Abstract
INTRODUCTION
Treatment of primary prostate cancer (CaP) is the withdrawal of androgens. However, CaP eventually progresses to grow in a castration-resistant state due to aberrant activation of androgen receptor (AR). Understanding the mechanisms leading to the aberrant activation of AR is critical to develop effective therapy. We have previously identified Rho GDP Dissociation Inhibitor alpha (GDIα) as a novel suppressor in prostate cancer. In this study, we examine the effect of GDIα on AR signaling in prostate cancer cells.
METHODS
GDIα was transiently or stably transfected into several prostate cancer cell lines including LNCaP, C4-2, CWR22Rv1, and DU145. The regulation of AR expression by GDIα was analyzed by qRT-PCR and Western blot. AR activity was measured by luciferase reporter assays and electrophoretic mobility shift analysis (EMSA). Immunofluorescence assay was performed to study AR nuclear translocation. The interaction between GDIα and AR was examined by co-immunoprecipitation assays.
RESULTS
In this study, we have identified GDIα as a negative regulator of AR signaling pathway. Overexpression of GDIα downregulates AR expression at both mRNA and protein levels. Overexpression of GDIα is able to prevent AR nuclear translocation and inhibit trans-activation of AR target genes. Co-immunoprecipitation assays showed that GDIα physically interacts with the N-terminal domain of AR.
CONCLUSIONS
GDIα suppresses AR signaling through inhibition of AR expression, nuclear translocation, and recruitment to androgen-responsive genes. GDIα regulatory pathway may play a critical role in regulating AR signaling and prostate cancer growth and progression.
Keywords: prostate cancer, RhoGDIα, AR
INTRODUCTION
Prostate cancer is the most diagnosed cancer among men in the United States. A very large portion of men with prostate cancer are treated successfully with androgen deprivation therapy (ADT). However, eventually most prostate cancer patients will relapse due to the progression of castration-resistant prostate cancer (CRPC) [1]. Understanding the molecular mechanisms leading to CRPC is critical to develop successful therapies to combat this lethal response [1–3]. Although circulating androgens remain very low or undetectable in the castrate environment in CRPC, androgen receptor (AR) is expressed at high levels and AR-regulated genes such as prostate-specific antigen (PSA) are also expressed [4], indicating AR signaling pathway is aberrantly activated in CRPC [5].
Rho GDP dissociation inhibitor (RhoGDI) is a cellular regulatory protein that acts primarily by controlling the cellular distribution and activity of Rho GTPases [6]. RhoGDIalpha (GDIα) is one of the GDI family mammalian members, which binds to most of the Rho GTPases including RhoA, Cdc42, and Rac1 [7,8]. GDIα binds to and negatively regulates Rho GTPases by shielding their membrane-anchoring domains and restricting them to a cytosolic non-active localization [9,10]. Overexpression of GDIα in various cell lines induces disruption of the cytoskeleton and inhibits cell motility, while downregulation of GDIα expression is associated with progression of bladder cancer and breast cancer [11–14].
We previously demonstrated that loss of GDIα expression is associated with prostate cancer progression [15]. Overexpression of GDIα inhibits prostate cancer cell growth both in vitro and in vivo [15]. In this study, we examined the effects of GDIα on AR signaling in prostate cancer cells and demonstrated that GDIα interacts with AR and suppresses AR signaling in prostate cancer cells.
MATERIALS AND METHODS
Cell Culture and Reagents
LNCaP, C4-2, and CWR22Rv1 prostate cancer cells were cultured in RPMI-1640 medium containing either 10% complete fetal bovine serum (FBS) or 10% charcoal-dextran-stripped FBS and 100 units/ml penicillin and 0.1 mg/ml streptomycin as described previously [16]. Cells were maintained at 378C in a humidified incubator with 5% CO2. LNCaP passage numbers <30 were used throughout the study. GDIα overexpressing C4-2 cells (C2 and C4) and DU145 cells (DU1B3 and DU3B1) were grown in RPMI 1640 media with 10% FBS containing G418 (300 μg/ml) [17]. For transfection studies, cells were transiently transfected with expression plasmids using Attractene transfection reagent (QIAGEN).
Preparation of Whole Cell Extracts
Cells were harvested, washed with PBS twice, and lysed in high-salt buffer (10 mM HEPES [pH 7.9], 0.25 M NaCl, 0.1% NP-40) supplemented with protease inhibitors (Roche, Basel, Switzerland). Protein concentration was determined with Coomassie Plus protein assay kit (Pierce, Pockford, IL).
Cytosolic and Nuclear Protein Preparation
Cell were harvested, washed with PBS twice, and resuspended in low-salt buffer (10 mM HEPES, 1.5 mM MgCl2, 10 mM KCl, and 0.1% NP40) supplemented with protease inhibitors (Roche) and incubated on ice for 30 min. The cytosolic lysate was cleared by centrifugation at 5 × 103 rpm for 5 min at 4°C. The pellets were collected as nuclear fraction. After washing twice with the low-salt buffer, nuclei were lysed in high-salt lysis buffer followed by mechanical disruption at 4°C for 1 hr. The nuclear fractions were cleared by centrifugation at 10,000 rpm for 15 min at 4°C. Protein concentration was determined using the Coomassie Plus protein assay kit (Pierce).
Western Blot Analysis
Equal amounts of cell protein extracts were loaded on 10 or 12% SDS–PAGE, and proteins were transferred to nitrocellulose membranes. After blocking in 5% non-fat milk in 1× PBS/0.1% Tween-20 at room temperature, membranes were washed with 1× PBS/0.1% Tween-20. The membranes were incubated over-night with primary antibodies at 4°C. Proteins were visualized by enhanced chemiluminescence kit (Millipore) after incubation with the appropriate horseradish peroxidase-conjugated secondary antibodies.
Real-Time PCR Analysis
Total RNA was extracted by TRIzol (Invitrogen, Carlsbad, CA) reagent. One microgram RNA was digested by RQ1 DNase (Promega, Madison, WI). The resulting product was reverse transcribed with random primers by Im-PromII Reverse transcriptase (Promega). The newly synthesized cDNA was used to perform real-time PCR. The reaction mixture contained 4 μl cDNA template and 0.5 μM specific primers for AR and PSA as described previously. b-Actin primer was used as an internal control. The expression levels of AR and PSA were normalized to β-actin. The experiments were repeated three times with triplicates.
Co-Immunoprecipitation
LNCaP cells were lysed with high salt buffer. Equal amounts of whole cell protein extracts (100 μg) were immunoprecipitated by 1 mg anti-GDIα antibody or control anti-IgG antibody in 30 μl A/G agarose with constant rotation overnight. The immunoprecipitants were washed with washing buffer (10 mM HEPES [pH 7.9], 1 mM EDTA, 150 mM NaCl, and 1% NP-40) twice. The immunoprecipitants were then eluted and the eluates were electrophoresed on 8% SDS–PAGE, transferred to nitrocellulose membranes, and probed with anti-AR antibodies.
Luciferase Assays
C4-2 and CWR22Rv1 cells were transfected with pGL3-PSA6.0-Luc and pGL4-AR-prom-Luc reporters, using Attractene (QIAGEN). Cell lysates were subjected to luciferase assays using the Luciferase Assay System (Promega).
Electrophoretic Mobility Shift Analysis (EMSA)
Nuclear extracts from C4-2 and LNCaP cells transfected with vector control or GDIα plasmid were prepared. EMSA were performed by incubating nuclear extracts (5–10 μg) with AR consensus binding motif 5′-GGACAGGGTGTTCT-3′ (Santa Cruz Biotechnology, Santa Cruz, CA) in incubation buffer containing 10 mM HEPES pH 7.9, 80 mM NaCl, 10% glycerol, 1 mM DTT, 1 mM EDTA, 100 μg/ml poly(dI-dC). The protein–DNA complexes were resolved on a 4.5% native polyacrylamide gel at room temperature, and the results were autoradiographed using Storm 840 Imaging system (Molecular Dynamics, Sunnyvale, CA).
RESULTS
Overexpression of GDIα Inhibits AR Transactivation
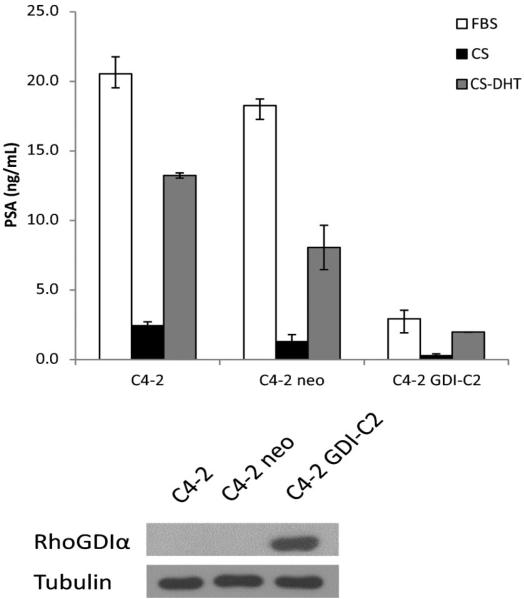
In our previous study, we showed that overexpression of GDIα in LNCaP-IL-6+ cells inhibits tumor growth accompanied by a significant decrease of PSA in the mouse sera bearing tumors, suggesting that GDIα inhibits AR signaling in prostate cancer cells [15]. To further characterize the effect of GDIα on AR signaling, we measured the levels of PSA by ELISA in C4-2 cells overexpressing GDIα compared to their parental cells. As shown in Figure 1, the levels of PSA protein expression in GDIα overexpressing C4-2 cells are significantly lower compared to their parental cells.
Fig. 1.
Overexpression of RhoGDIα downregulates prostate-specific antigen (PSA) protein expression. C4-2, C4-2 neo, and C4-2 GDI-C2 cells overexpressing RhoGDIα were cultured in FBS or charcoal-stripped FBS in the presence or absence of 1 nM DHT. Supernatants were collected and subjected to enzyme-linked immunosorbent assay (ELISA) for PSA proteins. Bottom panel shows immunoblot of whole cell extracts with RhoGDIα and tubulin antibodies.
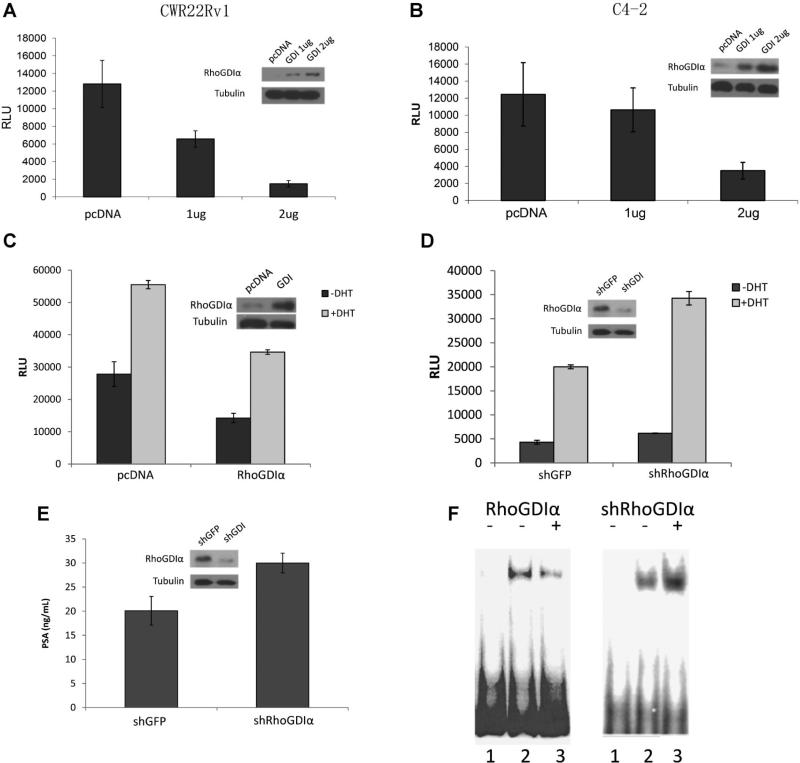
PSA is a typical androgen-regulated gene. To determine if GDIα also alters the expression of other androgen-regulated genes, we used PSA promoter, one of the best characterized androgen-responsive promoters as a model to examine the effects of GDIα on AR signaling. CWR22Rv1 cells and C4-2 cells were cotransfected with PSA-promoter luciferase reporter plasmid and different concentrations of GDIα or vector control. Overexpression of GDIα inhibited transactivation ability of AR in a dose-dependent manner in both CWR22Rv1 and C4-2 cells (Fig. 2A and B). GDIα overexpression also reduced DHT induced PSA promoter activity in C4-2 cells (Fig. 2C). We also knocked down GDIα expression using shRNA in LNCaP cells which express endogenous GDIα to test whether knock down of GDIα increases AR activity in LNCaP cells. LNCaP cells were co-transfected with PSA-luc and shRNA specific for GDIα while GFP shRNA was used as a control. Cells were cultured in the presence or absence of androgen and luciferase activities were determined. PSA luciferase activity was increased in LNCaP cells transfected with shRNA specific for GDIα compared to cells transfected with GFP shRNA control (Fig. 2D). Consistent with PSA-luc activity, knock down of GDIα expression by GDIα shRNA increased PSA protein expression in LNCaP cells (Fig. 2E).
Fig. 2.
Overexpression of RhoGDIα inhibits transactivation activity of the androgen receptor (AR). A,B: RhoGDIα inhibits transcriptional activation of PSA in CWR22Rv1 (A) and C4-2 (B) cells. CWR22Rv1 and C4-2 cells were co-transfected with PSA-6.0-Luc and RhoGDIα plasmids. Luciferase activity was measured. C: RhoGDIα inhibits DHT-induced PSA promoter activity. C4-2 cells were cultured in charcoal-stripped FBS condition and transfected with PSA-6.0-Luc plasmids and RhoGDIα plasmidin thepresence or absence of 1 nMDHT. Luciferase activity was measured. D: Knockdown of RhoGDIα expression increases PSA promoter activity. LNCaP cells were cultured in charcoal-stripped FBS condition and transfectedwith PSA-6.0-Luc plasmids and shRNA against RhoGDIα in thepresence or absence of 1 nM DHT. Luciferase activity was measured. E: Knockdown of RhoGDIα increases PSA protein expression. LNCaP cells were cultured in charcoal-stripped FBS in the presence or absence of 1 nMDHT. Supernatants were used for measurementof protein level of PSA by ELISA.Whole cell extracts were isolated and immunoblotted with RhoGDIα and tubulin antibodies. Inset Western blots show the expression of GDIα incells transfected with either GDIα or shRNA against GDIα. F: RhoGDIα decreases AR-ARE complex formation. EMSA was performed using radiolabeled ARE oligonucleotides with nuclear extracts isolated from C4-2 cells (left) transfected with vector control or RhoGDIα plasmid and LNCaP cells (right) transfected with shGFP or shRNA against RhoGDIα (Left: Lane 1, unbound probe; Lane 2, vector control; Lane 3, RhoGDIα. Right: Lane 1, unbound probe; Lane 2, control; Lane 3, shGDIα).
To determine whether GDIα modulates the binding of AR to the ARE DNA motif, EMSA analysis was performed. Overexpression of GDIα in C4-2 cells decreased the AR/ARE complex (Fig. 2F), whereas downregulation of GDIα in LNCaP cells using specific shRNA, increased the AR/ARE complex (Fig. 2F). These results suggested that GDIα decreased AR activation through inhibiting AR binding to androgen response element. Collectively, these results suggest that GDIα inhibits AR activity and AR-mediated PSA expression in prostate cancer cells.
Overexpression of GDIα Downregulates AR Expression
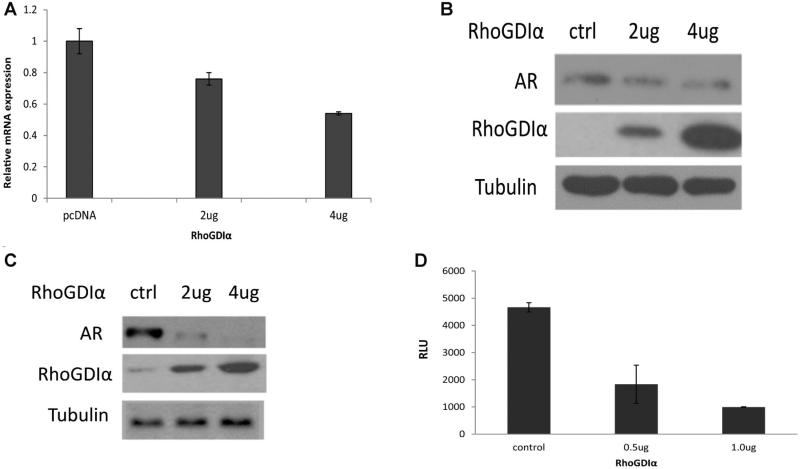
Since PSA is a typical AR-regulated gene, we next examined whether GDIα affects AR expression. GDIα was overexpressed in CWR22Rv1 cells that express low levels of endogenous GDIα protein [15]. The effect of GDIα overexpression on AR expression was analyzed. Total RNAs were isolated and AR mRNA levels were analyzed by qRT-PCR. Ectopic expression of GDIα in CWR22Rv1 cells decreased AR mRNA level (Fig. 3A). Consistent with the reduction in mRNA level, overexpression of GDIα downregulates AR protein level in CWR22Rv1 cells in a dose-dependent manner (Fig. 3B). Similar to CWR22Rv1 cells, overexpression of GDIα in C4-2 cells also downregulates AR expression (Fig. 3C). Since GDIα decreases AR steady state mRNA levels, we tested whether GDIα inhibits AR transcription. We transiently transfected CWR22Rv1 cells with a luciferase reporter containing the full-length promoter of AR and different concentrations of GDIα or vector control. Over-expression of GDIα inhibited AR transcription in CWR22Rv1 cells (Fig. 3D). Collectively, these data suggested that overexpression of GDIα inhibits AR expression, possibly at the transcriptional level.
Fig. 3.
RhoGDIα downregulates AR expression. A: Overexpression of RhoGDIα downregulates androgen receptor (AR) mRNA level in CWR22Rv1 cells in a dose-dependent manner. CWR22Rv1 cells were transfected with different amounts of RhoGDIα plasmids in FBS. Total RNAs were isolated for qRT-PCR analysis of AR mRNA expression. B,C: Overexpression of RhoGDIα inhibits AR protein expression in a dose-dependentmanner.CWR22Rv1 (B) or C4-2 (C) cells were transfected with different amounts of RhoGDIα in FBS condition. Total protein extracts were collected and subjected to Westernblot analysis. D: Overexpression of RhoGDIα inhibits AR promoter luciferase activity. CWR22Rv1 cells were co-transfected with AR promoter-driven luciferase reporter and RhoGDIα plasmids. Luciferase activity was measured.
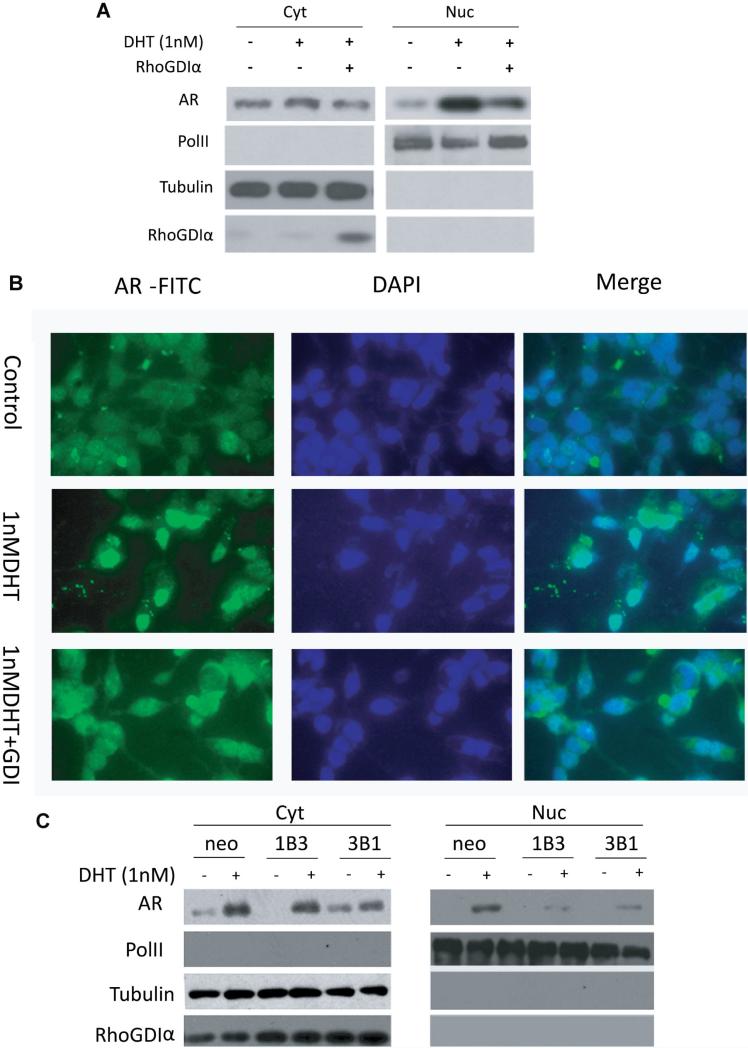
GDIα Inhibits AR Nuclear Translocation
Activated AR translocates into nucleus where it binds to androgen-responsive elements in the promoters of AR target genes. To examine whether GDIα downregulates AR activity through inhibition of AR nuclear translocalization, Western Blot analysis was performed using cell extracts from cytosolic and nuclear extracts of C4-2 cells transfected with vector control or GDIα plasmids. Nuclear levels of AR were decreased when GDIα was expressed compared to the controls (Fig. 4A). To further confirm these results, immunoflurescence was performed. In C4-2 cells, AR is mainly localized in cytoplasm in androgen-deprived conditions. AR nuclear translocation was significantly induced by 1 nM DHT. However, nuclear translocation of AR was partially blocked by over-expression of GDIα (Fig. 4B). To further examine whether GDIα inhibits AR nuclear localization in a dynamic manner, GDIα overexpressing stable clones from DU145 (neo, 1B3, and 3B1) were cultured in CSFBS condition and transfected with wt-AR in the presence or absence of 1 nM DHT for 6 hr. Cytoplasmic and nuclear proteins were isolated and analyzed by Western blot. The induction of AR nuclear translocation by DHT was reduced by overexpression of GDIα in 1B3 and 3B1 compared to the Neo control (Fig. 4C). Collectively, these results suggest that GDIα inhibits the nuclear translocation of AR.
Fig. 4.
RhoGDIα inhibits AR nuclear translocation. A: C4-2 cells were transfected with RhoGDIα plasmid in charcoal-stripped FBS in the presence or absence of1 nMDHT for 6 hr. Cytoplasmic and nuclear proteins were isolated and immunoblotted with AR, PolII, RhoGDIα, and tubulin antibodies. B: C4-2 cells were transfected with RhoGDIα plasmids and treated with control or1 nM DHT for 6 hr in charcoal-stripped FBS. Cells were processed for immunofluorescent staining with AR-FITC, andnuclei were stained with DAPI. C: DU145 stable clones overexpressing RhoGDIα (DUneo, DU1B3, and DU3B1) were cultured in charcoal-stripped FBS and transfected with wildtype-AR in the presence or absence of1 nMDHT for 6 hr. Cytoplasmic and nuclear proteins were isolated andimmunoblotted with AR, PolII, RhoGDIα, and tubulin antibodies. PolII and tubulin were used as loading controls for nuclear and cytoplasmic proteins, respectively.
GDIα Interacts With AR
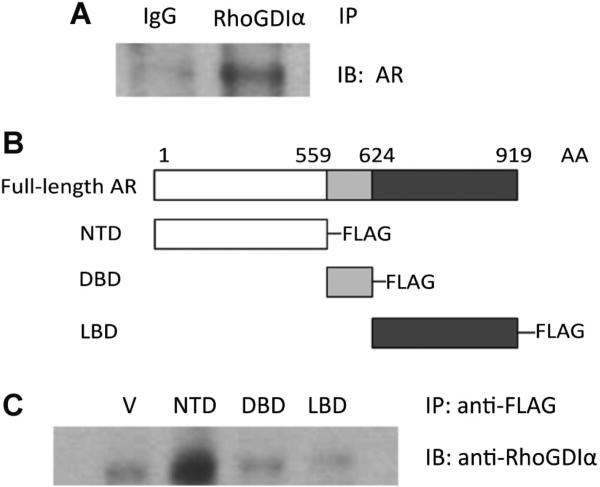
To determine whether GDIα interacts with AR, whole cell protein extracts from LNCaP cells were immunoprecipitated with anti-GDIα antibody or control rabbit IgG antibody. The resulting eluates were analyzed by Western blotting. Isotype-matched normal IgG was used as a control and the membranes were probed with anti-AR antibody. We observed that GDIα was able to co-immunoprecipitate with AR (Fig. 5A). These results suggest that GDIα may physically interact with AR.
Fig. 5.
RhoGDIα interacts with AR. A: Endogeneous RhoGDIα interacts with AR. Whole cell protein extracts from LNCaP cells were immunoprecipitated with antibodies against RhoGDIα or control IgG and probed for the presence of AR. B: Schematic representation of the functional domains of AR [919 amino acids (AA)]. C: Extracts from LNCaP cells expressing either the empty vector or flag-tagged NTD, DBD, and LBD of AR were immunoprecipitated with anti-Flag antibody and probed for the presence of RhoGDIα with anti-Rho GDIα antibody.
The AR protein contains an N-terminal transactivation domain (NTD), a DNA-binding domain (DBD) followed by a hinge region and a C-terminal ligand-binding domain (LBD) [18]. To test which domain of the AR protein interacts with GDIα, we cloned the three domains of the AR protein into Flag-tagged expression vectors by PCR amplification. We transfected Flag-tagged NTD/DBD/LBD-expressing plasmids (Fig. 5B) into LNCaP cells, immunoprecipitated with anti-Flag antibody and immunoblotted with anti-GDIα antibody (Fig. 5C). These experiments revealed that the NTD of AR co-precipitates with GDIα, suggesting that GDIα interacts with the NTD of AR.
DISCUSSION
AR activation in the absence of endocrine androgens is hypothesized to occur via multiple processes including: mutations or amplification of AR to induce hypersensitivity to low levels of intracrine androgens [19–23]; increased androgen biosynthesis [24–30]; the activation of alternate signal transduction pathways involving growth factor- and cytokine-mediated AR activation and downstream receptor tyrosine kinases [31–39]; the increased expression of AR coactivators [40–42]; the alteration of AR mRNA splicing to a constitutively activated form [19,21]; expression of TMPRSS2-ERG prostate cancer-specific gene fusion [43,44]; and the downregulation of specific micro-RNAs (miRNAs) [45,46]. Thus, the aberrant activation of AR plays a central role in CRPC, and understanding the molecular mechanisms leading to the aberrant activation of AR is critical to develop effective therapy. We have previously identified that downregulation of GDIα expression is associated with the progression to CRPC. In this study, we found that overexpression of GDIα inhibits AR expression and downregulates AR activity. The inhibition of AR activity correlates with induction of apoptosis and growth arrest by GDIα shown previously [15].
The emerging role of Rho GDI family members in cancer progression has been demonstrated in various cancer types including bladder cancer [13]. Lower levels of Rho GDI family members are often associated with breast cancer metastasis [14]. El Marzouk et al. [47] reported the direct interaction between GDIα and estrogen receptor a (ERα) influenced estrogen responsiveness in breast cancer. GDIα has also been shown to be involved in chemoresistance in breast cancer through its effects on ERa [48], indicating the potential roles of nuclear receptors in GDIα-mediated cellular functions. We demonstrated that GDIα interacts with AR and suppresses AR activity. We showed that overexpression of GDIα disrupts androgen signaling by inhibiting AR mRNA and protein expression and reducing the expression of AR target genes such as PSA. The inhibition of AR signaling pathway by GDIα has been demonstrated at three different levels: that GDIα (1) downregulates AR expression both at mRNA and protein level, (2) inhibits AR transactivation and prevents AR recruitment to the promoters of androgen-responsive genes, and (3) inhibits AR nuclear translocation.
GDIα is a central regulatory molecule of Rho GTPases. GDIα binds to most of the Rho GTPases and negatively regulates cellular distribution and activity of Rho proteins [49]. Several studies demonstrated that Rho GTPase signaling activates AR independent of ligand in prostate cancer cells [36,50]. Vav3, a Rho GTPase guanine nucleotide exchange factor, has been shown to be an AR coactivator that is up-regulated in human prostate cancer compared with benign tissue and in preclinical models of CRPC [50]. A recent study showed that Vav3 enhances the transcriptional activity of AR3 and another clinically relevant AR splice variant, ARv567es [51]. It has also been demonstrated that one major effector of RhoA, PRK1 associates with AR-NTD in vitro and in vivo, and androgen treatment causes its concomitant recruitment to both the PSA promoter and enhancer [52]. Recent study showed that RhoA activates AR signaling and increases the androgen responsiveness of select serum response factor target genes [53]. These studies demonstrate that Rho GTPases activate AR signaling and possibly promote prostate cancer cell growth and CRPC progression, supporting our data that GDIα suppresses AR signaling and inhibits prostate cancer cell growth. The inhibition of AR signaling by GDIα may also be explained by the reduction of activity of RhoA GTPases activity by GDIα and subsequent inhibition of androgen responsiveness by inhibiting the interaction between its downstream effectors and AR. Since GDIα interacts with RhoA GTPases and reduces RhoA GTPase activity in prostate cancer cells, and reports show that RhoA GTPases promote androgen hypersensitivity in prostate cancer cells [54], it is possible that GDIα-induced AR suppression may be mediated through reduction of RhoA GTPase activity by GDIα.
In summary, we demonstrate that GDIα suppresses AR signaling through inhibition of AR expression, nuclear translocation, and recruitment to androgen-responsive genes. GDIα regulatory pathway may play a critical role in regulating AR signaling and prostate cancer growth and progression.
ACKNOWLEDGMENTS
This work was supported in part by grants from VA Merits I01 BX000526, NIH CA 109441 and CA 140468.
REFERENCES
- 1.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: Progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Lorenzo G, Buonerba C, Autorino R, De Placido S, Sternberg CN. Castration-resistant prostate cancer: Current and emerging treatment strategies. Drugs. 2010;70(8):983–1000. doi: 10.2165/10898600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 3.Kim SJ, Kim SI. Current treatment strategies for castration-resistant prostate cancer. Korean J Urol. 2011;52(3):157–165. doi: 10.4111/kju.2011.52.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trapman J, Cleutjens KB. Androgen-regulated gene expression in prostate cancer. Semin Cancer Biol. 1997;8(1):29–36. doi: 10.1006/scbi.1997.0050. [DOI] [PubMed] [Google Scholar]
- 5.Kung HJ, Evans CP. Oncogenic activation of androgen receptor. Urol Oncol. 2009;27(1):48–52. doi: 10.1016/j.urolonc.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Olofsson B. Rho guanine dissociation inhibitors: Pivotal molecules in cellular signalling. Cell Signal. 1999;11(8):545–554. doi: 10.1016/s0898-6568(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 7.Dovas A, Couchman JR. RhoGDI: Multiple functions in the regulation of Rho family GTPase activities. Biochem J. 2005;390(Pt 1):1–9. doi: 10.1042/BJ20050104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JL, Erickson JW, Cerione RA. New insights into how the Rho guanine nucleotide dissociation inhibitor regulates the interaction of Cdc42 with membranes. J Biol Chem. 2009;284(35):23860–23871. doi: 10.1074/jbc.M109.031815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13(1):72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B. Rho GDP dissociation inhibitors as potential targets for anticancer treatment. Drug Resist Update. 2006;9(3):134–141. doi: 10.1016/j.drup.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 11.Barone I, Brusco L, Gu G, Selever J, Beyer A, Covington KR, Tsimelzon A, Wang T, Hilsenbeck SG, Chamness GC, Ando S, Fuqua SA. Loss of Rho GDIalpha and resistance to tamoxifen via effects on estrogen receptor alpha. J Natl Cancer Inst. 2011;103(7):538–552. doi: 10.1093/jnci/djr058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, Niho Y, Nishimune Y, Nishikawa S, Takai Y. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18(39):5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 13.Seraj MJ, Harding MA, Gildea JJ, Welch DR, Theodorescu D. The relationship of BRMS1 and RhoGDI2 gene expression to metastatic potential in lineage related human bladder cancer cell lines. Clin Exp Metastasis. 2000;18(6):519–525. doi: 10.1023/a:1011819621859. [DOI] [PubMed] [Google Scholar]
- 14.Jiang WG, Watkins G, Lane J, Cunnick GH, Douglas-Jones A, Mokbel K, Mansel RE. Prognostic value of rho GTPases and rho guanine nucleotide dissociation inhibitors in human breast cancers. Clin Cancer Res. 2003;9(17):6432–6440. [PubMed] [Google Scholar]
- 15.Zhu Y, Tummala R, Liu C, Nadiminty N, Lou W, Evans CP, Zhou Q, Gao AC. RhoGDIalpha suppresses growth and survival of prostate cancer cells. Prostate. 2012;72(4):392–398. doi: 10.1002/pros.21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadiminty N, Lou W, Sun M, Chen J, Yue J, Kung HJ, Evans CP, Zhou Q, Gao AC. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 2010;70(8):3309–3319. doi: 10.1158/0008-5472.CAN-09-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee SO, Lou W, Hou M, Onate SA, Gao AC. Interleukin-4 enhances prostate-specific antigen expression by activation of the androgen receptor and Akt pathway. Oncogene. 2003;22(39):7981–7988. doi: 10.1038/sj.onc.1206735. [DOI] [PubMed] [Google Scholar]
- 18.Heinlein CA, Chang C. Androgen receptor in prostate cancer. Endocr Rev. 2004;25(2):276–308. doi: 10.1210/er.2002-0032. [DOI] [PubMed] [Google Scholar]
- 19.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. doi: 10.1158/0008-5472.CAN-08-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. doi: 10.1158/0008-5472.CAN-08-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. doi: 10.1158/0008-5472.CAN-08-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linja MJ, Savinainen KJ, Saramaki OR, Tammela TL, Vessella RL, Visakorpi T. Amplification and overexpression of androgen receptor gene in hormone-refractory prostate cancer. Cancer Res. 2001;61(9):3550–3555. [PubMed] [Google Scholar]
- 23.Tilley WD, Buchanan G, Hickey TE, Bentel JM. Mutations in the androgen receptor gene are associated with progression of human prostate cancer to androgen independence. Clin Cancer Res. 1996;2(2):277–285. [PubMed] [Google Scholar]
- 24.Page ST, Lin DW, Mostaghel EA, Hess DL, True LD, Amory JK, Nelson PS, Matsumoto AM, Bremner WJ. Persistent intraprostatic androgen concentrations after medical castration in healthy men. J Clin Endocrinol Metab. 2006;91(10):3850–3856. doi: 10.1210/jc.2006-0968. [DOI] [PubMed] [Google Scholar]
- 25.Mostaghel EA, Page ST, Lin DW, Fazli L, Coleman IM, True LD, Knudsen B, Hess DL, Nelson CC, Matsumoto AM, Bremner WJ, Gleave ME, Nelson PS. Intraprostatic androgens and androgen-regulated gene expression persist after testosterone suppression: Therapeutic implications for castration-resistant prostate cancer. Cancer Res. 2007;67(10):5033–5041. doi: 10.1158/0008-5472.CAN-06-3332. [DOI] [PubMed] [Google Scholar]
- 26.Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: Mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22(2):243–258. doi: 10.1016/j.beem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marks LS, Mostaghel EA, Nelson PS. Prostate tissue androgens: History and current clinical relevance. Urology. 2008;72(2):247–254. doi: 10.1016/j.urology.2008.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratu-moral androgens in metastatic prostate cancer: A mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stanbrough M, Bubley GJ, Ross K, Golub TR, Rubin MA, Penning TM, Febbo PG, Balk SP. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 2006;66(5):2815–2825. doi: 10.1158/0008-5472.CAN-05-4000. [DOI] [PubMed] [Google Scholar]
- 30.Mohler JL, Gregory CW, Ford OH III, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–448. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 31.Visakorpi T. New pieces to the prostate cancer puzzle. Nat Med. 1999;5(3):264–265. doi: 10.1038/6472. [DOI] [PubMed] [Google Scholar]
- 32.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: A novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci USA. 1999;96(10):5458–5463. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen T, Wang LH, Farrar WL. Interleukin 6 activates androgen receptor-mediated gene expression through a signal transducer and activator of transcription 3-dependent pathway in LNCaP prostate cancer cells. Cancer Res. 2000;60(8):2132–2135. [PubMed] [Google Scholar]
- 34.Lee SO, Lou W, Hou M, de Miguel F, Gerber L, Gao AC. Inter-leukin-6 promotes androgen-independent growth in LNCaP human prostate cancer cells. Clin Cancer Res. 2003;9(1):370–376. [PubMed] [Google Scholar]
- 35.Hobisch A, Eder IE, Putz T, Horninger W, Bartsch G, Klocker H, Culig Z. Interleukin-6 regulates prostate-specific protein expression in prostate carcinoma cells by activation of the androgen receptor. Cancer Res. 1998;58(20):4640–4645. [PubMed] [Google Scholar]
- 36.Lyons LS, Rao S, Balkan W, Faysal J, Maiorino CA, Burnstein KL. Ligand-independent activation of androgen receptors by Rho GTPase signaling in prostate cancer. Mol Endocrinol. 2008;22(3):597–608. doi: 10.1210/me.2007-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frigo DE, Sherk AB, Wittmann BM, Norris JD, Wang Q, Joseph JD, Toner AP, Brown M, McDonnell DP. Induction of Kruppel-like factor 5 expression by androgens results in increased CXCR4-dependent migration of prostate cancer cells in vitro. Mol Endocrinol. 2009;23(9):1385–1396. doi: 10.1210/me.2009-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agoulnik IU, Bingman WE III, Nakka M, Li W, Wang Q, Liu XS, Brown M, Weigel NL. Target gene-specific regulation of androgen receptor activity by p42/p44 mitogen-activated protein kinase. Mol Endocrinol. 2008;22(11):2420–2432. doi: 10.1210/me.2007-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kazmin D, Prytkova T, Cook CE, Wolfinger R, Chu TM, Beratan D, Norris JD, Chang CY, McDonnell DP. Linking ligand-induced alterations in androgen receptor structure to differential gene expression: A first step in the rational design of selective androgen receptor modulators. Mol Endocrinol. 2006;20(6):1201–1217. doi: 10.1210/me.2005-0309. [DOI] [PubMed] [Google Scholar]
- 40.Yeh S, Miyamoto H, Shima H, Chang C. From estrogen to androgen receptor: A new pathway for sex hormones in prostate. Proc Natl Acad Sci USA. 1998;95(10):5527–5532. doi: 10.1073/pnas.95.10.5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao S, Lyons LS, Fahrenholtz CD, Wu F, Farooq A, Balkan W, Burnstein KL. A novel nuclear role for the Vav3 nucleotide exchange factor in androgen receptor coactivation in prostate cancer. Oncogene. 2012;31:716–727. doi: 10.1038/onc.2011.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Agoulnik IU, Weigel NL. Coactivator selective regulation of androgen receptor activity. Steroids. 2009;74(8):669–674. doi: 10.1016/j.steroids.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tomlins SA, Laxman B, Dhanasekaran SM, Helgeson BE, Cao X, Morris DS, Menon A, Jing X, Cao Q, Han B, Yu J, Wang L, Montie JE, Rubin MA, Pienta KJ, Roulston D, Shah RB, Varambally S, Mehra R, Chinnaiyan AM. Distinct classes of chromosomal rearrangements create oncogenic ETS gene fusions in prostate cancer. Nature. 2007;448(7153):595–599. doi: 10.1038/nature06024. [DOI] [PubMed] [Google Scholar]
- 44.Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679. [DOI] [PubMed] [Google Scholar]
- 45.Shi XB, Tepper CG, de Vere White RW. MicroRNAs and prostate cancer. J Cell Mol Med. 2008;12(5A):1456–1465. doi: 10.1111/j.1582-4934.2008.00420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sun T, Yang M, Kantoff P, Lee GS. Role of microRNA-221/-222 in cancer development and progression. Cell Cycle. 2009;8(15):2315–2316. doi: 10.4161/cc.8.15.9221. [DOI] [PubMed] [Google Scholar]
- 47.El Marzouk S, Schultz-Norton JR, Likhite VS, McLeod IX, Yates JR, Nardulli AM. Rho GDP dissociation inhibitor alpha interacts with estrogen receptor alpha and influences estrogen responsiveness. J Mol Endocrinol. 2007;39(4):249–259. doi: 10.1677/JME-07-0055. [DOI] [PubMed] [Google Scholar]
- 48.Su LF, Wang Z, Garabedian MJ. Regulation of GRIP1 and CBP Coactivator activity by Rho GDI modulates estrogen receptor transcriptional enhancement. J Biol Chem. 2002;277(40):37037–37044. doi: 10.1074/jbc.M111607200. [DOI] [PubMed] [Google Scholar]
- 49.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: Regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12(8):493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lyons LS, Burnstein KL. Vav3, a Rho GTPase guanine nucleotide exchange factor, increases during progression to androgen independence in prostate cancer cells and potentiates androgen receptor transcriptional activity. Mol Endocrinol. 2006;20(5):1061–1072. doi: 10.1210/me.2005-0346. [DOI] [PubMed] [Google Scholar]
- 51.Peacock SO, Fahrenholtz CD, Burnstein KL. Vav3 enhances androgen receptor splice variant activity and is critical for castration-resistant prostate cancer growth and survival. Mol Endocrinol. 2012 doi: 10.1210/me.2012-1165. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Metzger E, Muller JM, Ferrari S, Buettner R, Schule R. A novel inducible transactivation domain in the androgen receptor: Implications for PRK in prostate cancer. EMBO J. 2003;22(2):270–280. doi: 10.1093/emboj/cdg023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmidt LJ, Duncan K, Yadav N, Regan KM, Verone AR, Lohse CM, Pop EA, Attwood K, Wilding G, Mohler JL, Sebo TJ, Tindall DJ, Heemers HV. RhoA as a mediator of clinically relevant androgen action in prostate cancer cells. Mol Endocrinol. 2012;26(5):716–735. doi: 10.1210/me.2011-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bakin RE, Gioeli D, Sikes RA, Bissonette EA, Weber MJ. Constitutive activation of the Ras/mitogen-activated protein kinase signaling pathway promotes androgen hypersensitivity in LNCaP prostate cancer cells. Cancer Res. 2003;63(8):1981–1989. [PubMed] [Google Scholar]