pad.
Assessment of acute neurological function
After injury, the duration of suppression of the paw withdrawal reflex and the self-righting reflex were monitored as acute measures of injury severity. Self-righting was monitored by placing the animal on its back and recording the time from injury required to right itself three consecutive times. For sham-operated animals, the duration of suppression of righting response was measured from the time of reinstatement of the paw pinch reflex (the point at which the FPI was delivered in injured animals).
Measurement of cerebral perfusion
Cerebral perfusion was measured using a PIM3 scanning laser Doppler device. Due to the prolonged anesthesia required for these measures, perfusion measures were carried out in animals separate than those used for behavior. Following the creation of the burr hole, but prior to hub implantation, the cerebral cortex was scanned through the burr hole to establish pre-injury perfusion values. After injury, the animal was placed back into the stereotaxic frame and maintenance of anesthesia reinstated. The hub was gently removed and cerebral perfusion monitored every 10 min for the first hour after injury. Additional scans were performed 24 h after injury to determine if perfusion had normalized by this time point. Sham-operated animals were treated identically with the exception of the injury. Five consecutive scans were generated for each time point, the perfusion values of which were average for each animal.
Behavioral assessments
All behavioral tests were conducted by an experimenter blind to the treatment groups.
Vestibulomotor and motor functions
Three different motor skill tasks (beam balance, rotarod, and foot-fault) were used to determine animals' vestibulomotor and motor performances as described previously.21,22 Beginning on day 1 post-injury, animals were given three trials in the beam balance task during which the length of time spent balancing on the beam was recorded. For the foot fault task, the number of foot faults was evaluated by placing the animal on a wire grid (opening size of 2×2 cm) and counting the number of times a front paw missed and slipped below the plane of the grid out of a total of 50 steps. The rotarod procedure used was similar to that described by Hamm et al.22 The animal was placed upon the stationary rotarod device for a period of 10 sec, after which rotation was initiated (beginning speed of 3 rpm that was incrementally increased by 3 rpm every 10 sec until a maximum of 30 rpm was reached). The time spent on the device (maximum time of 20 sec at 30 rpm) was recorded in each of the two daily trials. A trial ended, and time was recorded, when the animal either fell off the device or held onto the rotating rod for two consecutive rotations without attempting to walk.
Spatial learning and short-term memory
A common component of neuropsychological tests used to diagnose mTBI (e.g., military acute concussion evaluation, MACE; automated neuropsychological assessment metrics, ANAM) is a short-term memory test. In order to assess short-term memory, we used a modified version of the Morris water maze task in which rats are trained to find the location of a hidden platform within a single training session, followed by a probe trial administered 30 min later.23,24 On day 5 post-injury, rats were given 10 consecutive training trials with an inter-trial interval (iti) of 4 min. Each trial was initiated by placing the rat into the water maze at one of four randomly chosen starting positions. The animal was allowed to search for the hidden platform for a period of 60 sec and the time to find the platform recorded. If the rat failed to find the hidden platform on any given trial, it was led there by the experimenter. Thirty min after the last training trial, animals were tested in a probe trial in which the platform was removed from the tank and the rat allowed to search for a period of 60 sec. Movement within the maze was monitored using a video camera linked to tracking software (Ethovision, Noldus Information Technology, Leesbury, VA, USA). Measures of memory including latency to first platform crossing, number of crossings, and quadrant preference were recorded.
Ex vivo diffusion tensor imaging (DTI)
At the conclusion of behavior testing, rats were given an overdose of sodium pentobarbital (100 mg/kg), transcardially perfused with ice-cold phosphate-buffered-saline (0.1 M PBS), followed by buffered 4% paraformaldehyde. Brains were carefully removed and stored in fixative at 4°C. Representative brains from each group were then submerged in Fomblin (Kurt J. Lesker Company, Livermore, CA) and images were acquired at the UTHealth MRI Core Facility using a Bruker Biospec 70/30 URS scanner, operating at field strength of 7 Tesla. A series of 3D EPI DTI scans (42 gradient directions) were obtained in axial orientation. These images were then imported into DTI Studio software to calculate maps of fractional anisotropy (FA), mean (MD), longitudinal (LD), and radial (RD) diffusivities. Regions of interest (ROIs) encompassing the genu of the corpus callosum, cingulum, internal and external capsules, fimbria, and cortex proximal to the injury site, were outlined in the ipsilateral hemisphere, and values compared between sham and FPI groups. Statistical comparisons were made using a two-way ANOVA, followed by a Bonferroni post-hoc analysis. Fiber tracking was carried out using MedINRIA 1.9 (http://www-sop.inria.fr/asclepios/software/MedINRIA/) from a representative sham and FPI rat using a FA threshold of 400 and a background threshold of 100.
Immunohistochemistry
Brains not used for DTI scanning were transferred to a 30% buffered sucrose solution for cryopreservation. Brains were sectioned on a cryostat in the coronal plane at 40 μm through the rostro-caudal extent. Sections were incubated in primary antibody solutions (0.1-0.5 μg/mL antibody, 2.5% normal goat serum in PBS) overnight at room temperature. Following extensive washing, sections were incubated for 1 h in PBS containing species-specific secondary antibodies linked to AlexaFluor dyes (Alexa488 or Alexa568; Invitrogen). Sections were mounted onto glass slides and coverslipped with Fluoromount-G to retard fading. Slides were examined using an upright microscope with epifluorescence capabilities. Images were captured using a MagnaFire camera using settings that remained constant across groups.
Silver staining
Silver staining was carried out on free-floating sections using a kit from FD Neurotechnologies (Columbia, MD) essentially as described by the vendor. Of exception was the impregnation time was extended from 4 min to 6 min in order to maximize the signal-to-noise ratio.
Statistical analyses
Statistical comparisons were carried out using SigmaStat (Systat Software, San Jose, CA). Across group comparisons of data collected over time (e.g., behavioral training and blood flow measures) were evaluated using a repeated measures two-way ANOVA, followed by post-hoc analysis. Group main, or interactions of group and time, differences were used to compare the groups. Single measure data (e.g., probe trial data) was statistically compared using a Student's t-test for unpaired variables, whereas within group comparisons (e.g., quadrant preference) were tested using a repeated measures one-way ANOVA.
Results
1.0 atm FPI did not cause vestibulomotor, motor, or spatial learning, and memory deficits
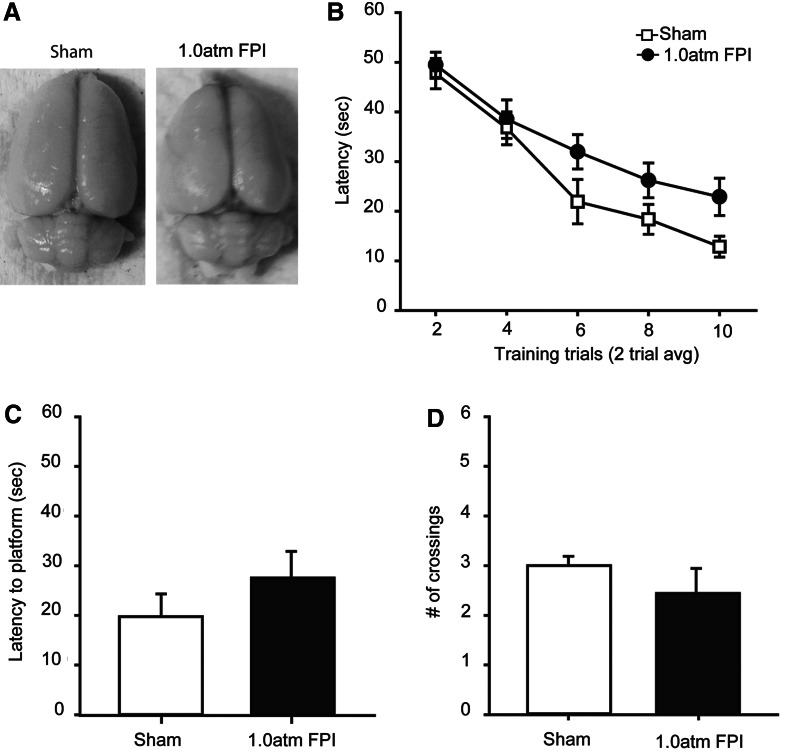
The majority of human mTBI patients are head CT negative, indicating no overt physical damage to the brain, yet many will suffer from neurological, cognitive, and behavioral dysfunctions <(see Jeter et al., this issue)>. In order to model these clinical observations, we employed 1.0 atm lateral FPI to examine if this injury gives rise to reproducible behavioral dysfunction in the absence of overt brain damage. We observed a suppression of the righting response of 4.1±0.2 min in the 1.0 atm injured rats (n=20), significantly longer than the 1.30±0.1 min observed in sham controls (n=17) (Student's t-test: p<0.001). When the brains of these animals were examined for signs of cortical contusion or tissue loss, no differences were seen between the 1.0 atm FPI brains and those from their sham-operated counterparts (Fig. 1A).
FIG. 1.
FPI of 1.0 atm did not cause learning or memory dysfunction when tested 5 days post-injury. (A) Representative pictures of brains from a sham and a 1.0 atm FPI injured animal. (B) Training curves in the abbreviated Morris water maze task for sham-operated controls (n=17) and 1.0 atm FPI (n=20) animals. During memory testing 30 min after training, 1.0 atm FPI rats were not significantly different than controls in their (C) latency to first platform crossing or in (D) the number of platform crossings during the 60 sec probe trial. Data are presented as the mean±SEM.
In order to determine the effect of 1.0 atm mFPI on vestibulomotor and motor functions, animals were tested on the balance beam, rotarod, and foot fault tasks, beginning on day 1 post-injury. No significant differences between the two groups was detected in either vestibulomotor (balance beam (day 1): sham, 56.30±2.01 sec; 1.0 atm FPI, 56.39±1.95 sec, Student's t-test: p=0.975), locomotor (rotarod (day 1): sham, 66.36±4.30 sec; 1.0 atm FPI, 73.33±7.63 sec, Student's t-test: p=0.401), or motor coordination (foot fault (days 1–3): ipsilateral two-way repeated measures ANOVA: F(2,66)=0.468, p=0.628; contralateral two-way repeated measures ANOVA: F(2,66)=0.311, p=0.734).
When learning was assessed using the abbreviated water maze task (on day 5 post-injury), a trend towards longer latencies to locate the hidden platform was observed in the 1.0 atm FPI animals compared to the sham controls (repeated measures two-way ANOVA: F(1,35)=3.51; p=0.069) (Fig. 1B). When short-term memory was assessed 30 min after the completion of training, there was no difference in latency to the previous location of the platform (Students t-test, p=0.42; Fig. 1C), nor in number of platform crossings (Student's t-test, p=0.56, Fig. 1D). Both sham (one-way repeated measures ANOVA: F(3,48)=7.85, p<0.001) and 1.0 atm FPI (one-way repeated measures ANOVA: F(3,57)=12.17, p<0.001) groups displayed significant quadrant preferences for the target quadrant (data not shown). As significant short-term memory impairments were not observed at 1 atm FPI, further characterization of this injury magnitude was not carried out.
1.5 atm FPI causes transient motor dysfunction
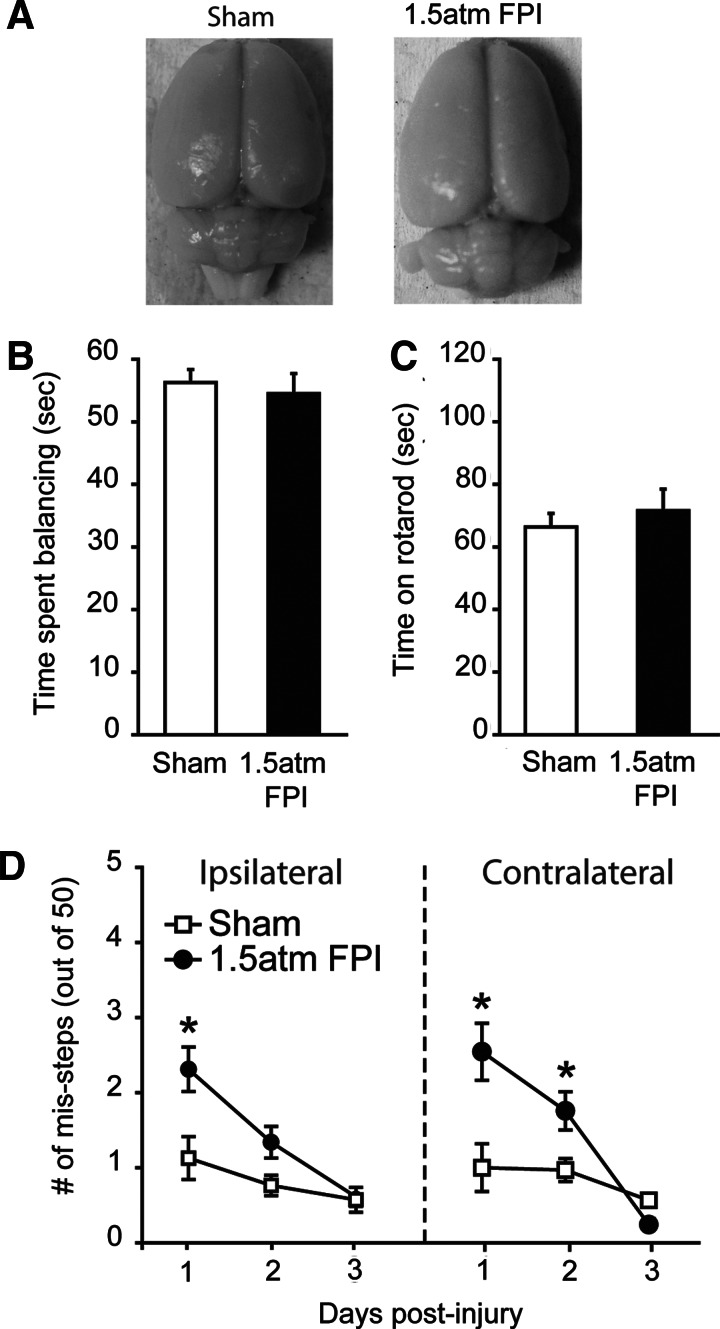
To determine if a higher injury magnitude could be used to mimic the cognitive deficits associated with uncomplicated mild TBI, groups of animals were injured using 1.5 atm FPI (n=11) or sham-operated (n=11) and the resulting pathobiologies compared. We observed a significantly longer suppression of the righting response in 1.5 atm injured rats as compared to sham animals (sham: 1.53±0.2 min; 1.5 atm mFPI: 7.35±0.3 min, Student's t-test: p<0.001). 1.5 atm animals also had significantly longer suppression of paw withdrawal reflexes (1.0 atm mFPI, 43.67±1.48 sec; 1.5 atm mFPI 90.73±7.28 sec, Student's t-test: p<0.001), further supporting increased injury. Similar to 1.0 atm FPI, 1.5 atm FPI did not cause overt damage to the brain (Fig. 2A). When tested for vestibulomotor and locomotor function, 1.5 atm FPI animals were not significantly impaired in their ability to perform the beam balance (sham: 56.28±2.08 sec; mFPI: 54.91±3.21 sec, Student's t-test: p=0.933; Fig. 2B) or the rotarod (sham: 66.36±4.30 sec; mFPI: 71.58±6.89 sec, Student's t-test: p=0.527; Fig. 2C) tasks. Transient deficits in motor coordination were observed, with 1.5 atm FPI animals making significantly more ipsilateral (two-way repeated measures ANOVA: F(2,39)=4.06, p=0.025) and contralateral (two-way repeated measures ANOVA: F(2,39)=8.56, p<0.001) foot-faults than did their sham-operated counterparts on days 1 and 2 post-injury (Fig. 2D). By day 3, the performance of the injured animals did not differ from the sham group.
FIG. 2.
FPI of 1.5 atm caused transient motor coordination deficits. (A) Representative pictures of brains from a sham and a 1.5 atm FPI injured animal. When tested on day 1 post-injury, 1.5 atm FPI animals (n=11) were not significantly different than shams (n=11) in their ability to perform the (B) beam balance, or (C) rotarod tasks. (D) In contrast, injured animals displayed transient ipsilateral and contralateral paw placement deficits that recovered by day 3 post-injury. Data are presented as the mean±SEM; *p<0.05.
1.5 atm FPI caused spatial learning and short-term memory dysfunction
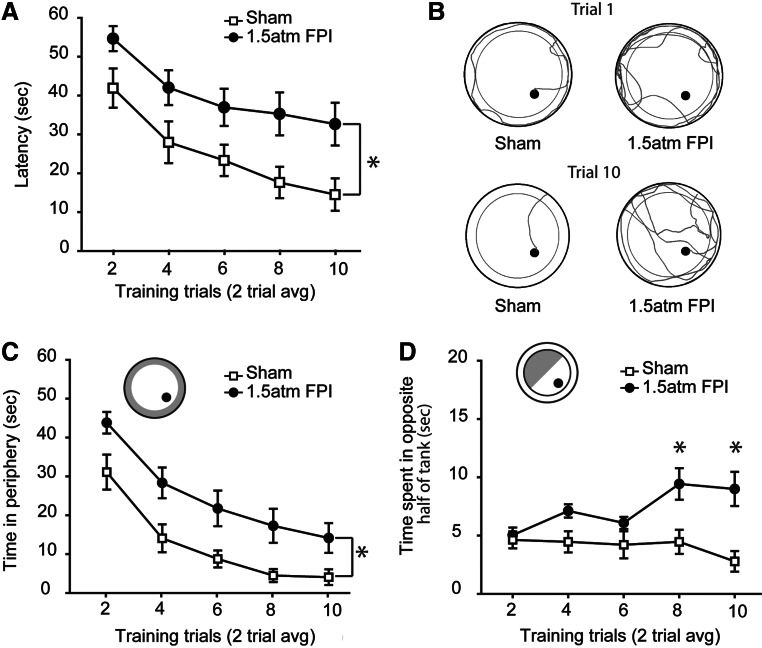
To test if 1.5 atm FPI causes learning and memory deficits, sham and injured animals were trained and tested in the abbreviated version of MWM task on day 5 post-injury. During training, there was a significant difference in the latency to find the hidden platform between the two groups (two-way repeated measures ANOVA: F(1,20)=25.25, p<0.001; Fig. 3A). The representative traces shown in Figure 3B indicate that, although sham-operated animals initially swam along the perimeter of the tank looking for a means of escape, by the end of training, they had learned the location of the hidden platform and took a direct path to its location. In contrast, 1.5 atm animals appeared to continue to spend more time in the periphery of the tank and had a less focused search pattern. Quantification of the swim paths revealed that over the course of training, 1.5 atm FPI injured animals entered (F(1,20)=22.84, p<0.001) and spent more time (F(1,20)=21.55, p<0.001; Fig. 3C) in the periphery of the tank. Further, 1.5 atm FPI rats spent significantly more time in the opposite half of the tank throughout training (F(1,20)=9.71, p=0.005; Fig. 3D). No significant differences in number of right or left turns were found between the groups, indicating no inherent circling bias.
FIG. 3.
FPI of 1.5 atm caused significant spatial learning deficits. (A) When trained in the abbreviated version of the Morris water maze task on day 5 post-injury, 1.5 atm FPI rats were significantly impaired in their ability to learn the location of the hidden platform compared to sham-operated controls. (B) Representative traces of the swimming paths taken by a sham and a 1.5 atm FPI rat at the beginning and end of training. Poor performance in the water maze task was in part due to injured animals spending more time searching in (C) the perimeter and (D) opposite half of the tank for a means of escape. Data are presented as the mean±SEM; *p=0.05.
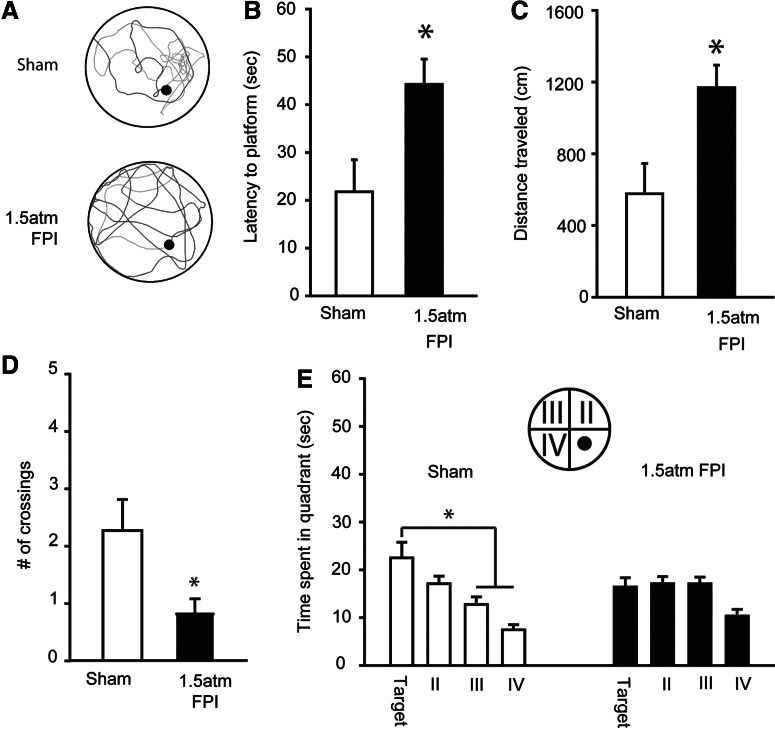
Short-term memory was assessed by a probe trial administered 30 min after the completion of training. Figure 4A shows representative traces from a sham and a 1.5 atm FPI rat, indicating the paths taken to first platform crossing and during the remainder of the 60 sec probe. The traces show that the sham-operated controls had a more localized search pattern, requiring less time to cross the platform location and resulting in more platform crossings than that observed in the trace from the 1.5 atm FPI animal. Consistent with this, latency (Student's t-test: p=0.016; Fig. 4B) and path length (Student's t-test: p=0.011; Fig. 4C) to first platform crossing were found to be significantly different between the sham and FPI groups. Throughout the 60 sec probe trial, 1.5 atm FPI animals crossed the platform fewer times than did sham controls (Student's t-test: p=0.025), indicating a less focused search pattern. Further, sham-operated controls spent more time searching in the quadrant in which the platform was located (one-way repeated measures ANOVA: F(3,30)=7.46, p<0.001), whereas the 1.5 atm FPI animals spent an equivalent amount of time exploring three of the four quadrants (one-way repeated measures ANOVA: F(3,30)=2.70, p=0.063; Fig. 4D). No differences in swim speed (sham, 26.91±0.88 cm/sec; 1.5 atm FPI, 28.04±1.10 cm/sec; Student's t-test: p=0.435) or latency to a visible platform (sham, 5.79±0.82 sec; 1.5 atm FPI, 8.77±1.54 sec; Student's t-test: p=0.103) was observed between the two groups.
FIG. 4.
FPI of 1.5 atm caused significant short-term memory deficits. (A) Representative traces of swimming paths taken by a sham and a 1.5 atm FPI rat in a probe trial given 30 min after training. Injured rats performed poorly in the short-term memory tasks as evidence by (B) longer latencies to first platform crossing, (C) longer path lengths to first platform crossing, (D) fewer number of platform crossings during the 60 sec probe trial, and (E) a lack of preference for searching in the quadrant in which the platform was located. Data are presented as the mean±SEM; *p<0.05.
1.5 atm FPI transiently altered cerebral perfusion
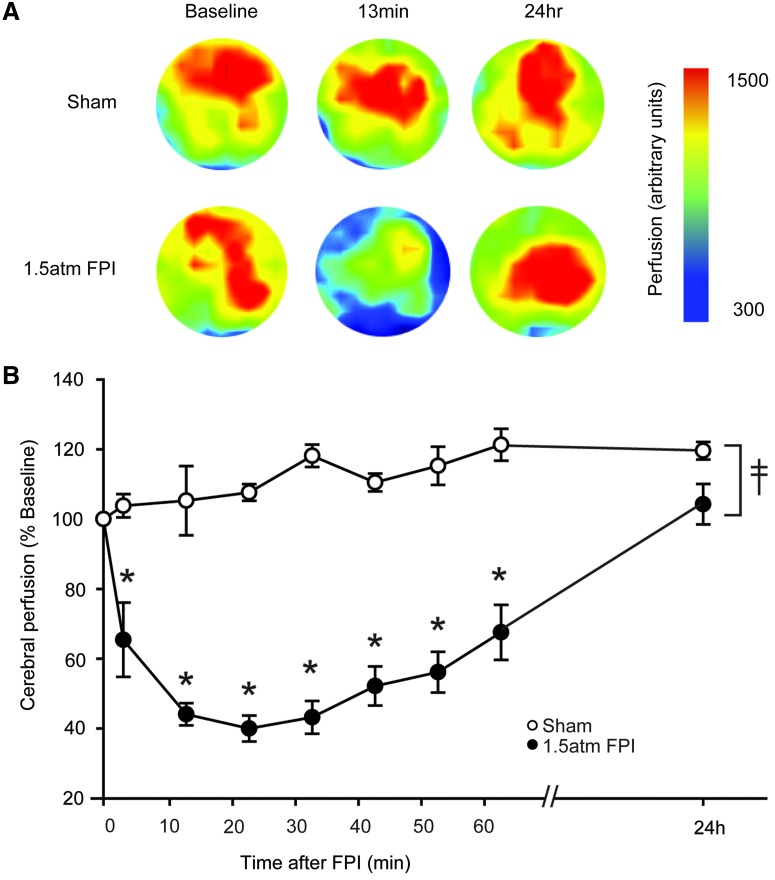
To examine the consequences of 1.5 atm FPI on cerebral perfusion, a Periscan PIM3 Laser Doppler Blood Perfusion Imager was used as a noninvasive means of monitoring microvasculature blood perfusion. Due to the low intensity of the laser and its inability to penetrate the skull, imaging was done through the craniotomy prepared for hub implantation. Sham animals received the crainiectomy in order to allow cerebral imaging to be performed. Scans were performed prior to injury (baseline), 3 min post-injury and every 10 min thereafter for the first hour after injury. Figure 5A shows representative color-coded images from a sham and a 1.5 atm FPI animal. An apparent decrease in cerebral blood perfusion can be observed 13 min after injury that returns to baseline by 24 h post-injury. The summary data in Figure 5B show that, compared to sham-operated controls (n=4), cerebral perfusion significantly decreases in the 1.5 atm FPI animals (n=6) (two-way repeated measures ANOVA: F(8,57)=14.337, p<0.001). The decrease could be detected as early as 3 min after injury, and persisted throughout the 1 h monitoring period. When examined the following day, cerebral perfusion had returned to baseline values.
FIG. 5.
FPI of 1.5 atm caused transient disturbances in cerebral perfusion. Cerebral perfusion at the site of injury was monitored using a Perimed PIM3 Laser Doppler Blood Perfusion Imager. (A) Representative colorized images indicating cerebral perfusion over time from a sham and a 1.5 atm FPI rat. (B) Injury (n=6) caused a significant decrease in cerebral perfusion, as indicated by lower perfusion values than detected in shams (n=4), throughout the 1 h contiguous monitoring period. Cerebral perfusion returned to baseline 24 h after injury. Color image is available online at www.liebertpub.com/neu
1.5 atm FPI did not cause overt cell loss or dendritic damage
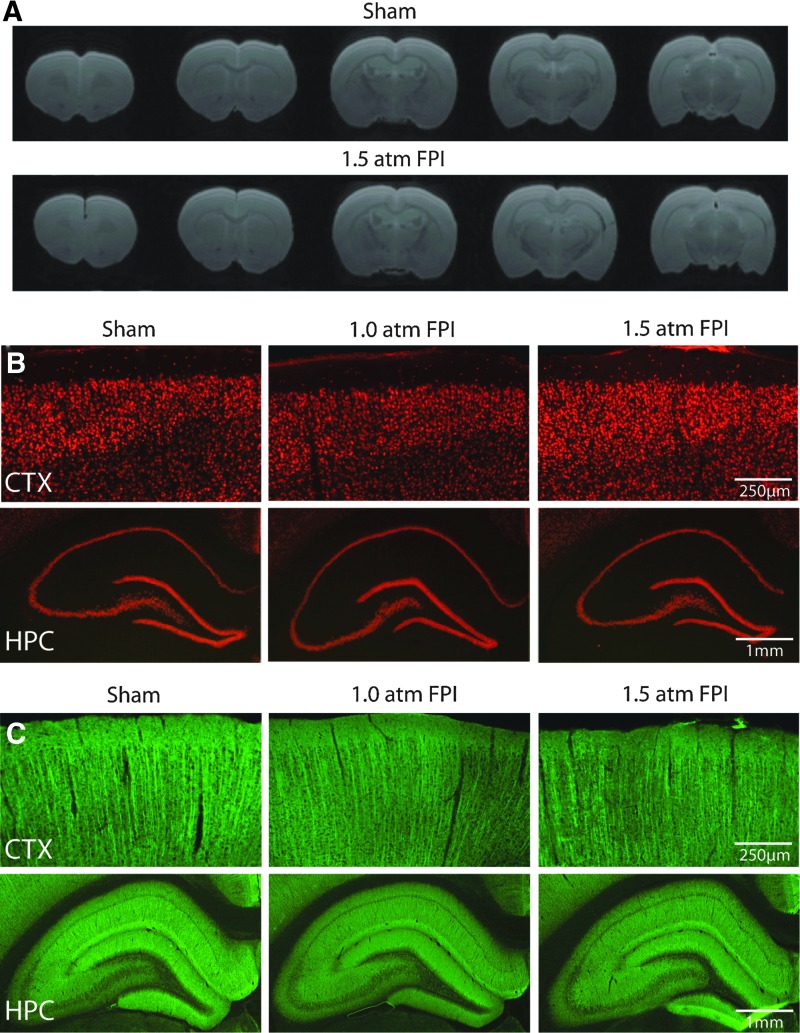
Patients with uncomplicated mild TBI are typically negative for findings on computerized tomography (CT) or MRI scans. To examine if histopathological changes associated with 1.5 atm FPI can be detected using imaging, the animals used for behavioral studies were transcardially perfused on day 15 post-injury with 4% paraformaldehyde and brains removed. Ex vivo Rapid Acquisition with Refocused Echoes (RARE) scans throughout the rostro-caudal extent of the cerebral cortex were obtained from sham and 1.5 atm FPI animals. No overt damage to the cerebral cortex, hemorrhage, or midline shifts was observed in 1.5 atm FPI rats compared to sham-operated controls (Fig. 6A). Examination of NeuN immunostained tissue confirmed that neither 1.0 atm nor 1.5 atm FPI caused changes in cortical or hippocampal neuron density or distribution (Fig. 6B). Likewise, dendritic integrity appeared to be unaffected by the injury, with no reproducible loss or reductions in microtubule-associated protein 2 (MAP2) immunoreactivity observed at the site of injury nor in the ipsilateral hippocampus (Fig. 6C), areas known to suffer dendritic loss following more severe forms of injury.25,26
FIG. 6.
FPI of 1.5 atm did not cause overt cell loss or dendritic damage. (A) Representative RARE images in the coronal plane from a sham and a 1.5 atm FPI rat. Representative photomicrographs of tissue sections collected 15 days post-injury from a sham, a 1.0 atm FPI, and a 1.5 atm FPI rat showing (B) NeuN and (C) microtubule-associated protein 2 (MAP-2) immunoreactivity from the ipsilateral cortex (CTX) and hippocampus (HPC). Color image is available online at www.liebertpub.com/neu
1.5 atm FPI increases indices of axonal damage
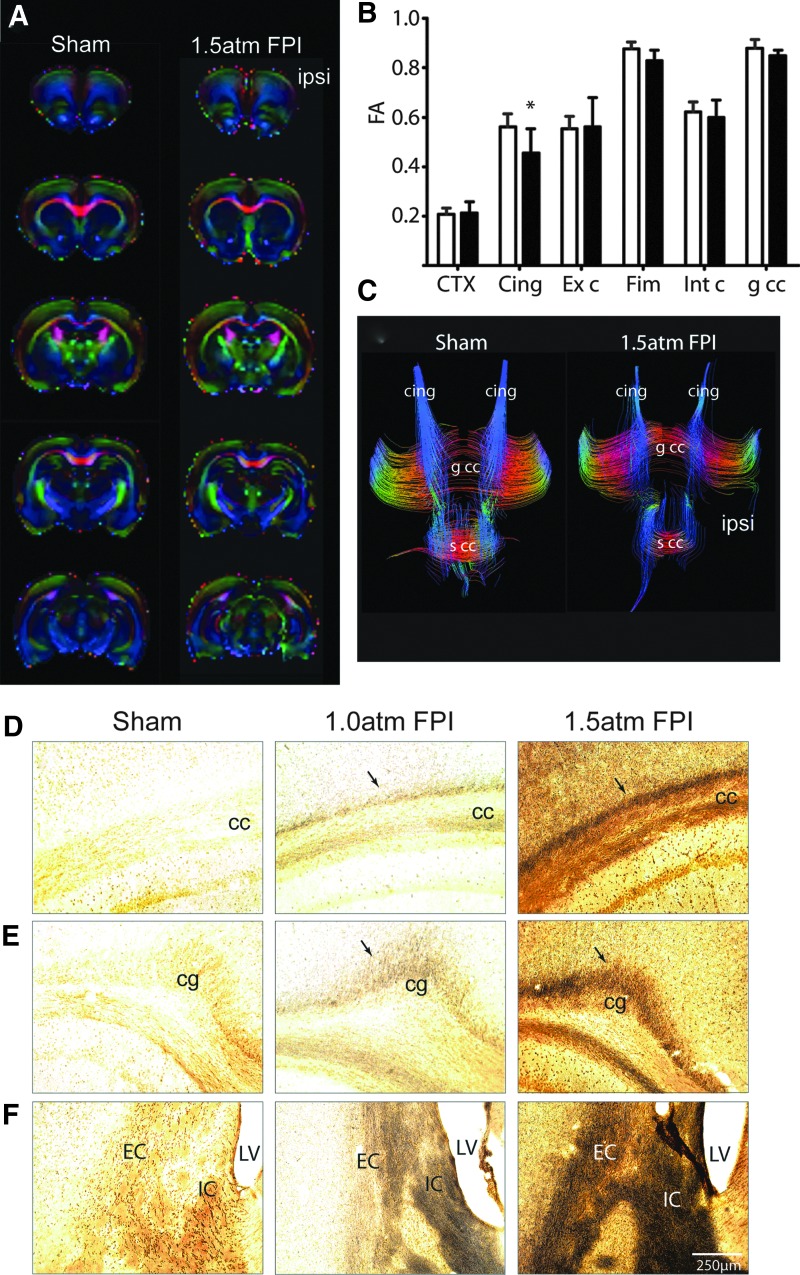
Recent imaging studies are beginning to show that mild TBI is associated with abnormal findings detected using Diffusion Tensor Imaging (DTI).27,28 To examine if 1.5 atm FPI causes axonal disturbances that can be detected using DTI, brains from sham (n=6) and 1.5 atm FPI (n=6) were used for ex vivo imaging. Representative colorized FA maps from a sham and an injured animal are shown in Figure 7A. ROI analysis revealed that 1.5 atm FPI caused a significant decrease in FA values in the cingulum (Fig. 7B). The representative tract tracings shown in Figure 7C demonstrate a reduction of the fibers with FA values over a threshold in the ipsilateral cingulum of the 1.5 atm FPI rat. When tissue sections from sham and FPI animals were used for silver staining, dramatic increases in silver impregnation were observed in the corpus callosum (Fig. 7D), the cingulum (cg; Fig. 7E), and the internal (IC) and external (EC) capsule (Fig. 7F) of 1.5 atm FPI rats. Although to a lesser extent, enhanced silver impregnation was also observed in these areas when silver staining was carried out using tissue sections from 1.0 atm rats (Fig. 7D–F).
FIG. 7.
Mild FPI increased markers of axonal damage. (A) Representative colorized FA maps in the coronal plane from a sham and a 1.5 atm FPI rat. (B) Summary data showing the quantification of FA values from defined ROIs corresponding to the cortex (CTX) immediately beneath the injury site, the cingulum (Cing), the external capsule (Ext c), the fimbria (Fim), the internal capsule (Int c), and the genu of the corpus callosum (g cc) from sham and 1.5 atm rats (n=6/group). (C) Representative images of tract tracings from a sham and a 1.5 atm rat, demonstrating the fibers passing through the area of the cingulum. Representative photomicrographs of silver-stained tissue sections collected 15 days post-injury from a sham, a 1.0 atm FPI, and a 1.5 atm FPI rat. 1.0 atm FPI, and to a greater degree 1.5 atm FPI, caused enhanced silver impregnation in fibers of (D) the cc, (E) the cingulum (cg), and (F) the internal (IC) and external (EC) capsule. LV, lateral ventricle. Color image is available online at www.liebertpub.com/neu
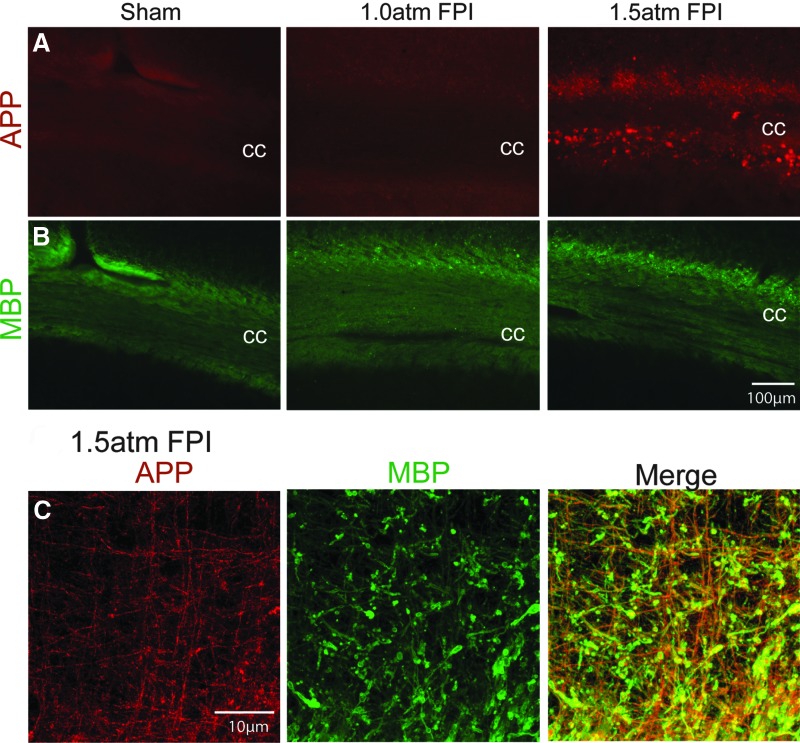
Further supporting a conclusion of axonal damage, we detected an increase in amyloid precursor protein (APP) immunoreactivity in the corpus callosum (cc) ipsilateral to the injury of 1.5 atm FPI animals (Fig. 8A). As it had been previously reported that unmyelinated axons in the corpus callosum are more vulnerable to TBI,29 we examined if the increased APP immunoreactivity we observed was localized to myelinated (Fig. 8B) or nonmyelinated axons using double-label immunohistochemistry. In contrast to our expectations, the representative confocal pictures shown in Figure 8C demonstrate that APP immunoreactivity (red) was predominantly located in MBP-positive (green) myelinated axons.
FIG. 8.
Mild FPI enhanced APP immunoreactivity. (A) 1.5 atm FPI, but not 1.0 atm FPI, increased amyloid precursor protein (APP) immunoreactivity in the ipsilateral corpus callosum (cc), but did not appear to (B) reduce myelin basic protein (MBP) immunostaining. (C) Representative confocal images showing that the enhanced APP immunoreactivity (red) observed in 1.5 atm FPI rats co-localizes with MBP (green)-positive myelinated fibers. Areas of co-localization appear yellow in the merged image. Color image is available online at www.liebertpub.com/neu
1.5 atm FPI increased markers of inflammation
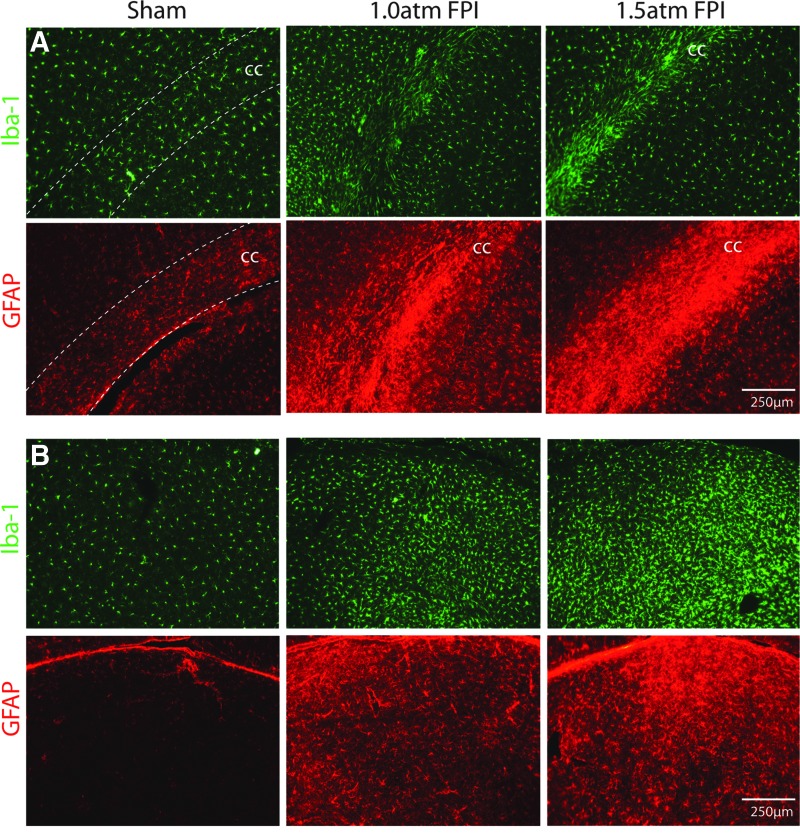
To examine inflammation after mild FPI, we investigated if enhanced Iba-1 (a marker of microglia) and GFAP (a marker of astrocytes) immunoreactivity, could be observed at 15 days after the injury. Figure 9A shows that enhanced Iba1 and GFAP immunoreactivity can be seen in the corpus callosum of both 1.0 atm FPI and 1.5 atm FPI rats compared to that seen in sham-operated controls. Similarly, a robust increase in Iba-1 and GFAP can be seen in the thalamus (Fig. 9B). These observations suggest that mild FPI caused an inflammatory response that persisted for at least 2 weeks after injury.
FIG. 9.
FPI of 1.5 atm increased Iba-1 and GFAP immunoreactivity. Representative photomicrographs of tissue sections taken 15 days after sham surgery, 1.0 atm FPI or 1.5 atm FPI are shown. (A) Images of the ipsilateral corpus callosum (cc) showing that 1.5 atm FPI increased Iba-1 (a marker of microglia) and GFAP (a marker of astrocytes) immunoreactivity, suggesting ongoing microglial and astrocytic activation, respectively. (B) Similar increases in Iba-1 and GFAP immunoreactivity were observed in the thalamus, suggesting a prolonged period of neuroinflammation. Color image is available online at www.liebertpub.com/neu
Discussion
In the present study, we employed 1.0 atm and 1.5 atm FPI levels to investigate the neurobehavioral and histopathological changes triggered by mTBI. Our results show the following five key findings: 1) Neither 1.0 atm nor 1.5 atm mFPI caused visible contusion injury to the brain; 2) Neither injury caused visible hippocampal or neocortical neuronal loss, a finding consistent with previous reports; 3) 1.5 atm, but not 1.0 atm, FPI caused transient motor and significant learning and short-term memory impairments; 4) Both 1.0 atm and 1.5 atm FPI caused a local inflammatory response in the corpus callosum and the thalamus that persisted for at least 2 weeks after the injury; and 5) Both 1.0 atm and 1.5 atm FPI caused axonal damage in the corpus callosum, and internal and external capsules, findings consisted with clinical findings.
One of the defining features of mTBI is the temporary loss or alteration of consciousness. While consciousness is difficult to assess in rodents, investigators have employed the duration of suppression of righting response as a rodent correlate for the period of loss of consciousness. Using this measure, we found that 1.0 atm and 1.5 atm FPI injury resulted in durations of suppression of righting responses of 2.8 min and 5.8 min over sham, respectively. This period of suppression is consistent with a classification of mild from other studies.13,16 In addition to loss of consciousness, some mTBI patients display problems with balance and motor function in the subacute phase of injury. Although neither 1 atm FPI nor 1.5 atm FPI caused vestibulomotor deficits, 1.5 atm FPI caused temporary motor deficits on both the ipsilateral and contralateral sides. Although our lateral FPI was centered on the right parietal cortex, motor deficits on both sides are consistent with the reported diffuse nature of injury caused by FPI. These deficits were related to visually coordinated behavior, as indicated by increased foot fault errors, but not gross locomotion as both injured and sham animals performed equally well on a rotarod task.
Previous studies in rats, cats, and pigs documented that FPI caused alterations of regional blood flow.31–33 For example, Ginsberg et al.32 reported that in neocortical regions ipsilateral to the trauma, blood flow was depressed by 44% in moderately-injured FPI rats as compared to that measured in sham-operated controls. Using a scanning laser-doppler device, we observed that mild FPI also results in a significant reduction in ipsilateral cerebral perfusion within minutes of injury that lasted throughout the 1 h monitoring period, returning to normal values by 24 h post-injury. At present, it is not clear if this suppression was due to impaired cerebral autoregulation or systemic physiological changes such as decreased arterial blood pressure, changes in blood carbon dioxide or oxygen levels, or decreased heart or respiratory rates. Previous studies that have monitored the systemic response to FPI have not observed significant influences on cardiovascular parameters. For example, Yamakami and McIntosh34 observed that regional cerebral blood flow after moderate FPI is reduced and occurs in the absence of changes in mean arterial blood pressure, heart rate, or blood gas levels. Similarly, DeWitt and colleagues observed significant reductions in both global and regional cerebral blood flow, but not in other cardiovascular parameters or blood chemistry, after moderate FPI.35,36 Although these physiological measures were not carried out in the present study, these findings suggest that local vascular changes may underlie the decreased cerebral perfusion we observed. While the consequences of this reduced cerebral perfusion is not known at present, it has been suggested that reduced blood flow may make the injured brain more vulnerable to a subsequent mild TBI or ischemic insult. Consistent with this, Robertson and colleagues reported that hypotension, when initiated soon after cortical impact injury, results in marked brain damage and contusion.37
The temporal lobe, especially the hippocampus, is vulnerable to TBI. Consistent with this, mTBI patients often exhibit short-term declarative memory deficits (e.g., recall of a list of words after a short delay) that can be observed for days after the injury, and in some cases much longer. In order to test FPI animals in a short-term task analogous to that used in humans, we employed an abbreviated water maze task to test hippocampal-dependent spatial learning and short-term memory recall. In this task, animals were given a single training session (consisting of 10 trials) on day 5 post-injury, followed by a probe trial 30 min later. Although we report changes in molecular pathways including inflammatory pathways after 1 atm FPI <(Redell et al., this issue)>, we did not observe significant learning or memory deficits in these animals when tested 5 days post injury. This observation, however, does not preclude the possibility that cognitive deficits would be observed if testing were carried out at an earlier time point or using a more sensitive task. For example, Shultz et al. reported that 1.2 atm FPI rats have a significant impairment in reversal learning in the water maze task16 when tested 24 h after injury. Consistent with this, we have observed that 1.2 atm FPI animals have significant learning deficits when trained in the abbreviated Morris water maze task (F(1,18)=8.47, p=0.009) on day 3 post-injury (Supplementary Fig. S1; supplementary data are available online at www.liebertpub.com/neu). These animals, when tested for short-term memory 30 min after training, were also dysfunctional in their ability to locate the hidden platform as indicated by longer latencies to first platform crossing and a lack of strong preference for the target quadrant. While testing at these early time points may be suitable for evaluating drugs that only require administration for a period of 1–2 days, for pharmacological agents that require longer than 2 days for effectiveness (e.g., lithium), testing animals at these time points may not be optimal.38
In contrast to that seen at 1.0 atm FPI, 1.5 atm FPI caused significant learning and short-term memory deficits when animals were tested in the abbreviated Morris water maze task on day 5 post-injury. This observation raises the possibility that this injury may result in hippocampal neuron loss and/or neuronal dysfunction. In agreement with a previous report by Eakin and Miller,13 we did not observe visible hippocampal cell loss after 1.5 atm FPI. These investigators, however, did observe that injured animals had a reduction in the number of neurons having spatiotemporal activity, suggesting that deficits in spatial learning and memory as a result of 1.5 atm FPI may result from altered place cell firing rather than loss of hippocampal neurons. As one of our goals was to evaluate the consequences of mild FPI using testing procedures reflective of those typically performed in humans, we did not test memory recall with a longer delay.
Concussions are not typically associated with gross gray matter damage,39,40 but have recently been shown to cause diffused axonal injury as detected by DTI. For example, mild TBI patients have reduced FA values in both the genu and splenium of the corpus callosum, the internal and external capsule, the left superior cerebellar peduncle, the cingulum, the hippocampus, the thalamus, the dorsolateral prefrontal cortex, and other brain regions.5,27,41–47 Interestingly, depending on the time points examined, either increases or decreases in FA values have been observed, a finding thought to be related to the development of axonal swelling that occurs early after injury.48 Quantification of FA values showed a significant decrease in the cingulum of 1.5 atm FP brains 15 days post-injury. However, differences in FA values did not reach significance in other regions of interest (Fig. 8). Using more sensitive histopathological analysis, we observed enhanced APP immunoreactivity in the corpus callosum of 1.5 atm FPI animals, suggesting axonal injury. This was further supported by the detection of silver-impregnated axons in this fiber bundle, as well as in the internal and external capsule, the cingulum, and the thalamus. Previous ultrastructural analysis and electrophysiological recordings have suggested that unmyeliniated axons are more vulnerable to TBI.29,49 However, Creed et al.50 reported a reduction of compound action potential (CAP) amplitude after TBI that was restricted to myelinated axons. Consistent with this later study, our double immunostaining showed that the enhanced APP immunoreactivity seen in the corpus callosum after 1.5 atm FPI occurred predominantly in myelinated axons. At present, it is unclear why the more severe form of FPI used by Reeves resulted in unmyelinated axon injury, whereas the lower magnitude of FPI used in this study damaged myelinated axons.
One of the key findings in the present study is that mild FPI, both 1.0 atm and 1.5 atm, resulted in a dramatic increase in Iba-1 and GFAP immunoreactivities within the corpus callosum and the thalamus, suggesting persistent inflammation. This is consistent with our genomic studies <(Redell et al., this issue)> that found that mild FPI increases the message and protein levels of proinflammatory cytokines and chemokines in the injured cortex. The increases in Iba-1 and GFAP we observed were protracted in nature, lasting for over 2 weeks after mild FPI. In monkeys, increases in microglial activation have been observed for up to a year after injury.51 Recently, an imaging study using the positron emission tomography (PET) ligand (R)PK11195 has reported activated microglia in TBI patients as long as 17 years after moderate-severe injury.52 Although the consequences of microglial activation in the chronically injured brain is not known, it is possible that the duration of this inflammatory response may increase the vulnerability of the brain to a second injury or ischemic event. Future studies will be required to determine the duration of microglial and astrocytic activation after mild FPI, and to determine if these changes represent ongoing developing pathology, or are a component of the repair process.
In summary, we found that both 1.0 atm and 1.5 atm FPI injury caused transient suppression of acute neurological functions that occur in the absence of visible contusion to the cerebral cortex. Further, we show that 1.5 atm injury led to transient motor disturbances, and significant impairments in spatial learning and short-term memory. Consistent with previous studies, 1.5 atm FPI does not cause visible neuronal loss in the hippocampus or in the neocortex, but gives rise to a robust inflammatory response. Measures of axonal injury (e.g., DTI, APP immunoreactivity, and silver staining) identified several areas with axonal disturbances, including the cingulum, the corpus callosum, and the internal and external capsules. These findings are consistent with the deficits and pathologies associated with mild TBI in humans, and support the use of mild FPI as a model to evaluate putative therapeutic options.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Bruce Lyeth of the University of California, Davis for kindly providing the hubs used to mount the rats to the FPI device. We also thank the Imaging Core of the Mission Connect Mild TBI Translational Consortium under the direction of Dr. Ponada Narayana for their assistance with the MRI. The work performed in the authors' laboratories were made possible by grants from DOD (W81XWH-08-2-0134) and TIRR Foundation.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alexander MP. Mild traumatic brain injury: Pathophysiology, natural history, and clinical management. Neurology. 1995;45:1253–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 2.Binder LM. A review of mild head trauma. Part II: Clinical implications. J Clin Exp Neuropsychol. 1997;19:432–457. doi: 10.1080/01688639708403871. [DOI] [PubMed] [Google Scholar]
- 3.d'Hemecourt P. Subacute symptoms of sports-related concussion: Outpatient management and return to play. Clin Sports Med. 2011;30:63–72. doi: 10.1016/j.csm.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 4.Ruff RM. Camenzuli L. Mueller J. Miserable minority: Emotional risk factors that influence the outcome of a mild traumatic brain injury. Brain Inj. 1996;10:551–565. doi: 10.1080/026990596124124. [DOI] [PubMed] [Google Scholar]
- 5.Mac Donald CL. Johnson AM. Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364:2091–2100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharp DJ. Ham TE. Investigating white matter injury after mild traumatic brain injury. Curr Opin Neurol. 2011;24:558–563. doi: 10.1097/WCO.0b013e32834cd523. [DOI] [PubMed] [Google Scholar]
- 7.Wilde EA. Ramos MA. Yallampalli R, et al. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev Neuropsychol. 2010;35:333–351. doi: 10.1080/87565641003696940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamm RJ. Dixon CE. Gbadebo DM, et al. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J Neurotrauma. 1992;9:11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 9.Kochanek PM. Clark RS. Ruppel RA, et al. Biochemical, cellular, and molecular mechanisms in the evolution of secondary damage after severe traumatic brain injury in infants and children: Lessons learned from the bedside. Pediatr Crit Care Med. 2000;1:4–19. doi: 10.1097/00130478-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 10.McIntosh TK. Neurochemical sequelae of traumatic brain injury: Therapeutic implications. Cerebrovasc Brain Metab Rev. 1994;6:109–162. [PubMed] [Google Scholar]
- 11.Povlishock JT. Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: A review of current thoughts. J Neurotrauma. 1995;12:555–564. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- 12.Abdel Baki SG. Kao HY. Kelemen E. Fenton AA. Bergold PJ. A hierarchy of neurobehavioral tasks discriminates between mild and moderate brain injury in rats. Brain Res. 2009;1280:98–106. doi: 10.1016/j.brainres.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 13.Eakin K. Miller JP. Mild traumatic brain injury is associated with impaired hippocampal spatiotemporal representation in the absence of histological changes. J Neurotrauma. 2012;29:1180–1187. doi: 10.1089/neu.2011.2192. [DOI] [PubMed] [Google Scholar]
- 14.Gurkoff GG. Giza CC. Hovda DA. Lateral fluid percussion injury in the developing rat causes an acute, mild behavioral dysfunction in the absence of significant cell death. Brain Res. 2006;1077:24–36. doi: 10.1016/j.brainres.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 15.Henninger N. Dutzmann S. Sicard KM. Kollmar R. Bardutzky J. Schwab S. Impaired spatial learning in a novel rat model of mild cerebral concussion injury. Exp Neurol. 2005;195:447–457. doi: 10.1016/j.expneurol.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 16.Shultz SR. MacFabe DF. Foley KA. Taylor R. Cain DP. A single mild fluid percussion injury induces short-term behavioral and neuropathological changes in the Long-Evans rat: Support for an animal model of concussion. Behav Brain Res. 2011;224:326–335. doi: 10.1016/j.bbr.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Wu A. Ying Z. Gomez-Pinilla F. Vitamin E protects against oxidative damage and learning disability after mild traumatic brain injury in rats. Neurorehabil Neural Repair. 2010;24:290–298. doi: 10.1177/1545968309348318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dixon CE. Lyeth BG. Povlishock JT, et al. A fluid percussion model of experimental brain injury in the rat. J Neurosurg. 1987;67:110–119. doi: 10.3171/jns.1987.67.1.0110. [DOI] [PubMed] [Google Scholar]
- 19.Floyd CL. Golden KM. Black RT. Hamm RJ. Lyeth BG. Craniectomy position affects Morris water maze performance and hippocampal cell loss after parasagittal fluid percussion. J Neurotrauma. 2002;19:303–316. doi: 10.1089/089771502753594873. [DOI] [PubMed] [Google Scholar]
- 20.Kelley BJ. Farkas O. Lifshitz J. Povlishock JT. Traumatic axonal injury in the perisomatic domain triggers ultrarapid secondary axotomy and Wallerian degeneration. Exp Neurol. 2006;198:350–360. doi: 10.1016/j.expneurol.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 21.Dash PK. Orsi SA. Zhang M, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS One. 2010;5:e11383. doi: 10.1371/journal.pone.0011383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamm RJ. Pike BR. O'Dell DM. Lyeth BG. Jenkins LW. The rotarod test: An evaluation of its effectiveness in assessing motor deficits following traumatic brain injury. J Neurotrauma. 1994;11:187–196. doi: 10.1089/neu.1994.11.187. [DOI] [PubMed] [Google Scholar]
- 23.Blum S. Moore AN. Adams F. Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzowski JF. McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colicos MA. Dixon CE. Dash PK. Delayed, selective neuronal death following experimental cortical impact injury in rats: Possible role in memory deficits. Brain Res. 1996;739:111–119. doi: 10.1016/s0006-8993(96)00819-0. [DOI] [PubMed] [Google Scholar]
- 26.Dash PK. Mach SA. Moore AN. The role of extracellular signal-regulated kinase in cognitive and motor deficits following experimental traumatic brain injury. Neuroscience. 2002;114:755–767. doi: 10.1016/s0306-4522(02)00277-4. [DOI] [PubMed] [Google Scholar]
- 27.Arfanakis K. Haughton VM. Carew JD. Rogers BP. Dempsey RJ. Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 28.Shenton ME. Hamoda HM. Schneiderman JS, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reeves TM. Phillips LL. Povlishock JT. Myelinated and unmyelinated axons of the corpus callosum differ in vulnerability and functional recovery following traumatic brain injury. Exp Neurol. 2005;196:126–137. doi: 10.1016/j.expneurol.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 30.Armstead WM. Kiessling JW. Kofke WA. Vavilala MS. Impaired cerebral blood flow autoregulation during posttraumatic arterial hypotension after fluid percussion brain injury is prevented by phenylephrine in female but exacerbated in male piglets by extracellular signal-related kinase mitogen-activated protein kinase upregulation. Crit Care Med. 2010;38:1868–1874. doi: 10.1097/CCM.0b013e3181e8ac1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bedell EA. DeWitt DS. Prough DS. Fentanyl infusion preserves cerebral blood flow during decreased arterial blood pressure after traumatic brain injury in cats. J Neurotrauma. 1998;15:985–992. doi: 10.1089/neu.1998.15.985. [DOI] [PubMed] [Google Scholar]
- 32.Ginsberg MD. Zhao W. Alonso OF. Loor-Estades JY. Dietrich WD. Busto R. Uncoupling of local cerebral glucose metabolism and blood flow after acute fluid-percussion injury in rats. Am J Physiol. 1997;272:H2859–H2868. doi: 10.1152/ajpheart.1997.272.6.H2859. [DOI] [PubMed] [Google Scholar]
- 33.Long JB. Gordon J. Bettencourt JA. Bolt SL. Laser-Doppler flowmetry measurements of subcortical blood flow changes after fluid percussion brain injury in rats. J Neurotrauma. 1996;13:149–162. doi: 10.1089/neu.1996.13.149. [DOI] [PubMed] [Google Scholar]
- 34.Yamakami I. McIntosh TK. Effects of traumatic brain injury on regional cerebral blood flow in rats as measured with radiolabeled microspheres. J Cereb Blood Flow Metab. 1989;9:117–124. doi: 10.1038/jcbfm.1989.16. [DOI] [PubMed] [Google Scholar]
- 35.Yuan XQ. Prough DS. Smith TL. Dewitt DS. The effects of traumatic brain injury on regional cerebral blood flow in rats. J Neurotrauma. 1988;5:289–301. doi: 10.1089/neu.1988.5.289. [DOI] [PubMed] [Google Scholar]
- 36.DeWitt DS. Smith TG. Deyo DJ. Miller KR. Uchida T. Prough DS. L-arginine and superoxide dismutase prevent or reverse cerebral hypoperfusion after fluid-percussion traumatic brain injury. J Neurotrauma. 1997;14:223–233. doi: 10.1089/neu.1997.14.223. [DOI] [PubMed] [Google Scholar]
- 37.Robertson CS. Cherian L. Shah M, et al. Neuroprotection with an erythropoietin mimetic peptide (pHBSP) in a model of mild traumatic brain injury complicated by hemorrhagic shock. J Neurotrauma. 2012;29:1156–1166. doi: 10.1089/neu.2011.1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dash PK. Johnson D. Clark J, et al. Involvement of the glycogen synthase kinase-3 signaling pathway in TBI pathology and neurocognitive outcome. PLoS One. 2011;6:e24648. doi: 10.1371/journal.pone.0024648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraus MF. Susmaras T. Caughlin BP. Walker CJ. Sweeney JA. Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 40.Sterr A. Herron KA. Hayward C. Montaldi D. Are mild head injuries as mild as we think? Neurobehavioral concomitants of chronic post-concussion syndrome. BMC Neurol. 2006;6:7. doi: 10.1186/1471-2377-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Inglese M. Makani S. Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 42.Lipton ML. Gulko E. Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- 43.Maruta J. Suh M. Niogi SN. Mukherjee P. Ghajar J. Visual tracking synchronization as a metric for concussion screening. J Head Trauma Rehabil. 2010;25:293–305. doi: 10.1097/HTR.0b013e3181e67936. [DOI] [PubMed] [Google Scholar]
- 44.Rutgers DR. Fillard P. Paradot G. Tadie M. Lasjaunias P. Ducreux D. Diffusion tensor imaging characteristics of the corpus callosum in mild, moderate, and severe traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:1730–1735. doi: 10.3174/ajnr.A1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh M. Jeong J. Hwang D. Sungkarat W. Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn Reson Imaging. 2010;28:22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wilde EA. McCauley SR. Barnes A, et al. Serial measurement of memory and diffusion tensor imaging changes within the first week following uncomplicated mild traumatic brain injury. Brain Imaging Behav. 2012;6:319–328. doi: 10.1007/s11682-012-9174-3. [DOI] [PubMed] [Google Scholar]
- 47.Wilde EA. McCauley SR. Hunter JV, et al. Diffusion tensor imaging of acute mild traumatic brain injury in adolescents. Neurology. 2008;70:948–955. doi: 10.1212/01.wnl.0000305961.68029.54. [DOI] [PubMed] [Google Scholar]
- 48.Bazarian JJ. Zhong J. Blyth B. Zhu T. Kavcic V. Peterson D. Diffusion tensor imaging detects clinically important axonal damage after mild traumatic brain injury: A pilot study. J Neurotrauma. 2007;24:1447–1459. doi: 10.1089/neu.2007.0241. [DOI] [PubMed] [Google Scholar]
- 49.Reeves TM. Phillips LL. Lee NN. Povlishock JT. Preferential neuroprotective effect of tacrolimus (FK506) on unmyelinated axons following traumatic brain injury. Brain Res. 2007;1154:225–236. doi: 10.1016/j.brainres.2007.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Creed JA. DiLeonardi AM. Fox DP. Tessler AR. Raghupathi R. Concussive brain trauma in the mouse results in acute cognitive deficits and sustained impairment of axonal function. J Neurotrauma. 2011;28:547–563. doi: 10.1089/neu.2010.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nagamoto-Combs K. McNeal DW. Morecraft RJ. Combs CK. Prolonged microgliosis in the rhesus monkey central nervous system after traumatic brain injury. J Neurotrauma. 2007;24:1719–1742. doi: 10.1089/neu.2007.0377. [DOI] [PubMed] [Google Scholar]
- 52.Ramlackhansingh AF. Brooks DJ. Greenwood RJ, et al. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.