Abstract
We report that polyclonal CD8regs generated in one week ex-vivo with anti-CD3/28 beads and cytokines rapidly developed suppressive activity in vitro sustained by TGF-β. In immunodeficient mice, these CD8regs demonstrated a markedly protective, IL-10 dependent activity against a xeno-GVHD. They expressed IL-2Rα/β, Foxp3, TNFR2, and the negative co-stimulatory receptors CTLA-4, PD-1, PD-L1 and Tim-3. Suppressive activity in vitro correlated better with TNFR2 and PD-L1 than Foxp3. Blocking studies suggested that TNF enhanced PD-L1 expression and the suppressive activity of the CD8regs generated. Unlike other polyclonal CD4 and CD8 Tregs, these CD8regs preferentially targeted allogeneic T cells, but they lacked cytotoxic activity against them even after sensitization. Unlike CD4regs, these CD8regs could produce IL-2 and proliferate while inhibiting target cells. If these CD8regs can persist in foreign hosts without impairing immune surveillance, they could serve as a practical remission-inducing product for the treatment of autoimmune diseases, graft-versus-host disease, and allograft rejection.
Introduction
The principal function of the immune system is to eliminate microbial invaders, but unfortunately, not all T and B lymphocytes with the potential to cause autoimmune diseases are eliminated. Once these self-reactive cells are persistently activated, present therapeutic agents can arrest disease progression, but cure has been elusive. This can be explained by the tight homeostatic control of immune system. Each action triggers a counter response to modulate and eventually terminate the response. Thus, therapeutic agents directed against pathogenic cells or signaling pathways may also target the counter response needed for termination.
Since regulatory T cells (Tregs) control pathogenic self-reactive cells and maintain immunologic homeostasis, there has been great interest in exploring their therapeutic potential for autoimmune diseases [1]. Clinical trials exploring the therapeutic potential of regulatory T cells for human immune-mediated diseases have begun using expanded endogenous CD4+CD25+Foxp3+ Tregs isolated from blood [2]. However, these Tregs are difficult to expand from the small numbers isolated, and their functional properties decrease after large expansion [3]. Moreover, the pathogenic memory T cells, which are predominant in established autoimmune disease and allograft rejection, may be resistant to suppression by CD4regs [4, 5].
The suppressive effects of CD8+ cells on normal and pathologic immune responses have been known for decades [6–8]. Although unlike CD4regs, there are few thymus-derived CD8regs [9], many subsets have been generated from peripheral CD8 cells. Early workers reported that CD8 cells activated with antigens and TGF-β developed suppressive activity. Later TCR transgenic CD8+ cells activated with TGF-β became Foxp3+ and developed potent suppressive activity that could be distinguished from their cytolytic effects [8]. CD8regs can be divided into cells recognizing MHC class I antigens, and those with a predominantly non-cytotoxic mechanism of action [8, 10–12].
Human CD8regs occur spontaneously in vivo. Examples include appearance following rejection-free cardiac transplant recipients[13], in patients with type 1 diabetes following treatment with anti-CD3[14] or in lupus patients in remission following autologous stem-cell transplantation [15]. These Tregs can also be induced ex-vivo. In 1994 we reported that human CD8+ cells mitogen-activated with TGF-β developed suppressive activity [16]. Based on the methodology we used to induce polyclonal CD4+CD25+Foxp3+ Tregs with protective activity in vivo [17], our objective was to induce CD8+ cells ex-vivo to become suppressor cells. We have generated human CD8regs phenotypically resembling exhausted CD8 cells (14) that have marked protective activity in vivo. Similar to mouse donor CD8+Foxp3+ Tregs that spontaneously arise following transplantation of MHC mismatched lymphoid cells [18–20], these polyclonal human CD8regs cells preferentially targeted alloantigens which may explain their strong protective effects.
2. Materials and methods
2.1 Mice
NOD/scid/IL2R common γ chain−/− (NSG) mice were obtained from Jackson Laboratory (Bar Harbor, ME). The mice were bred and housed under specific pathogen-free conditions in microisolator cages and given unrestricted access to autoclaved food and sterile water. Animals of both sexes were used for experiments at 8–12 weeks of age. The mice received a single dose of 150 cGy gamma irradiation from a linear accelerator before injection of human PBMC on the same day. All experiments were performed according to the guidelines of the Institutional Animal Committee of the University of Southern California.
2.2 Monoclonal antibodies, cytokines and cytokine antagonists used
The following FITC, PE, Cyc or APC conjugated human antibodies were used for flow cytometric analysis: From BD Pharmingen (San Diego, CA): CD3(HIT3a), CD4 (RM4-5), CD28 (CD28.2), CD45RA (L48), CD45RO (UCHL1), CD122 (Mik-β3), CD86 (2331[FUN1]), CD103 (Ber-ACT8), CD274, PD-L1 (M1H1), CTLA-4 (BNI3), HLA-DR (G46-6), Granzyme A (CB9), Granzyme B (GB11), mouse IgG1 (MOPC-21), IgG2a (G155-178), IgG2b (27-35), from Biolegend, (San Diego, CA): CD8 (SK1), CD25 (M-A251), PD-1 (EH12.2H7), CD274, PD-L1 (29E.2A3), Tim3 (F38-2E2), HLA-ABC (Wb/32) from eBiosciences (San Diego, CA): Foxp3 (206D), and from R&D Systems, Inc (Minneapolis, MN): TNF-RII (22235). We obtained unconjugated PD-1 (MIH4) and PD-L1 (MIH1) and CTLA-4 (BNI3.1) from BD Pharmingen as a generous gift from Noel Warner. TNFR-Fc (Enbrel) was obtained from Amgen, (Thousand Oaks, CA). Other agents purchased from BD Pharmingen included recombinant human IL-2 (MQ1-17H12), IFN-γ (B27), from HumanZyme (Chicago, IL); recombinant human TGF-β1, from Invitrogen (Carlsbad, CA): anti-human CD3/CD28-conjugated Dynabeads, carboxyfluorescein succinimidyl ester (CFSE), and AIM-V serum-free medium from GIBCO Invitrogen, Life Technologies, (Grand Island, NY); RPMI 1640 medium, Cellgro Mediatech, (Manassas, VA) Fetal Bovine Serum (FBS) Atlanta Biologicals, (Lawrenceville, GA).
2.3 Isolation of human nTregs and generation of human iTreg cells ex vivo
PBMC were prepared from heparinized venous blood of healthy adult volunteers by Ficoll-Hypaque density gradient centrifugation. All protocols that involved human blood donors were approved by the IRB at the University of Southern California. T cells were prepared by E rosetting and negative selection of non-T cells as described previously to a purity of >95% [21]. The T cells were incubated with unconjugated mouse anti-CD4 (OKT4), anti-CD45RO (UCHL1), anti-HLA-DR (L243), and anti-CD 11b (OKM1) (American Type Tissue Culture Collection, Bethesda, MD) and depleted with goat anti-mouse IgG coated beads (Dynabeads, Life Technologies, Grand Island, New York). This isolation procedure was repeated to increase the purity to >90%. The naïve CD8 cells were stimulated with CD3/28 beads at 1:5 ratio (one bead to 5 cells) + rhIL-2 (50U/ml) CD8Medium or with TGFβ1 (5ng/ml) CD8TGFβ in AIM-V serum-free medium containing Hepes buffer (10 mM). sodium pyruvate (1mM), glutamine and penicillin and streptomycin in 24 or 48 well plates. On day 3, cells were split and fresh culture medium with IL-2 (30–50 U/ml) and was added to the wells. Additional IL-2 (50U/ml) was added the day before harvest at day 5 or 6, and the beads were removed. In experiments to assess cytokine production, the CD8 cells were stimulated with PMA and Ionomycin for 6 hours. Brefeldin A was added one hour later and the cells were permeabilized (Fix and Perm kit™ (BD) and stained for IL-2, IFN-γ, TNF-α and IL-17. Intracellular cytokine production was determined by flow cytometry. In some experiments we determined the effect of TNFR-Fc and anti-PD-L1 on the generation of CD8regs and their suppressive activity.
2.4 Flow Cytometry
The effect of the CD8+ cell conditioning procedures on their phenotype was assessed by comparative studies with fresh, unstimulated cells. Each subset was stained with mAbs to markers indicated above, and analyzed on a FACS Calibur flow cytometer using Cell Quest Software (Becton Dickinson). For Foxp3, CTLA-4 and granzymes A and B the cells were also permeabilized for intracellular staining. Histograms also showing isotype control staining were prepared using FloJo Software (Treestar Inc. Ashland, OR).
2.5 Suppressive assays of CD4+ Treg cells in vitro and in vivo
CD8Medium or CD8TGFβ (Tregs) were added to 1.5 × 105 autologous or allogeneic CD4+ CD25 depleted cells (responder T cells) in ratios of 1:2, 1:4 and 1:8 in 96 well flat bottomed plates (Greiner Bio-one (Monroe, NC)). The cells were stimulated with anti-CD3/28 beads (bead:responder ratio 1:2 and 1:4) for 3 to 4 days in RPMI 1640 culture medium (Cellgro Mediatech) with 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA) The CD4 responder cells, and in some experiments the CD8 cells were labeled with CFSE as previously described. Antigen-presenting cells were omitted. Cell division was monitored by CFSE dilution. Suppressive activity was the calculated as the percentage of cycling CD4 responder cells cultured with CD8 cells divided by the percentage of cycling responder cells cultured alone × 100.
The model to assess suppressor activity in vivo was the protection of immunodeficient NSG mice from a rapidly fatal human anti-mouse GVHD as described previously [22]. Twenty ×106 human PBMC with 5 × 106 allogeneic or autologous CD8Medium or CD8TGFβ in 0.2ml were injected IV into the tail vein of NSG mice sublethally irradiated with 150 cGy. The positive control was mice injected with PBMC only. The negative control for suppression was mice injected with PBMC and un-stimulated CD8 cells. The animals were weighed every 2 to 3 days and euthanized when they lost 20% of their original weight. In other experiments the effect of decreasing IL-10 and TGF-β signaling on the protective effects of CD8regs was determined by injecting the mice IP with the ALK5 TGF-βR1 inhibitor (LY-364947, Sigma-Aldrich, St. Louis, MO) and anti IL-10R (Taconic, Germantown, NY, clone:YL03.1B1.39-34ABS), 0.5mg IP weekly.
2.6 Cytotoxicity assay
Cytotoxic killer cells were generated by stimulating naïve CD8 cells with allogeneic monocyte-derived mature DCs [23] at a 30:1 ratio (T cells: DCs). Cells were harvested at day 6 or 7 of culture, and spun through a density gradient to remove dead cells. Target cells were total T cells from the allogeneic donor activated with concanavalin A (Sigma) 5μg/ml for 4 days. We used three color flow cytometry based upon a method previously described to determine cytotoxic activity [24]. Each CD8 subset was incubated with CFSE-labeled allogeneic concanavalin A blasts for 4 hours, at a 30:1 effector to target cell ratio. Cytotoxicity was determined by staining of Annexin V and 7-AAD using a kit supplied by eBioscience and following the manufacturer’s instructions. Target cells killed were double stained by Annexin V and 7-AAD, and specific cytotoxicity was determined after correction for background staining by the following formula: (observed cytotoxicity − minimum cytotoxicity) / (maximum cytotoxicity − minimum cytotoxicity) × 100. Annexin single positive cells undergoing early apoptosis are also predestined for cell death, but by metabolic pathways distinct from cytolysis.
2.7 Statistical Analysis
Flow cytometry and cytokine data were analyzed using Student’s 2-tailed t-tests using Graph Pad Prism Software. Comparison values of p <0.05 were considered statistically significant. Survival was determined using the Log-Rank test of Kaplan-Meier survival curves.
3 Results
3.1 CD8+ cells stimulated with anti-CD3 and anti-CD28 coated beads have strong protective activity in humanized mice and preferentially target allogeneic T cells
Because in vitro suppressor assays may not predict the protective effects of Tregs in vivo [25] we elected to use immunodeficient mice to study the suppressive effects of human naïve CD8+ cells stimulated with anti-CD3/28 coated beads, IL-2 ± TGF-β. Since first reported by Mutis and coworkers [22], we and others have used this assay to investigate the protective effects of expanded endogenous CD4+CD25+Foxp3+ Tregs and CD4 iTregs induced ex-vivo with IL-2, TGF-β and retinoic acid [17]. Since these mice cannot reject human T cells, they develop an ultimately fatal graft-versus host disease. We and others have reported that endogenous and CD4regs generated ex-vivo enhance survival by 50 to 100% (Table 1).
Table 1.
Comparison of human CD4 expanded endogenous Tregs and induced Tregs with CD8 Tregs in preventing human anti-mouse GVHD in immunodeficient mice
| Treg subset
|
Treg/PBMC ratio
|
50% survival (Days) PBMC PBMC+Tregs
|
Percent increase in survival
|
|
|---|---|---|---|---|
| June C (2008)[38] | ||||
| Expanded CD4 nTregs (artificial APC [aAPC}, IL-2 and rapamycin) | 1:5 | 25 | 50+ | 100+ |
| Fowler D (2010)[41] | ||||
| Expanded CD4 nTregs (IL-2, TGF-β Rapamycin) | 1:20 | 37 | 75 | 102 |
| Blazar B (2011)[40] | ||||
| Expanded CD4 nTregs (aAPC, IL-2) | ||||
| (x1) | 1:1 | 39 | 55 | 41 |
| (x3) | 1:2 | 46 | 53 | 15 |
| (x4) | 1:2 | 46 | 60 | 30 |
| Blazar B (2011)[39] | ||||
| CD4 iTregs (IL-2, TGF-β, Rapa) | 1:3 | 25 | 45 | 80 |
| CD4 nTregs (x1) | 1:3 | 38 | 55 | 45 |
| CD4 nTregs (expanded 40 days) | 1:1 | 48 | 62 | 29 |
| Horwitz DA (2010)[17] | ||||
| CD4 iTregs (IL-2, TGF-β, atRA) | 1:4 | 11 | 24 | 118 |
| Expanded CD4 nTregs (x1) | 1:4 | 11 | 20 | 80 |
| Horwitz DA (Present Report) | ||||
| CD8 iTregs (IL-2) | 1:4 | 11 | 44 | 300 |
| CD8 iTregs (IL-2, TGF-β) | 1:4 | 11 | 42 | 282 |
Abbreviations: aAPC, artificial antigen-presenting cells; atRA, all trans retinoic acid; nTregs, endogenous Tregs (both thymus-derived natural and those induced in vivo); iTregs, Tregs induced ex-vivo
Unless aAPC are indicated, CD4+ cells were stimulated with anti-CD3/28 coated beads.
We previously reported that intravenous transfer of twenty million human PBMC into lightly irradiated NOD SCID IL-2R common gamma chain deficient (IL-2R ɣc−/−) (NSG) mice resulted in rapid engraftment of human T cells in the lungs, liver, bone marrow and spleen leading to death of the animal in two weeks [17]. This model was also used in the present study. Where we and others had reported that CD4Tregs doubled survival (Table 1), transfer of a similar number of conditioned CD8+ cells quadrupled the survival of the mice. Unlike CD4regs which are TGF-β dependent, conditioning CD8+ cells without this cytokine had similar protective effects. Control untreated CD8+ cells completely lacked protective activity (Fig. 1A). Adding retinoic acid to IL-2 and TGF-β did not enhance survival further (result not shown).
Figure 1. Allogeneic polyclonal CD8regs induced ex-vivo are more protective in vivo against a human anti-mouse graft versus host disease than CD4regs, and have a cytokine-dependent mechanism of action.
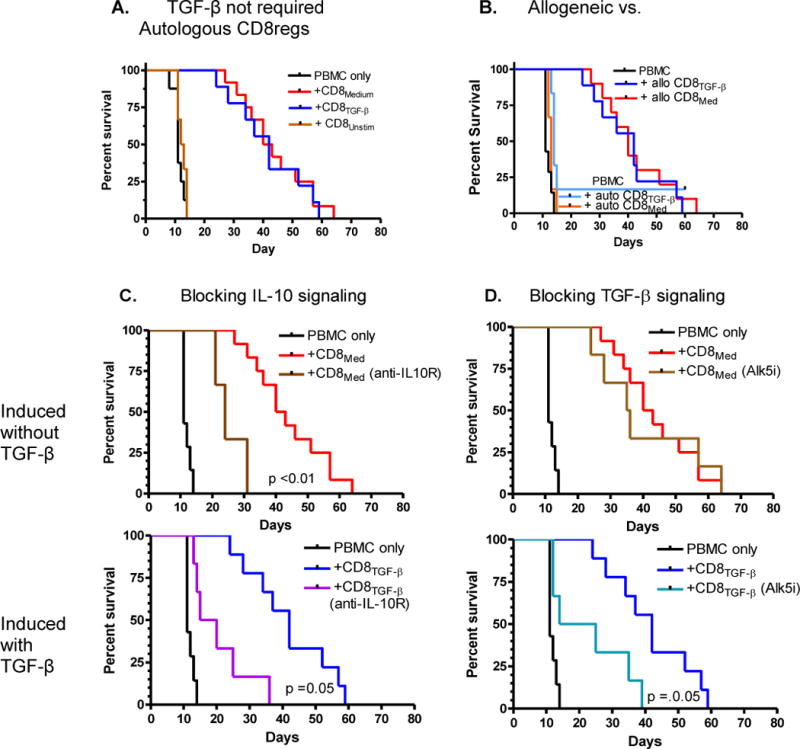
Lightly irradiated immunodeficient NOD SCID IL-2R common gamma chain deficient (IL-2R ɣc−/−) (NSG) mice were injected intravenously with 20×106 human PBMC. A. Mice were also injected IV with 5×106 allogeneic CD8+ cells stimulated for 1 week with anti-CD3/28 beads, IL-2 (CD8Med), or with TGF-β (CD8TGF-β) (n >9/group). B. Mice were injected with 5×106 autologous or allogeneic CD8Med or CD8TGF-β. C. NSG mice injected with similar numbers of PBMC ± CD8Med, or CD8TGF-β in mice that received weekly injections of anti-IL-10R (0.5mg). D. Similar to C. except mice received weekly injections of Alk5 TGF-βR1 signaling inhibitor (0.5mg).
Four mice were sacrificed at days 59 and 60 for histologic inspection. The characteristic liver inflammatory lesions as reported earlier [17] were not observed, but there were some mononuclear infiltrates in the lungs. Some human CD8+ cells were observed in the spleen and bone marrow, but not CD4+ cells. Thus, the CD8+ cells had blocked the marked engraftment of human T cells in these mice and greatly prolonged the life of these animals. These CD8+ cells can be, therefore, called suppressor/regulatory cells or CD8regs.
We then compared the effects of CD8regs on autologous or allogeneic PBMC transferred to the mice. Because of the large numbers of human PBMC needed to cause rapid GVHD, we used PBMC allogeneic to the CD8+ cells in the initial experiments. Previously, in our studies with CD4regs, we had observed the protective effects were similar on both [17]. However, this was not the case with CD8regs we had generated. With one exception, neither CD8regs generated with TGF-β nor without this cytokine could increase the survival of mice that had received autologous T cells (Fig. 1B). Thus, the CD8regs appeared to depend on alloantigen stimulation provided by their target cells for their protective effects.
Since we had observed that blocking IL-10 and TGF-β signaling abrogated the protective effects of iCD4regs and iCD8regs in a mouse chronic GVHD [26], similar studies were conducted with this model. We found that weekly injections of anti-IL-10R antibodies inhibited the protective effects of CD8regs conditioned with and without TGF-β (Fig 1C). One difference between the two CD8reg subsets was that blocking TGF-β signaling through TGF-βR1 partially inhibited the protective effects of CD8regs conditioned with TGF-β, but had no effect on CD8regs conditioned without this cytokine (Fig. 1D).
3.2 CD8+ cells stimulated with anti-CD3/28 beads strongly express IL-2Rαβ chains, TGF-β dependent Foxp3, TNFR2, and negative co-stimulatory molecules including PD-L1
Like CD4+CD25+Foxp3+ Tregs [17], CD8+ cells stimulated with anti-CD3/28 beads strongly express CD25 and CD122. Thirty to 40% of stimulated CD8+ cells displayed Foxp3. This was enhanced by adding TGF-β, but adding an alk5 TGF-βR1 signaling inhibitor decreased Foxp3 to baseline levels expressed to ~20% (Fig 2A). TGF-β enhanced Foxp3, however, was not stable. Sustained high levels required the addition of >20U/ml IL-2 every three days. As shown in Fig 2B, Foxp3 expression decreased if lower amounts were added. Thus, as demonstrated for CD4regs, both IL-2 and TGF-β have important roles in Foxp3 expression by CD8+ cells [27].
Figure 2. Foxp3 is expressed by activated human CD8+ cells, enhanced by TGF-β, and sustained by IL-2.
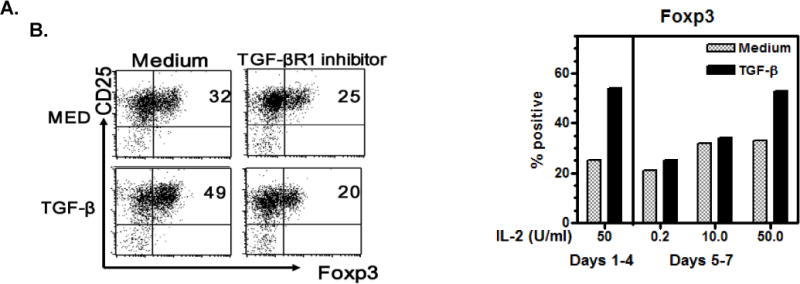
A. Naïve CD8 cells were stimulated with anti-CD3/28 coated beads (1 bead per 5 cells) with IL-2 (50U/ml) ± TGF-β1 (5ng/ml) and an alk5 TGF-βR1 signaling inhibitor (10μM) for 5 days. The cells were permeabilized, stained for Foxp3 and analyzed by flow cytometry. B. CD8 cells were stimulated as above with IL-2 50U/ml for 4 days, washed and IL-2 added back in the amounts indicated. Foxp3 was determined after culture for 2 more days. Here 50U/ml of IL-2 was required to sustain Foxp3 expression.
Anti-CD3/28 CD8+ stimulated cells also expressed TNFR2, and the negative co-stimulatory molecules CTLA-4, PD-1, PD-L1, Tim-3. Fig. 3A shows representative FACS histograms along with the mean ± SEM of >4 subjects. Although >85% of the activated CD8+ cells rapidly expressed PD-L1 with anti-PD-L1 mAb clone 29E.2A3, the maximum values were somewhat less with clone M1H1. The addition of TGF-β enhanced only PD-1. As reported by others, TGF-β, strongly upregulates CD103 on CD8 cells [28]. In addition, TGF-β attenuated positive co-stimulatory molecules induced by anti-CD3 that included HLA-DR, CD80 and CD86. Moreover, although anti-CD3 stimulated CD8+ cells can become cytolytic [29], TGF-β strongly down-regulated baseline levels of both Granzyme A and Granzyme B (Fig. 3B).
Figure 3. Phenotypic characteristics of CD8+ cells activated with anti-CD3/28 beads and IL-2.
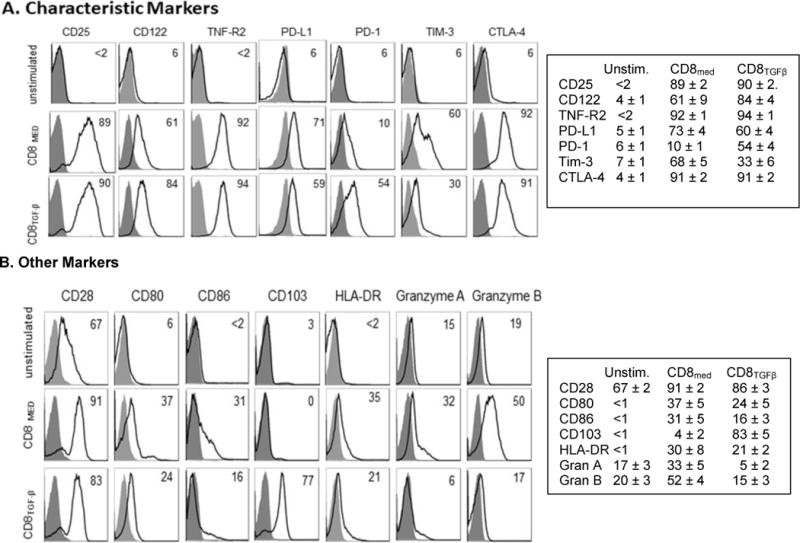
A. B. TGF-β has positive and negative effects on the phenotype of CD8+ cells stimulated with anti-CD3/28 beads and IL-2. Flow cytometry histograms comparing markers expressed by unstimulated naive CD8 cells with cells stimulated with anti-CD3/28 coated beads, IL-2 (50U/ml) ± TGF-β1, for 5 days. Isotype controls are shaded. The panels show a representative histogram and the mean ± SEM for at least three donors.
3.3 CD8regs sustained by TGF-β preferentially target allogeneic T cells
Similar to the in vivo studies, TGF-β was not needed for the generation of the inhibitory effects of anti-CD3/28 activated CD8 cells. Fig. 4A shows that within 2 days after activation, CD8+ cells had developed strong in vitro suppressive activity. However, by day 5 the suppressive activity by CD8+ cells stimulated without TGF-β began to decline while those with added TGF-β did not. Suppressive activity remained intact for at least 20 days in cultures supplemented with IL-2 only (result not shown). A likely explanation for this effect is the ability of TGF-β to protect CD8 cells from apoptosis [27].
Figure 4. Suppressive effects of CD8regs in vitro.
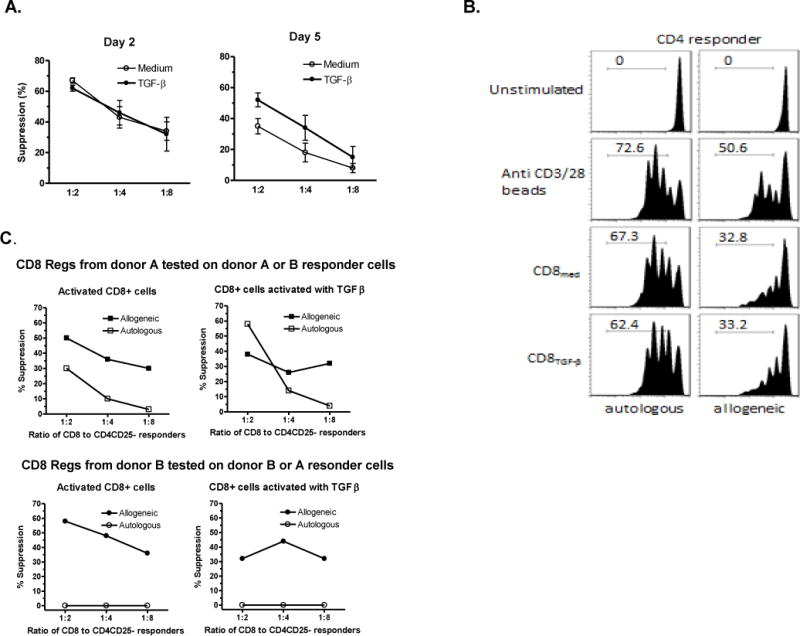
A. TGF-β is not required for generation, but sustains suppressive activity. CD8 cells stimulated ± TGF-β for 2 days or 5 days were mixed with allogeneic naïve CFSE-labeled CD4+CD25- cells in the ratios shown, and restimulated with anti-CD3/28 beads (1 bead per 2 responder cells). After 4 days dilution of CD4 CFSE was assessed by flow cytometry. The data indicates the mean ± SEM of 5 separate experiments and shows equivalent suppression at 2 days, but at 5 days a loss of activity by CD8 cells conditioned without TGF-β. B. CD8regs preferentially target allogeneic T cells. Naïve CD8+ and CD4+CD25- cells isolated from peripheral blood mononuclear cells of two separate donors were stimulated with anti-CD3/28 beads, IL-2 ± TGF-β for 5 days and assessed for their ability to suppress the proliferation of CD4+ cells. In secondary cultures, the conditioned CD8 cells were cultured with thawed autologous or allogeneic CFSE-labeled CD4 responder cells labeled with CFSE in a 1:4 ratio and stimulated with anti-CD3/28 beads (1 bead per 2 CD4+CD25- responder cells) for 4 days. The histogram shows preferentially targeting of allogeneic CD4+ cells. In this experiment the CD8 cells were also labeled with CFSE and their proliferative activity is shown in Fig. 5A. C. Using the protocol described in panel B, this experiment is representative of the variability of the suppressive effects against autologous and allogeneic CFSE-labeled CD4+ cells at various suppressor to responder ratios.
Also consistent with the in vivo protective effects described above was that suppressive activity in vitro against allogeneic CD4+ cells was greater than against autologous cells (Fig. 4 B&C). This characteristic distinguishes these CD8regs from other polyclonal Tregs. Their activity against autologous CD4+ cells was donor variable. With some CD8regs had suppressive effects at high Treg to Tresponder ratios, but with other donors they completely lacked activity against autologous CD4+ cells as shown in Fig. 4C.
3.4 CD8regs are not anergic and lack cytolytic activity against T cells
One of the characteristic features of Foxp3+ CD4regs is that they are anergic and one of their suppressive mechanisms is consuming IL-2 produced by other T cells [30]. Figure 5A shows that unlike CD4regs, CD8regs labeled with CFSE can proliferate after restimulation with anti-CD3/28 beads. CD8regs induced with TGF-β divided even greater when cultured with CD4responder cells. This proliferation did not correlate with suppressive activity since only allogeneic CD4 responder cells were markedly suppressed in this experiment (Fig. 4B).
Figure 5. Characteristics of CD8regs induced with immobilized anti-CD3 and anti-CD28.
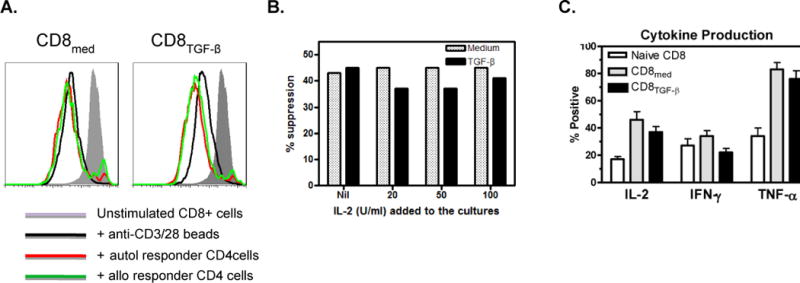
A. CD8regs are not anergic. The protocol described in Figure 4 was used for these experiments. After generation, CD8Medium and CD8TGF-β were labeled with CSFE and re-stimulated with anti-CD3/28 beads with or without autologous or allogeneic CD4responder cells. As shown, CD8Medium proliferate in response to secondary anti-CD3/28 stimulation, and this proliferation was enhanced when CD8TGF-β were when cultured with CD4 responder cells. The suppressive effects of these CD8regs on autologous and allogeneic CD4 responder cells are shown in Fig. 4B.
B. IL-2 does not inhibit suppressive activity. IL-2 was added in the concentrations shown to CD8regs mixed with CFSE-labeled responder CD4+ cells in suppressor assays in a ratio of 1:4. The experiment shown is representative of 4 similar experiments where IL-2 had no effect on CD8reg suppressive activity.
C. Comparison of cytokine production between CD8Medium and CD8TGF-β and unstimulated CD8+ cells. Using protocols described above, unstimulated CD8+ cells and those conditioned for 5 days were cultured with phorbol myristate acetate and ionomycin for 6 hours. Brefeldin A was added for the last 5 hours. The cells were permeabilized, stained for the cytokines shown, and intracellular cytokine production assessed by flow cytometry. In each of 6 experiments performed, the conditioned CD8+ cells produced more IL-2 and TNF-α than unstimulated CD8+ cells.
While the addition of IL-2 abolishes the suppressive activity of CD4regs [31], IL-2 had no effect on the suppressive activity of CD8 cells (Fig. 5B). Moreover, unlike CD4regs which cannot produce IL-2 [31], CD8regs retained their ability to produce IL-2, IFN-γ and TNF-α. In fact, the percentage of IL-2 and TNF-α producing cells increased following conditioning (Fig. 5C). The ability to produce IL-2 and proliferate while inhibiting other T cells contrasts the suppressive properties of these CD8regs from CD4regs. Since IL-10 has an important role in the mechanism of natural and induced human CD8reg subsets[32, 33], we assessed IL-10 production, but only documented low levels (results not shown).
One of the principal activities of CD8+ cells is to recognize and kill MHC I non-identical cells. It was especially important, therefore, to investigate the possible cytotoxic activity of these CD8regs. We used a method similar to that described in our report that human naïve CD4+ cells alloactivated with IL-2 and TGF-β developed the capacity to suppress CD8+ cells from becoming killer cells [21]. We stimulated CD8+ cells with allogeneic non-T cells or mature dendritic cells, and assessed killer activity against CFSE-labeled matched allogeneic concanavalin A activated T cells. Instead of using radioisotopes, however, we documented apoptotic cell death by target cells double stained by annexin V and 7AAD [24]. The FACS profile and baseline staining is shown in Supplementary Fig. 1. Target cell death following 4 hour incubation with CD8 subsets, and specific cytotoxic activity calculated by a formula indicated in the Methods, is shown in Fig. 6. Neither CD8+ cells activated with or without TGF-β acquired to ability to kill allogeneic human T lymphoblasts. (Fig. 6A). This was not surprising since others have shown that anti-CD3 and anti-CD3/28 induced CD8+ cell killing of lymphoblast–like P815 cells [29] or tumor target cells [34, 35] is Fc receptor dependent. By contrast, Mazur et al failed to induce naïve CD8+ cord blood cells expanded with anti-CD3/28 to become killer cells [36]. Thus, anti-CD3/28 antibodies induce IL-2 production and proliferative activity, but not strong cytolytic activity. Although allogeneic stimulator cells can induce naïve CD8+ cells to become antigen-specific killer cells without CD4 cell help [37] (Fig. 6 A,B), cytotoxic activity of the anti-CD3/28 induced CD8regs remained negligible even after subsequent allo-sensitization of (Fig. 6B).
Figure 6. Unlike alloantigen-stimulated naïve CD8+ cells, CD8regs lack cytotoxic activity against allogeneic T cells.
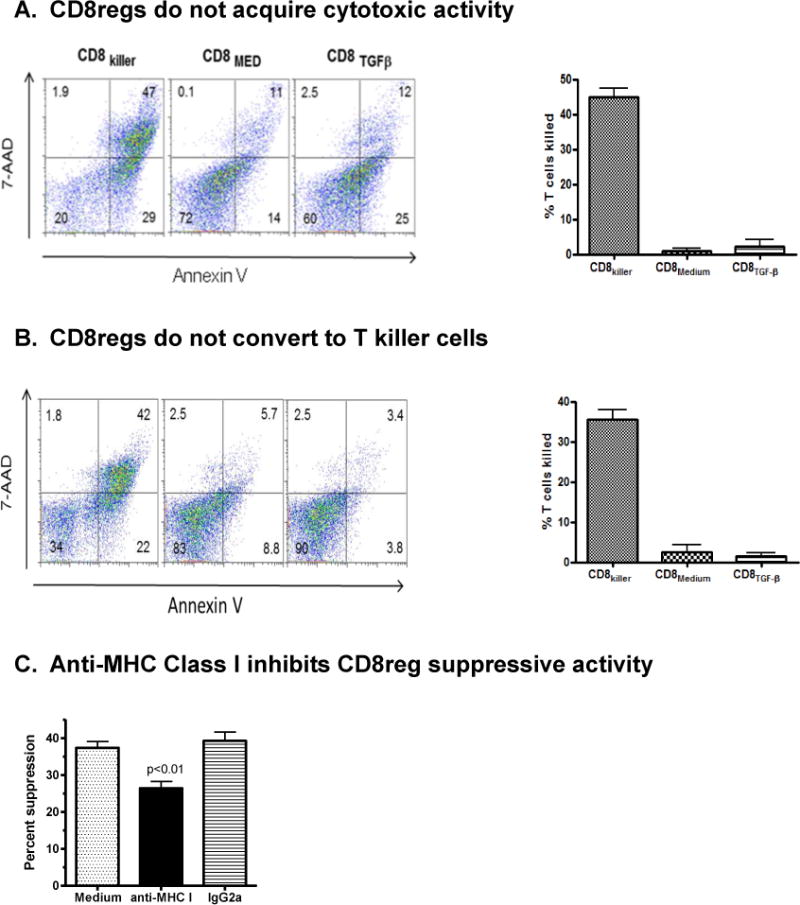
A. Naïve CD8 cells were cultured ratio for 7 days with CD3/CD28 beads, IL-2 and with or without TGF-β to generate CD8regs. CD8 killer cells were generated by culturing CD8 cells with allogeneic mature dendritic cells at a 30:1 T cell:DC ratio. Each CD8 cell subset was then mixed with CFSE-labeled concanavalin activated T cells from the DC donor for 4 hours, at a 30:1 effector to target cell ratio. Killing of target cells defined by CFSE-labeled cells double stained for Annexin V and 7-AAD was then determined by flow cytometry. Shown is representative example. Scatter plots showing the characteristics of the controls used is shown in Supplementary Fig. 1. The bar graph indicates the mean and SEM of specific killing for each CD8 subset of the four healthy donors studied. This was calculated using the formula given in Methods. P values, <0.001, compared CD8killer with each CD8reg subset.
B. To determine whether CD8regs can be converted into cytotoxic killer cells by allogeneic stimulation, CD8regs were harvested at day 7, then co-cultured with irradiated allogeneic non-T cells for 6 days or DCs and examined for cytotoxity as described above. A representative example of Annexin V and 7AAD staining from one of three healthy donors studied and a summary of the calculated CTL activity of the three donors is shown in the bar graph. In these experiments the positive controls were CD8 cells stimulated with allogeneic non-T cells for 6 days. The flow cytometry data for baseline and maximal annexin V and 7AAD staining is shown in supplemental Fig. 1.
C. To confirm that that CD8regs could recognize foreign alloantigens expressed by the target CD4 cells, anti-human HLA A,B,C or isotype control IgG2a (10mg/ml) was added to the in vitro suppressor assay using the protocol described in Fig. 4A. The bar graph shows the mean ± SEM suppressive activity of three donors. Anti-HLA class I antibodies significantly decreased suppressive activity compared with control IgG (p.<0.01).
3.5 Characteristic expression of TNFR2 and PDL-1 by these CD8regs
As indicated above, CD8+ cells stimulated with anti-CD3/28 beads rapidly expressed both TNFR2 and PDL-1. Unlike the rapid expression of Foxp3, however, this was not dependent on TGF-β (Fig 3A). To determine the significance of this finding, we isolated CD8 cells single and double positive cells expressing these receptors by cell sorting and assessed the suppressive activity of these cells and control sham sorted and double negative cells. Since, most cells expressed TNFR2 and PDL-1 by day 4 (result not shown), we sorted CD8+ cells bearing these receptors after 2 days of culture. Their FACS profile is shown in Figure 7A. In three separate experiments we observed that TNFR2 PDL-1 double positive cells exhibited much stronger suppressive activity than control sham sorted cells. One of these is shown in (Fig. 7B). Either TNFR2 or PDL-1 single positive cells had modest activity, but the double negative cells had none. The results were similar whether or not the CD8+ cells were conditioned with TGF-β. Therefore, these CD8regs characteristically express TNFR2 and PD-L1.
Figure 7. The role of TNFR2 and PD-L1 displayed by CD8Medium and CD8TGF-β in the generation and expression of suppressive activity.
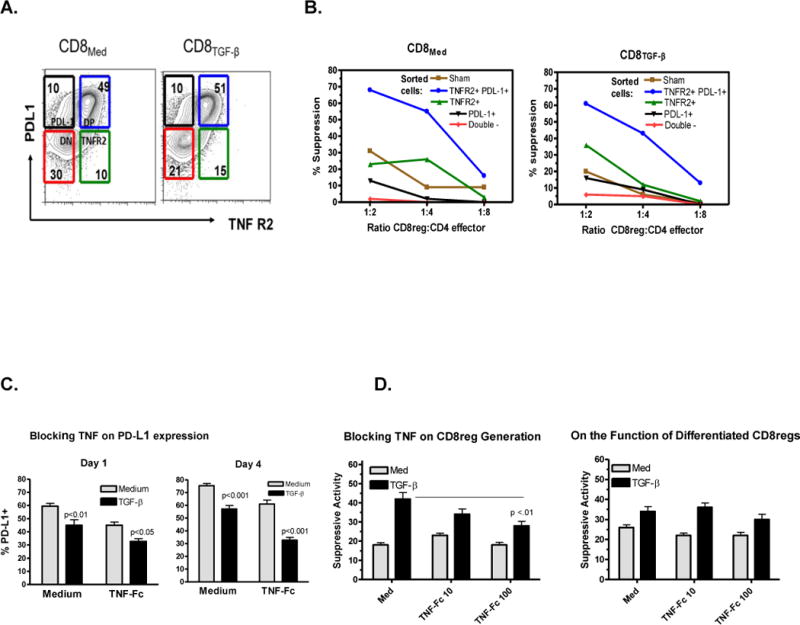
A. Rapid expression of both PD-L1 and TNFR2. CD8+ cells were stimulated with anti-CD3/28 beads and IL-2 ± TGF-β as described above for 2 days, stained for TNFR2 and PD-L1, and sorted into TNFR2+ PD-L1+, TNFR2+ PD-L1−, TNFR2− PD-L1+, and TNFR2− PD-L1− fractions by cell sorting. Each fraction was tested for suppressive activity in vitro. An additional control was sham sorted cells.
B. Suppressive activity of each sorted CD8+ cell subset on allogeneic CD4 responder cell proliferation. The assay was set up and performed at various suppressor to responder cell ratios as described above. The results shown are representative of three separate experiments where the TNFR2/PD-L1 double positive cells had markedly stronger suppressive activity than sham sorted controls (p<0.01), and the double negate cells lacked any activity.
C. TNF upregulates PD-L1: CD8 cells were stimulated ± TGF-β as described above with TNFR2-Fc (50 ug/ml) and examined for PD-L1 expression after culture for 1 or 4 days. The bars indicate the mean ± SEM of three separate experiments. P values are indicated.
D. Effect of blocking TNF in the generation of CD8regs and the suppressive activity of differentiated CD8regs. The doses of TNFR2-Fc added are indicated using protocols described above. The bars indicate the mean ± SEM of three separate experiments. P values are indicated.
We next looked for a relationship between TNFR2 and PD-L1. We obtained evidence that TNF upregulated PD-L1 expression. As shown in Fig. 7C, blocking TNF with soluble TNF receptors (TNFR2-Fc) significantly decreased PD-L1 expression. When these CD8 cells were tested for functional activity, there was a significant decrease in TGF-β induced enhancement of suppressive activity (Fig. 7D). By contrast, adding TNFR2-Fc to fully differentiated CD8regs did not affect their suppressive activity. We have also added anti-PD-L1 to CD8regs across a wide dose range (0.1 to 20mg/ml), but have not observed consistent effects on suppressive activity (results not shown).
4. Discussion
Our finding that polyclonal human CD8regs generated ex-vivo with anti-CD3/28 beads, IL-2 and TGF-β preferentially targeted allogeneic T effector cells, and had markedly greater protective activity in vivo than that reported for CD4regs may have clinical relevance. The functional properties of natural and induced human CD8 subsets have been generally assessed by in vitro suppressor assays [11, 12]. However, since these methods may not correlate well with protective effects in vivo [25], we and others have used a human anti-mouse GVHD to assess human Treg activity. These studies have indicated that both expanded endogenous CD4regs and those induced ex-vivo can double the survival of the mice [17, 38–41], (see Table 1). CD8regs quadrupled survival. Independently, another group has just reported that allogeneic CD8regs generated by another method markedly inhibit GVHD in a humanized mouse model [42].
The present CD8regs have a characteristic phenotype. Besides markers associated with Tregs such as Foxp3, CD25, CD122, CTLA-4, and TNFR2, these Tregs expressed the negative co-stimulator receptors PD-L1, PD-1, and Tim3. These negative co-stimulatory receptors are characteristically expressed by “exhausted” CD8+ cells following certain viral infections [43]. However, while IL-2 receptors become dim on exhausted CD8+ cells [43], the CD8regs described here are CD25bright and TGF-β enhanced CD122 expression. The autoantibody-suppressing CD8regs that appear in lupus patients following autologous stem cell transplantation have a phenotype quite similar to the CD8regs we have induced ex-vivo. They express Foxp3, PD-1, PD-L1, CTLA-4 and CD103 [15]. It is likely that the similar profile of negative co-stimulatory receptors expressed by CD8regs induced with and without TGF-β relates to their similar protective effects.
The present study suggests that both IL-2 and TGF-β are needed for the generation of clinically useful human CD8regs. This is consistent with the essential role of these cytokines in the generation and maintenance of CD4+Foxp3+ Tregs [44, 45]. As with human CD4regs, TGF-β enhanced Foxp3 expression by CD8+ cells, an effect that was IL-2 dependent. The percentage of Foxp3+ cells expressed by the present CD8regs induced with anti-CD3/28 beads was markedly higher than reported by others Those who have used SEB or anti-CD3 ± anti-CD28 have observed <30% of human CD8+Foxp3+ cells even with TGF-β [46–48]. Others who expanded CD8+ CCR7+ cells with IL-15 reported that these cells were Foxp3+ [49]. Our range was 45 to 65% which we believe is due to the combination of anti-CD3 and anti-CD28 immobilized on beads and frequent IL-2 supplementation.
Since activated human T cells can transiently express Foxp3 [50], it has not been established whether this transcription factor has the same essential role in generating human CD8regs that it presumably has in mice. This may not be the case. In this study, Foxp3 expressed by CD8+ cells did not correlate with suppressive activity in vitro or in vivo and required exogenous IL-2 to maintain expression. In addition, others have reported TCR stimulated mouse CD8+ cells that express Foxp3 lack suppressive activity [51]. Finally, using GFP to sort mouse Foxp3+ cells, we have observed that both TGF-β induced Foxp3+ and Foxp3− CD8 cells have equivalent protective activity in vivo [52]. However, we cannot exclude the possibility that the Foxp3+ cells accounted for most of the suppressive activity observed.
TGF-β had other important effects on the human CD8+ cells besides enhancing Foxp3 expression. First, TGF-β induced CD8 cells to express CD103. Another group has also reported this finding, but they described alloantigen-induced CD8+CD103+CD28− cells that were predominantly antigen-specific [11]. CD8+CD103 Tregs may traffic to skin and mucous membranes [53]. Although CD8+CD28− cells possess suppressive activity [54] and can comprise 1/3 of isolated CD8+ cells, following anti-CD3/28 stimulation almost all of the cells harvested were CD28+ (Fig. 3). Second, TGF-β was required for the maintenance of function in vitro. CD8regs induced with TGF-β expanded at least 10 fold with IL-2 supplements and maintained their suppressive activity for at least 4 weeks (results not shown). Thirdly, blocking TGF-β signaling in vivo diminished the protective effect of TGF-β conditioned CD8regs. The increased stability of these CD8regs may endow them to have even more protective function in established disease than in disease prevention [27].
Our TNF blocking studies with TNFR2-Fc strongly correlating TNFR2 with the generation of suppressive activity suggests that this receptor may have the same important functional role for CD8regs as it does for both murine and human CD4regs [55]. Human thymic CD8+Foxp3+ Tregs also express TNFR2, are anergic in vitro, and do not produce cytokines [9]. The induced CD8regs described here are unlike their thymic counterparts in that they produce IL-2 and TNF, and proliferate in response to TCR stimulation in vitro. Others have concluded that TNFR2 expressed by CD8regs induced with anti-CD3 correlated better than CD25 with suppressive activity and, thus, is a marker for this potent Treg subpopulation [48]. The TNF signaling through TNFR2 that generates these CD8regs, therefore, may serve to counterbalance the well-known pro-inflammatory effects of this cytokine. Blocking TNF by anti-TNF therapy in rheumatoid arthritis also induces CD4regs that may restrain the progression of IL-17-associated inflammation in RA via regulation of monocyte-derived IL-6 [56].
The rapid induction of both TNFR2 and PD-L1 on CD8 cells has not been reported previously. Both PD-1 and PD-L1 are instrumental in the maintenance of peripheral tolerance [57]. Here TGF-β enhanced PD-1 expression by anti-CD3/28 stimulated CD8+ cells, and our evidence that TNF enhanced PD-L1 expression on CD8regs is a novel observation. Recently, others have reported that blocking TNF decreased PD-L1 expression by monocytes [58]. Previously others have reported that overexpressing PD-L1 in Th1 cells converted these cells to CD4regs [59]. In this study anti-PD-L1 did not affect in vitro suppressive activity. Others have reported a relationship between PD-L1 and IL-10 production [60, 61], and in this study the suppressive activity of the CD8regs in vivo was IL-10 dependent.
Although CD8+ cells can generally recognize and kill allogeneic target cells, we found that the CD8regs generated in this study were unable to kill activated T cells. While it is well known that TGF-β inhibits granzyme expression and the development of killer cells [62], in this study, CD8regs induced without TGF-β also lacked cytotoxic effects. This is consistent with the results of others who have induced polyclonal CD8regs ex-vivo [46–49, 62, 63]. Even anti-CD3 induced CD8regs that expressed TNFR2 had a non-cytotoxic mechanism of action [64]. By contrast, cytotoxic CD8regs recognizing MHC class I determinants have generally express NKG2A [10, 11]. This marker was not expressed by our CD8regs (result not shown).
The mechanism of action of these CD8regs remains to be defined. Because of the observed plasticity of Tregs [1], we considered the possibility that non-cytotoxic CD8regs could become killer cells following exposure to allogeneic cells. However, cytotoxic effects were not observed. Although the in vivo protective activity of these CD8regs was IL-10 dependent, we detected only low levels of IL-10 in vitro. It is possible, however, that the interaction of one or more of the negative co-stimulatory molecules expressed by the CD8regs with their respective ligands on other cells could induce these cells to produce IL-10. Clearly, CD8regs that target allogeneic cells may have an important biological function. As stated above, not all allogeneic CD8+ cells derived from stem cell transplants cause GVHD. Some become CD8+Foxp3+ Tregs and are not rejected [18–20]. One group reported that these CD8regs were even more potent than CD4regs [19].
There are limitations to this study. Because GVHD in the xenograft system is CD4 dependent [65] and their activation is dependent upon cross presentation by the human antigen-presenting cells transferred, this is not a good model to study human class I restricted responses. We have used this model in humanized mice because in vitro suppressive assays may not reflect protective activity of Tregs in vivo. Another group, however, has recently humanized mice with PBMC and reported that allo-specific CD8regs generated with CD40-activated B cells protected these mice from donor T cell-induced anti-human GVHD. This model better reflects human immunemediated disease. Importantly, these CD8regs induced long-term tolerance effectively without compromising general immunity [42]. Although generated differently, the CD8regs reported by these investigators expressed Foxp3, CD28 and CTLA-4 and required IL-2 for induction and maintenance.
There are also limitations of mouse models to study human immune regulation. We and others have previously observed that although IL-2 and TGF-β induce mouse CD4 cells to become Tregs that are protective in vivo [66], another agent such as retinoic acid must be added to this combination to rapidly induce human naïve CD4+ cells to have similar activity [17]. In the present experiments, the preferential effect of human polyclonally-induced CD8regs on allogeneic T cells is not observed in mouse models.
5. Conclusions
The finding that the polyclonal CD8regs generated with anti-CD3/28 beads preferentially target allogeneic cells raise the possibility that Tregs from an MHC class I mismatched donor could be used for T cell immunotherapy. Allogeneic cells transferred from one unrelated individual to another should be rejected. However, since the suppressive activity of the transferred CD8regs may be continuously strengthened by contact with the recipient’s cells, they could prevent the recipient from mounting an immune response against them. If appropriately MHC class I mismatched CD8regs Tregs can be administered without being rejected and maintain their protective activity without serious adverse side effects, they have the potential to revolutionize our treatment of autoimmune diseases, graft-versus-host disease, and allograft rejection.
Supplementary Material
Highlights.
A method to generate polyclonal human CD8regs rapidly ex-vivo that preferentially target allogeneic cells.
Evidence that these CD8regs had stronger protective activity in vivo than that reported for CD4regs
Expression of TNFR2 and PD-L1 correlated better than Foxp3 with non-cytotoxic suppressive activity.
The possibility that allogeneic polyclonal Tregs can be used as a therapeutic product
Acknowledgments
This study was supported in part by grants from the National Institutes of Health (R43 AI084359), the Arthritis Foundation, Southern California Chapter, Athelos-Neostem Inc., the Treadwell Foundation and funds from a Schwab Charitable Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authorship Contributions:
David A. Horwitz: Designed research, analyzed data, and wrote the manuscript Stephanie Pan: Performed research
Julie Wang: Performed research
Jing-Ni Oh: Performed research and analyzed data
Moegan Chen: Analyzed data
Song Guo Zheng: Supervised research, analyzed data, critiqued manuscript
J. Dixon Gray: Performed research, analyzed data, critiqued manuscript
Conflict of Interest: Dr. Horwitz is a consultant for Athelos, a company that partially funded the research.
References
- 1.Miyara M, Wing K, Sakaguchi S. Therapeutic approaches to allergy and autoimmunity based on FoxP3+ regulatory T-cell activation and expansion. J Allergy Clin Immunol. 2009;123:749–755. doi: 10.1016/j.jaci.2009.03.001. quiz 756–747. [DOI] [PubMed] [Google Scholar]
- 2.Rosenblum MD, Gratz IK, Paw JS, Abbas AK. Treating human autoimmunity: current practice and future prospects. Science translational medicine. 2012;4:125sr121. doi: 10.1126/scitranslmed.3003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann P, Boeld TJ, Eder R, Huehn J, Floess S, Wieczorek G, Olek S, Dietmaier W, Andreesen R, Edinger M. Loss of FOXP3 expression in natural human CD4+CD25+ regulatory T cells upon repetitive in vitro stimulation. Eur J Immunol. 2009;39:1088–1097. doi: 10.1002/eji.200838904. [DOI] [PubMed] [Google Scholar]
- 4.Afzali B, Mitchell PJ, Scotta C, Canavan J, Edozie FC, Fazekasova H, Lord GM, John S, Barber LD, Hernandez-Fuentes MP, Lechler RI, Lombardi G. Relative resistance of human CD4(+) memory T cells to suppression by CD4(+) CD25(+) regulatory T cells. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1734–1742. doi: 10.1111/j.1600-6143.2011.03635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, Wood KJ, Turka LA, Jones ND. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci U S A. 2007;104:19954–19959. doi: 10.1073/pnas.0704397104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmann M, Beverley PC, Dunkley M, Kontiainen S. Different Ly antigen phenotypes of in vitro induced helper and suppressor cells. Nature. 1975;258:614–616. doi: 10.1038/258614a0. [DOI] [PubMed] [Google Scholar]
- 7.Cantor H, Shen FW, Boyse EA. Separation of helper T cells from suppressor T cells expressing different Ly components. II. Activation by antigen: after immunization, antigen-specific suppressor and helper activities are mediated by distinct T-cell subclasses. J Exp Med. 1976;143:1391–1340. doi: 10.1084/jem.143.6.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapp JA, Bucy RP. CD8+ suppressor T cells resurrected. Human immunology. 2008;69:715–720. doi: 10.1016/j.humimm.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 9.Cosmi L, Liotta F, Lazzeri E, Francalanci M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S, Annunziato F. Human CD8+CD25+ thymocytes share phenotypic and functional features with CD4+CD25+ regulatory thymocytes. Blood. 2003;102:4107–4114. doi: 10.1182/blood-2003-04-1320. [DOI] [PubMed] [Google Scholar]
- 10.Lu L, Cantor H. Generation and regulation of CD8(+) regulatory T cells. Cellular & molecular immunology. 2008;5:401–406. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koch SD, Uss E, van Lier RA, ten Berge IJ. Alloantigen-induced regulatory CD8+CD103+ T cells. Human immunology. 2008;69:737–744. doi: 10.1016/j.humimm.2008.08.281. [DOI] [PubMed] [Google Scholar]
- 12.Konya C, Goronzy JJ, Weyand CM. Treating autoimmune disease by targeting CD8(+) T suppressor cells. Expert opinion on biological therapy. 2009;9:951–965. doi: 10.1517/14712590903020759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ciubotariu R, Vasilescu R, Ho E, Cinti P, Cancedda C, Poli L, Late M, Liu Z, Berloco P, Cortesini R, Suciu-Foca Cortesini N. Detection of T suppressor cells in patients with organ allografts. Human immunology. 2001;62:15–20. doi: 10.1016/s0198-8859(00)00226-3. [DOI] [PubMed] [Google Scholar]
- 14.Bisikirska B, Colgan J, Luban J, Bluestone JA, Herold KC. TCR stimulation with modified anti-CD3 mAb expands CD8+ T cell population and induces CD8+CD25+ Tregs. The Journal of clinical investigation. 2005;115:2904–2913. doi: 10.1172/JCI23961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Bertucci AM, Ramsey-Goldman R, Burt RK, Datta SK. Regulatory T cell (Treg) subsets return in patients with refractory lupus following stem cell transplantation, and TGF-beta-producing CD8+ Treg cells are associated with immunological remission of lupus. J Immunol. 2009;183:6346–6358. doi: 10.4049/jimmunol.0901773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gray JD, Hirokawa M, Horwitz DA. The role of transforming growth factor beta in the generation of suppression: an interaction between CD8+ T and NK cells. J Exp Med. 1994;180:1937–1942. doi: 10.1084/jem.180.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu L, Zhou X, Wang J, Zheng SG, Horwitz DA. Characterization of protective human CD4CD25 FOXP3 regulatory T cells generated with IL-2, TGF-beta and retinoic acid. PLoS One. 2010;5:e15150. doi: 10.1371/journal.pone.0015150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, Tanaka Y, Kambayashi T. Cell-autonomous role of TGFbeta and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood. 2012;119:5575–5583. doi: 10.1182/blood-2011-07-367987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robb RJ, Lineburg KE, Kuns RD, Wilson YA, Raffelt NC, Olver SD, Varelias A, Alexander KA, Teal BE, Sparwasser T, Hammerling GJ, Markey KA, Koyama M, Clouston AD, Engwerda CR, Hill GR, MacDonald KP. Identification and expansion of highly suppressive CD8(+)FoxP3(+) regulatory T cells after experimental allogeneic bone marrow transplantation. Blood. 2012;119:5898–5908. doi: 10.1182/blood-2011-12-396119. [DOI] [PubMed] [Google Scholar]
- 20.Beres AJ, Haribhai D, Chadwick AC, Gonyo PJ, Williams CB, Drobyski WR. CD8+ Foxp3+ regulatory T cells are induced during graft-versus-host disease and mitigate disease severity. J Immunol. 2012;189:464–474. doi: 10.4049/jimmunol.1200886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamagiwa S, Gray JD, Hashimoto S, Horwitz DA. A role for TGF-beta in the generation and expansion of CD4+CD25+ regulatory T cells from human peripheral blood. J Immunol. 2001;166:7282–7289. doi: 10.4049/jimmunol.166.12.7282. [DOI] [PubMed] [Google Scholar]
- 22.Mutis T, van Rijn RS, Simonetti ER, Aarts-Riemens T, Emmelot ME, van Bloois L, Martens A, Verdonck LF, Ebeling SB. Human regulatory T cells control xenogeneic graft-versus-host disease induced by autologous T cells in RAG2−/−gammac−/− immunodeficient mice. Clinical cancer research: an official journal of the American Association for Cancer Research. 2006;12:5520–5525. doi: 10.1158/1078-0432.CCR-06-0035. [DOI] [PubMed] [Google Scholar]
- 23.O’Neill DW, Bhardwaj N. Differentiation of peripheral blood monocytes into dendritic cells. In: Coligan John E, et al., editors. Current protocols in immunology. 2005. Chapter 22. Unit 22F 24. [DOI] [PubMed] [Google Scholar]
- 24.Derby E, Reddy V, Kopp W, Nelson E, Baseler M, Sayers T, Malyguine A. Three-color flow cytometric assay for the study of the mechanisms of cell-mediated cytotoxicity. Immunology letters. 2001;78:35–39. doi: 10.1016/s0165-2478(01)00226-7. [DOI] [PubMed] [Google Scholar]
- 25.Tang Q, Henriksen KJ, Bi M, Finger EB, Szot G, Ye J, Masteller EL, McDevitt H, Bonyhadi M, Bluestone JA. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng SG, Wang JH, Koss MN, Quismorio F, Jr, Gray JD, Horwitz DA. CD4+ and CD8+ regulatory T cells generated ex vivo with IL-2 and TGF-beta suppress a stimulatory graft-versus-host disease with a lupus-like syndrome. J Immunol. 2004;172:1531–1539. doi: 10.4049/jimmunol.172.3.1531. [DOI] [PubMed] [Google Scholar]
- 27.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 28.Wang D, Yuan R, Feng Y, El-Asady R, Farber DL, Gress RE, Lucas PJ, Hadley GA. Regulation of CD103 expression by CD8+ T cells responding to renal allografts. J Immunol. 2004;172:214–221. doi: 10.4049/jimmunol.172.1.214. [DOI] [PubMed] [Google Scholar]
- 29.De Jong R, Brouwer M, Rebel VI, Van Seventer GA, Miedema F, Van Lier RA. Generation of alloreactive cytolytic T lymphocytes by immobilized anti-CD3 monoclonal antibodies. Analysis of requirements for human cytolytic T-lymphocyte differentiation. Immunology. 1990;70:357–364. [PMC free article] [PubMed] [Google Scholar]
- 30.Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nature immunology. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- 31.Shevach EM. Biological functions of regulatory T cells. Advances in immunology. 2011;112:137–176. doi: 10.1016/B978-0-12-387827-4.00004-8. [DOI] [PubMed] [Google Scholar]
- 32.Rifa’i M, Shi Z, Zhang SY, Lee YH, Shiku H, Isobe K, Suzuki H. CD8+CD122+ regulatory T cells recognize activated T cells via conventional MHC class I-alphabetaTCR interaction and become IL-10-producing active regulatory cells. International immunology. 2008;20:937–947. doi: 10.1093/intimm/dxn052. [DOI] [PubMed] [Google Scholar]
- 33.Gilliet M, Liu YJ. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J Exp Med. 2002;195:695–704. doi: 10.1084/jem.20011603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flens MJ, Mulder WM, Bril H, von Blomberg van de Flier MB, Scheper RJ, van Lier RA. Efficient expansion of tumor-infiltrating lymphocytes from solid tumors by stimulation with combined CD3 and CD28 monoclonal antibodies. Cancer immunology, immunotherapy: CII. 1993;37:323–328. doi: 10.1007/BF01518455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum S, Milesi R, Tratkiewicz J, Olive D, Gallati H, Cerottini JC, von Fliedner V. Rapid induction of cytolytic T cells via CD28 stimulation for cellular immunotherapy. Therapeutic immunology. 1994;1:143–152. [PubMed] [Google Scholar]
- 36.Mazur MA, Davis CC, Szabolcs P. Ex vivo expansion and Th1/Tc1 maturation of umbilical cord blood T cells by CD3/CD28 costimulation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14:1190–1196. doi: 10.1016/j.bbmt.2008.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young JW, Steinman RM. Dendritic cells stimulate primary human cytolytic lymphocyte responses in the absence of CD4+ helper T cells. J Exp Med. 1990;171:1315–1332. doi: 10.1084/jem.171.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carroll RG, Carpenito C, Shan X, Danet-Desnoyers G, Liu R, Jiang S, Albelda SM, Golovina T, Coukos G, Riley JL, Jonak ZL, June CH. Distinct effects of IL-18 on the engraftment and function of human effector CD8 T cells and regulatory T cells. PLoS One. 2008;3:e3289. doi: 10.1371/journal.pone.0003289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR. Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. American journal of transplantation: official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2011;11:1148–1157. doi: 10.1111/j.1600-6143.2011.03558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR. Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Science translational medicine. 2011;3:83ra41. doi: 10.1126/scitranslmed.3001809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Amarnath S, Costanzo CM, Mariotti J, Ullman JL, Telford WG, Kapoor V, Riley JL, Levine BL, June CH, Fong T, Warner NL, Fowler DH. Regulatory T cells and human myeloid dendritic cells promote tolerance via programmed death ligand-1. PLoS biology. 2010;8:e1000302. doi: 10.1371/journal.pbio.1000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zheng J, Liu Y, Liu Y, Liu M, Xiang Z, Lam KT, Lewis DB, Lau YL, Tu W. Human CD8+ regulatory T cells inhibit GVHD and preserve general immunity in humanized mice. Science translational medicine. 2013;5:168ra169. doi: 10.1126/scitranslmed.3004943. [DOI] [PubMed] [Google Scholar]
- 43.Wherry EJ. T cell exhaustion. Nature immunology. 2011;12:492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 44.Furtado GC, Curotto de Lafaille MA, Kutchukhidze N, Lafaille JJ. Interleukin 2 signaling is required for CD4(+) regulatory T cell function. J Exp Med. 2002;196:851–857. doi: 10.1084/jem.20020190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Horwitz DA, Zheng SG, Wang J, Gray JD. Critical role of IL-2 and TGF-beta in generation, function and stabilization of Foxp3(+)CD4(+) Treg. Eur J Immunol. 2008;38:912–915. doi: 10.1002/eji.200738109. [DOI] [PubMed] [Google Scholar]
- 46.Mahic M, Henjum K, Yaqub S, Bjornbeth BA, Torgersen KM, Tasken K, Aandahl EM. Generation of highly suppressive adaptive CD8(+)CD25(+)FOXP3(+) regulatory T cells by continuous antigen stimulation. Eur J Immunol. 2008;38:640–646. doi: 10.1002/eji.200737529. [DOI] [PubMed] [Google Scholar]
- 47.Siegmund K, Ruckert B, Ouaked N, Burgler S, Speiser A, Akdis CA, Schmidt-Weber CB. Unique phenotype of human tonsillar and in vitro-induced FOXP3+CD8+ T cells. J Immunol. 2009;182:2124–2130. doi: 10.4049/jimmunol.0802271. [DOI] [PubMed] [Google Scholar]
- 48.Ablamunits V, Bisikirska B, Herold KC. Acquisition of regulatory function by human CD8(+) T cells treated with anti-CD3 antibody requires TNF. Eur J Immunol. 2010;40:2891–2901. doi: 10.1002/eji.201040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suzuki M, Jagger AL, Konya C, Shimojima Y, Pryshchep S, Goronzy JJ, Weyand CM. CD8+CD45RA+CCR7+FOXP3+ T cells with immunosuppressive properties: a novel subset of inducible human regulatory T cells. J Immunol. 2012;189:2118–2130. doi: 10.4049/jimmunol.1200122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gavin MA, Torgerson TR, Houston E, DeRoos P, Ho WY, Stray-Pedersen A, Ocheltree EL, Greenberg PD, Ochs HD, Rudensky AY. Single-cell analysis of normal and FOXP3-mutant human T cells: FOXP3 expression without regulatory T cell development. Proc Natl Acad Sci U S A. 2006;103:6659–6664. doi: 10.1073/pnas.0509484103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayer CT, Floess S, Baru AM, Lahl K, Huehn J, Sparwasser T. CD8+ Foxp3+ T cells share developmental and phenotypic features with classical CD4+ Foxp3+ regulatory T cells but lack potent suppressive activity. Eur J Immunol. 2011;41:716–725. doi: 10.1002/eji.201040913. [DOI] [PubMed] [Google Scholar]
- 52.Ya LX, An-Ping, Horwitz DA, Zheng SG. CD4+Foxp3+CD103+ Regulatory Cells Generated ex-vivo with TGF-beta suppress autoimmunity through IL-10 dependent Mechanisms. Arthritis and rheumatism. 2012;64:S1057–S1057. [Google Scholar]
- 53.Jenkinson SE, Whawell SA, Swales BM, Corps EM, Kilshaw PJ, Farthing PM. The alphaE(CD103)beta7 integrin interacts with oral and skin keratinocytes in an E-cadherin-independent manner*. Immunology. 2011;132:188–196. doi: 10.1111/j.1365-2567.2010.03352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Suciu-Foca N, Manavalan JS, Cortesini R. Generation and function of antigen-specific suppressor and regulatory T cells. Transplant immunology. 2003;11:235–244. doi: 10.1016/S0966-3274(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 55.Chen X, Subleski JJ, Hamano R, Howard OM, Wiltrout RH, Oppenheim JJ. Co-expression of TNFR2 and CD25 identifies more of the functional CD4+FOXP3+ regulatory T cells in human peripheral blood. Eur J Immunol. 2010;40:1099–1106. doi: 10.1002/eji.200940022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang XA, Zhang R, Zhang S, Deng S, Jiang D, Zhong J, Yang L, Wang T, Hong S, Guo S, She ZG, Zhang XD, Li H. Interferon Regulatory Factor 7 Deficiency Prevents Diet-induced Obesity and Insulin Resistance. American journal of physiology. Endocrinology and metabolism. 2013 doi: 10.1152/ajpendo.00505.2012. [DOI] [PubMed] [Google Scholar]
- 57.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ou JN, Wiedeman AE, Stevens AM. TNF-alpha and TGF-beta Counter-Regulate PD-L1 Expression on Monocytes in Systemic Lupus Erythematosus. Scientific reports. 2012;2:295. doi: 10.1038/srep00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Amarnath S, Mangus CW, Wang JC, Wei F, He A, Kapoor V, Foley JE, Massey PR, Felizardo TC, Riley JL, Levine BL, June CH, Medin JA, Fowler DH. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Science translational medicine. 2011;3:111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fujimura T, Ring S, Umansky V, Mahnke K, Enk AH. Regulatory T cells stimulate B7-H1 expression in myeloid-derived suppressor cells in ret melanomas. The Journal of investigative dermatology. 2012;132:1239–1246. doi: 10.1038/jid.2011.416. [DOI] [PubMed] [Google Scholar]
- 61.Geng L, Deng J, Jiang G, Song P, Wang Z, Jiang Z, Zhang M, Zheng S. B7-H1 up-regulated expression in human hepatocellular carcinoma tissue: correlation with tumor interleukin-10 levels. Hepato-gastroenterology. 2011;58:960–964. [PubMed] [Google Scholar]
- 62.Kapp JA, Honjo K, Kapp LM, Xu X, Cozier A, Bucy RP. TCR transgenic CD8+ T cells activated in the presence of TGFbeta express FoxP3 and mediate linked suppression of primary immune responses and cardiac allograft rejection. International immunology. 2006;18:1549–1562. doi: 10.1093/intimm/dxl088. [DOI] [PubMed] [Google Scholar]
- 63.Smyth MJ, Strobl SL, Young HA, Ortaldo JR, Ochoa AC. Regulation of lymphokine-activated killer activity and pore-forming protein gene expression in human peripheral blood CD8+ T lymphocytes. Inhibition by transforming growth factor-beta. J Immunol. 1991;146:3289–3297. [PubMed] [Google Scholar]
- 64.Ablamunits V, Herold KC. Generation and function of human regulatory CD8+ T cells induced by a humanized OKT3 monoclonal antibody hOKT3gamma1 (Ala-Ala) Human immunology. 2008;69:732–736. doi: 10.1016/j.humimm.2008.08.290. [DOI] [PubMed] [Google Scholar]
- 65.Wilson J, Cullup H, Lourie R, Sheng Y, Palkova A, Radford KJ, Dickinson AM, Rice AM, Hart DN, Munster DJ. Antibody to the dendritic cell surface activation antigen CD83 prevents acute graft-versus-host disease. J Exp Med. 2009;206:387–398. doi: 10.1084/jem.20070723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Horwitz DA, Zheng SG, Gray JD. Natural and TGF-beta-induced Foxp3(+)CD4(+) CD25(+) regulatory T cells are not mirror images of each other. Trends Immunol. 2008;29:429–435. doi: 10.1016/j.it.2008.06.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


