Abstract
Objective
This study evaluated whether worsened outcomes in sex mismatch are related to mismatch of organ size in heart transplantation.
Background
Sizing for organ allocation in heart transplantation currently incorporates only body weight differences between the donor and recipient. Weight correlates poorly to cardiac size, and donor–recipient weight differences are not associated with differential survival. Heart size correlates with sex, and donor–recipient sex mismatch conveys worse-than-expected outcomes.
Methods
We performed a retrospective cohort study of 31,634 donor–recipient adult heart transplant pairings from the United Network for Organ Sharing transplantation registry. We used predictive models to calculate the predicted total heart mass (pHM) for recipient and donor pairs. We assessed organ size mismatch by calculating the percent difference between the donor and recipient pHM as [(pHMrecipient − pHMdonor)/(pHMrecipient)]*100.
Results
The most-undersized pHM septile demonstrated higher mortality during the first year post-transplantation (hazard ratio [HR]: 1.27; p < 0.001), which remained robust in adjusted models (HR: 1.25; p = 0.03). Survival did not vary across septiles of weight differences. On univariate analysis, sex mismatch was associated with higher mortality in male patients, but not in female patients. Controlling for differences in pHM reversed these associations. Adjusted models demonstrated worse survival associated with sex mismatch in female patients (1-year HR: 1.28; p = 0.02) but no difference in male patients (1-year HR, 1.00; p = 1.0).
Conclusions
Differences in donor–recipient pHM modulated the survival associated with donor–recipient sex mismatch and identified donor heart undersizing as an otherwise occult and potentially preventable cause of mortality following orthotopic heart transplantation.
Keywords: heart size mismatch, heart transplant outcomes, heart transplantation, sex matching in transplantation
Over 3,700 people worldwide undergo heart transplantation each year (1). Organ allocation for heart transplantation currently incorporates only body weight into sizing considerations. This sizing method assumes a direct correlation between body weight and cardiac size. It functions poorly to predict outcomes (2,3). Heart size varies by sex, and studies of heart transplantation have consistently observed reduced survival associated with donor organ sex mismatch, particularly for male recipients of female organs (3–9).
Recent studies utilizing cardiac magnetic resonance have provided prediction models of cardiac mass that incorporate height, weight, age, and sex (10,11). These prediction models provide estimates of heart size that differ significantly from estimates using body weight alone. For example, the predicted heart masses (pHM) of a man and a woman both 55 years of age, 80 kg in weight, and 1.75 m tall yield a difference in pHM of 19%. Applying these measures again, a man would have to weigh 20 kg (25%) less than an otherwise similar woman to yield an equivalent pHM. It is therefore likely that the current practice of matching donor organs to recipients based on body weight differences fails to discriminate substantial size mismatches.
Using the United Network of Organ Sharing database of heart transplantation, we performed a retrospective cohort study to examine the relationship between size matching and clinical outcomes and to ascertain whether mismatched size accounts for differential survival with sex-mismatched organs.
Methods
Data source
We analyzed data from adult (age ≥18 years) first-time heart transplant recipients who underwent transplantation between October 1989 and June 2011. Data were extracted from the Standard Transplant Analysis and Research files, provided by the United Network of Organ Sharing. These files were compiled from individual centers and entered by data entry personnel using an electronic system with built-in data validation processes. This study was approved by the institutional review board at the University of Maryland, Baltimore, Maryland.
Study design
Our primary interest was in heart size matching. Size was evaluated using pHM values generated by combining right and left ventricular mass. We used previously published equations for left ventricular and right ventricular mass (10,11), as follows:
-
(1)
Predicted left ventricular mass(g)
= a · Height0.54 (m) · Weight0.61 (kg),
where a = 6.82 for women and 8.25 for men; and
-
(2)
Predicted right ventricular mass(g)
= a · Age−0.32 (years) · Height1.135 (m) · Weight0.315 (kg),
where a = 10.59 for women and 11.25 for men.
The difference in pHM was calculated according to the percent difference in pHM between the donor heart and the recipient heart, which we defined as [(pHMrecipient − pHMdonor)/(pHMrecipient)]*100. To facilitate comparison with the conventional standard of size matching, percent differences in body weight were calculated similarly. Patients missing the information needed to calculate the percent difference in pHM were not included in analysis. The data were inspected for outliers and implausible values. These were recoded as null datapoints. Values changed to null included: weight >130 or <40 kg, body mass index >40 or <15 kg/m2 , systolic < diastolic values, cardiac output >10 l/min, creatinine >5 mg/dl, height >210 or <140 cm, blood urea nitrogen >100 mg/dl, and differences in weight matching between donor and recipient >100% or <−75%. The primary outcome of the study was risk of death after transplantation using Kaplan-Meier survival and Cox proportional hazards survival models, censored at 1 and 5 years. Because the primary causes of death varied by time after transplantation, we did not assume that survival effects would remain continuous over time. Furthermore, although graphic evaluation of Schoenfeld residuals yielded no visibly detectable violation of the proportionality assumptions, formal goodness-of-fit testing was able to demonstrate imperfect function. We chose these time points as they both represent survival periods of clinical significance and periods dominated respectively by early and late phase hazards (8). To further inform considerations of potential mechanisms contributing to survival differences, we examined survival at 30 days and uncensored survival as secondary endpoints. We also examined rejection as a secondary endpoint, as graft rejection and treatment of rejection episodes convey significant post-transplantation risk, and rejection may differ according to sex matching. Rejection was evaluated through a dichotomous characterization of treatment given for acute rejection in the first post-transplantation year.
Statistical analysis
As both oversizing and undersizing of hearts represent mismatch, we did not assume the effect of sizing on outcomes to be linear and we performed categorical analyses centered on the best-matched categories. We partitioned differences in weight and pHM between donor and recipients into 7 quantiles (septiles). We chose septiles to optimize analysis without impairing presentation of results. An odd number of quantiles was needed so that a “best-matched” center quantile would be available. For an a priori alternative analytical approach, we combined quantiles differing from the centered septile (quantile 4) with p ≥ 0.2 into a single “best-matched” category (online supplement). We compared baseline characteristics across pHM septiles by Kruskal-Wallis (continuous variables) and chi-square tests (categorical variables). We performed a time-to-event analysis using the Kaplan-Meier method to demonstrate unadjusted effects on mortality. We applied multivariate Cox proportional hazards survival models to assess the independent effect of pHM differences on survival. Using logistic regression models, we evaluated data for associations with acute rejection within the first year. We based our selection of variables for inclusion in the adjusted model on either prior publication (1) or association (p ≤ 0.2) with 1-year mortality in unadjusted assessments. These variables included recipient age, serum creatinine, total bilirubin, presence of diabetes, pulmonary vascular resistance index, hospitalized status, localization in an intensive care unit, mechanical assistance through ventricular assist or extracorporeal membranous oxygenation, inotrope use, primary indication for transplantation, donor age, donor cause of death, ischemic time, and transplantation era. Categories of sex matching were also included in the adjusted models. For all analyses, we considered p ≤ 0.05 significant. Data are presented as medians with interquartile ranges, and hazard ratios (HR) with 95% confidence intervals (CI). We performed statistical analysis using STATA 11 SE software (StataCorp LP, College Station, Texas).
Results
The initial dataset consisted of 42,765 first-time orthotopic heart transplantations in adult recipients. Observations removed prior to analysis included 739 transplantations involving multiple organs, and 138 transplantations associated with implausible differences in body weight between donor and recipient. Data necessary to calculate the difference in pHM were available for 31,634 subjects, and this constituted the study cohort. Septiles consisted of 4,519 subjects each. Seventy-seven percent of recipients were male, and the median age was 55 years. Seventy-one percent of donors were male, and the median donor age was 29. Overall mortality rates at 1 and 5 years were 12% and 23%, respectively. Study population characteristics for septile 1 (most undersized donor), septile 4 (best matched), and septile 7 (most oversized donor) are presented in Table 1.
Table 1.
Demographics, By Size Category*
| Most Undersized (Septile 1) |
Best Fit (Septile 4) |
Most Oversized (Septile 7) |
p Value† | |
|---|---|---|---|---|
| Size matching | ||||
| % Heart mass difference | −22 (−27 to −19) | 0 (–1 to 2) | 30 (25 to 40) | 0.0001 |
| % Weight difference | −18 (−28 to −9) | −4 (−9 to 1) | 31 (15 to 46) | 0.0001 |
| Sex matching | <0.001 | |||
| FD/FR (%) | 3.5% | 15% | 9% | |
| MD/MR (%) | 21% | 77% | 40% | |
| MD/FR (%) | <1% | 2% | 51% | |
| FD/MR (%) | 75% | 6% | <1% | |
| Recipient factors | ||||
| Male sex | 96% | 83% | 40% | <0.001 |
| Age (yrs) | 54 (46 to 60) | 55 (47 to 61) | 54 (45 to 60) | 0.0001 |
| Hypertension treatment | 39% | 39% | 33% | <0.001 |
| BMI (kg/m2) | 27 (24 to 30) | 26 (23 to 29) | 23 (21 to 27) | 0.0001 |
| Diabetes (%) | 8% | 11% | 9% | <0.001 |
| Serum creatinine (mg/dl) | 1.2 (1.0 to 1.5) | 1.2 (1.0 to 1.5) | 1.1 (0.9 to 1.4) | 0.0001 |
| Serum bilirubin (mg/dl) | 0.8 (0.6 to 1.3) | 0.8 (0.5 to 1.3) | 0.8 (0.5 to 1.3) | 0.3 |
| PRA peak class I | 20 (5 to 43) | 20.5 (7 to 42) | 32 (10 to 65) | 0.0001 |
| PRA peak class II | 18 (7 to 51) | 22 (6 to 60) | 29 (10 to 65) | 0.0002 |
| Hemodynamics | ||||
| MPAP (mm Hg) | 29 (21 to 37) | 28 (21 to 36) | 30 (22 to 37) | 0.03 |
| CI (l/min/m2) | 2.2 (1.8 to 2.6) | 2.2 (1.8 to 2.7) | 2.2 (1.8 to 2.7) | 0.005 |
| PVRI (Woods units/m2) | 0.96 (0.62 to 1.47) | 1.02 (0.66 to 1.58) | 1.38 (0.89 to 2.10) | 0.0001 |
| PAWP (mm Hg) | 20 (13 to 27) | 19 (13 to 26) | 20 (14 to 26) | 0.01 |
| Acuity | ||||
| Hospitalized status | 56% | 50% | 60% | <0.001 |
| Intensive care unit location | 40% | 35% | 45% | <0.001 |
| Inotropic support received | 46% | 43% | 50% | <0.001 |
| Mechanical assist support or ECMO | 18% | 20% | 17% | <0.001 |
| IABP | 4.8% | 5.2% | 6.7% | <0.001 |
| Mechanically ventilated | 2.9% | 2.3% | 3.5% | 0.002 |
| Indication | ||||
| CAD | 51% | 49% | 37% | <0.001 |
| DCM | 77% | 78% | 78% | 0.2 |
| DCM (nonischemic) | 40% | 42% | 51% | <0.001 |
| Other | 9% | 9% | 13% | <0.001 |
| Donor factors | ||||
| Male donor | 21% | 79% | 91% | <0.001 |
| Age donor | 36 (23 to 46) | 28 (20 to 40) | 26 (19 to 38) | 0.0001 |
| BMI donor | 24 (21 to 27) | 25 (22 to 28) | 26 (23 to 30) | 0.0001 |
| Donor cause of death | <0.001 | |||
| Anoxia | 9% | 8% | 9% | |
| Stroke | 43% | 23% | 20% | |
| Head trauma | 42% | 63% | 65% | |
| CNS tumor | 1% | 1% | 1% | |
| Other diagnosis | 5% | 6% | 5% | |
| Transplantation factors | ||||
| Ischemic time (h) | 3.1 (2.4 to 3.8) | 3.0 (2.3 to 3.7) | 2.9 (2.2 to 3.6) | 0.0001 |
| Transplantation year | <0.001 | |||
| Prior to 1994 | 17% | 14% | 13% | |
| 1995–1999 | 31% | 25% | 26% | |
| 2000–2005 | 26% | 26% | 25% | |
| After 2005 | 26% | 36% | 36% | |
| Rejection and death rates | ||||
| Treated for acute rejection in first year | 45% | 38% | 38% | <0.001 |
| 1-yr mortality | 14.2% | 11.4% | 12.3% | <0.001 |
| 5-yr mortality | 26.3% | 22.2% | 23.0% | <0.001 |
Data are median (interquartile range) or percentages.
n =4,519 in each septile.
p Values reflect differences across all 7 quantiles, not only the 3 detailed in the table.
BMI = body mass index; CNS = central nervous system; CAD = coronary artery disease; CI = cardiac index; DCM = dilated cardiomyopathy; ECMO = extra-corporeal membranous oxygenation; FD = female donor; FR = female recipient; IABP = intra-aortic balloon pump; MD = male donor; MPAP = mean pulmonary arterial pressure; MR = male recipient; PAWP = pulmonary artery wedge pressure; PVRI = pulmonary vascular resistance index.
Sex
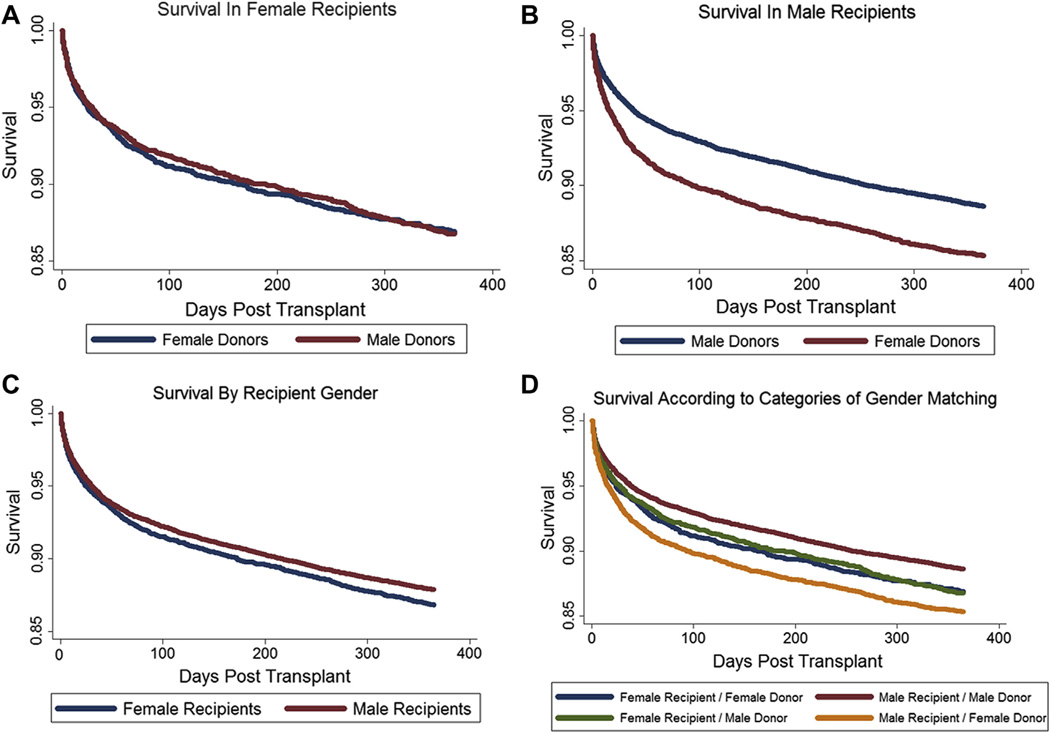
There were 22,439 (70.9%) sex-matched recipient–donor pairs (18,805 male recipients of male donors and 3,634 female recipients of female donors), and 9,195 (29.1%) sex-mismatched pairs (5,608 male recipients of female donors and 3,587 female recipients of male donors). Unadjusted survival in female recipients was worse than in males (HR: 1.09; p = 0.02). Survival differences among female recipients were not associated with donor sex (HR: 1.01; p = 0.9). Male recipients of female organs demonstrated worse survival than did male recipients of male organs (HR: 1.32; p < 0.001) (Fig. 1).
Figure 1. Unadjusted Kaplan-Meier Graphs of Survival in the first Year.
(A) Survival according to donor sex in female recipients (hazard ratio [HR]: 1.00; 95% confidence interval [CI], 0.88 to 1.14; p = 1.0). (B) Survival according to donor sex in male recipients (HR: 1.32; 95% CI: 1.22 to 1.43; p < 0.001). (C) Survival according to recipient sex (HR: 1.09; 95% CI: 1.01 to 1.18; p = 0.02). (D) Survival according to categories of sex matching.
Sizing by difference in weight
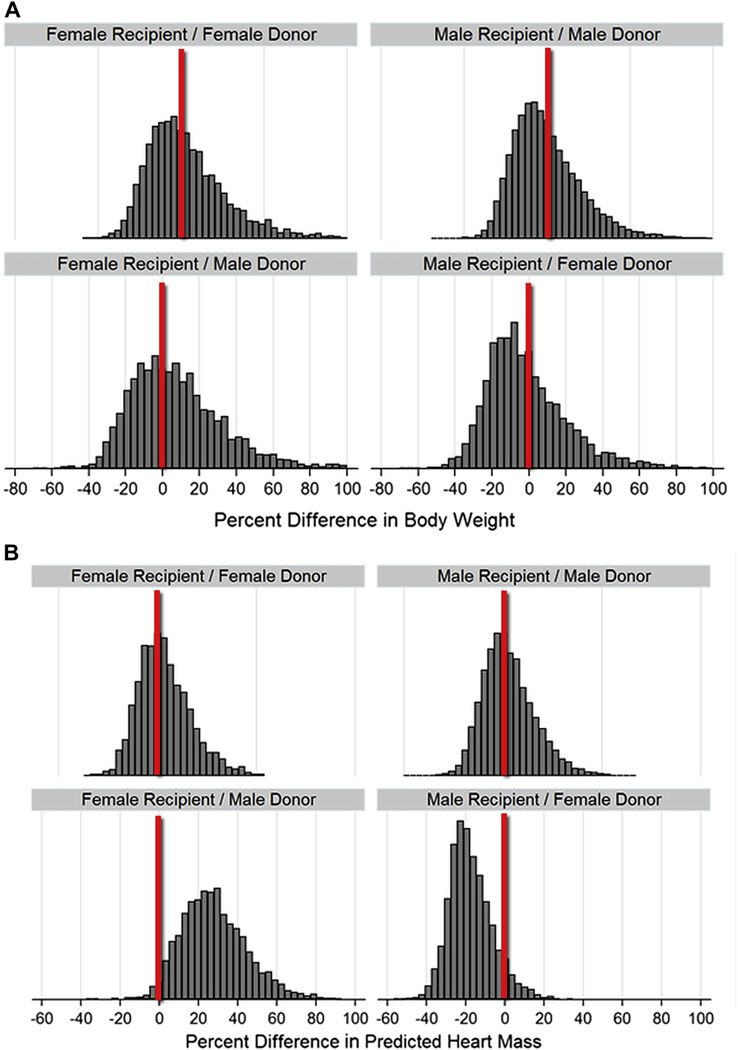
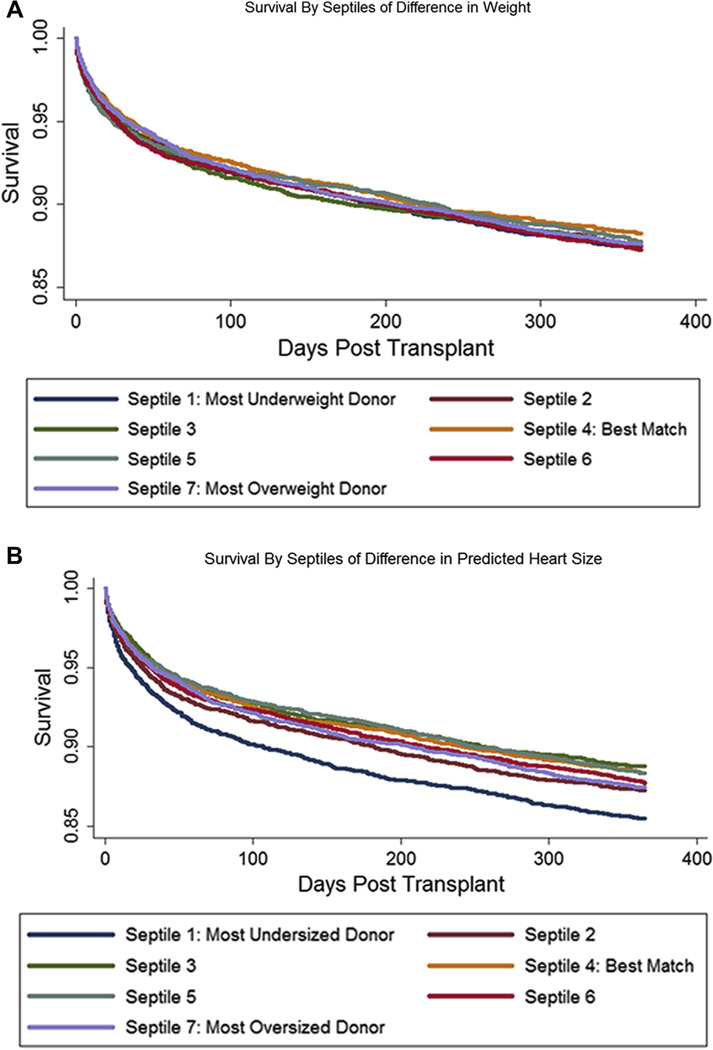
Consistent with the common practice of listing organ preference within 30% of recipient body weight, 86% of donor weights were within 30% of the corresponding recipient’s weight. Donor–recipient weight differences were distributed similarly across categories of sex matching (Fig. 2). Unadjusted and adjusted survival rates were similar in the 7 quantiles of weight differences when compared against the best–weight-matched quantile (Fig. 3, Online Table S1), reconfirming that weight differences do not predict survival differences.
Figure 2. Histograms of Donor/Recipient Differences, by Categories of Sex Matching.
(A) Differences in body weight. (B) Differences in predicted heart mass. The differences in size matching become apparent in the sex-mismatched categories, with tendency toward oversizing in female recipient/male donor matchings and undersizing in male recipient/female donor matchings.
Figure 3. Unadjusted Kaplan-Meier Graphs of Survival, by Septiles of Matching by Body Weight Versus pHM.
(A) Survival by body weight. Compared with best-matched quantile, no significant differences were found (hazard ratio [HR], most-underweight quantile: 1.07; 95% CI: 0.95 to 1.21; p = 0.25). (B) Survival by predicted heart mass (pHM). Compared with best-matched quantile, most-undersized had reduced survival (HR: 1.27; 95% CI: 1.12 to 1.43; p < 0.001). Abbreviations as in Figure 1.
Sizing by difference in pHM
We noted several variances in subject characteristics across quantiles of pHM difference (Table 1). Measures of acuity were generally higher, and the indication for transplantation more commonly nonischemic dilated cardiomyopathy in the oversized quantile. In contrast, ischemic disease was the primary indication for transplantation in the most undersized quantile. Male recipients of female organs were uncommon (<1%) in the most oversized quantile, whereas female recipients of male organs were uncommon (<1%) in the most undersized quantile.
In contrast to recipient–donor weight differences, differences in pHM were distributed differently according to categories of sex matching. The hearts of female recipients of male organs were often oversized, and the hearts of male recipients of female organs were often undersized by this metric (Fig. 2).
Unadjusted survival analysis according to septiles of difference in pHM demonstrated worse survival in the most-undersized quantile (HR: 1.27; p < 0.001) (Fig. 3, Table 2). This excess hazard persisted with a similar point estimate in fully adjusted models censored at 1 year (HR: 1.25; p = 0.03). Similarly, 5-year unadjusted hazard (HR: 1.18; p < 0.001) persisted in fully adjusted models (HR: 1.20; p = 0.01). Thirty-day and uncensored adjusted HRs were 1.11 (p = 0.5) and 1.20 (p = 0.002), respectively (Online Table S2).
Table 2.
Survival by Quantiles of Difference in pHM
| Unadjusted Models (N = 31,413) |
Adjusted Models (N = 17,529)* |
|||||||
|---|---|---|---|---|---|---|---|---|
| Survival 1 yr |
Survival 5 yrs |
Survival 1 yr |
Survival 5 yrs |
|||||
| HR (95% CI) |
p Value | HR (95% CI) |
p Value | HR (95% CI) |
p Value | HR (95% CI) |
p Value | |
| Quantiles | ||||||||
| 1 (most-undersized donor) | 1.27 (1.13 to 1.43) | <0.001 | 1.18 (1.09 to 1.28) | <0.001 | 1.25 (1.02 to 1.54) | 0.03 | 1.20 (1.04 to 1.39) | 0.01 |
| 2 | 1.10 (0.98 to 1.24) | 0.1 | 1.07 (0.98 to 1.17) | 0.1 | 1.14 (0.95 to 1.36) | 0.1 | 1.10 (0.97 to 1.25) | 0.1 |
| 3 | 0.96 (0.85 to 1.09) | 0.5 | 0.94 (0.86 to 1.03) | 0.2 | 1.02 (0.85 to 1.23) | 0.8 | 1.04 (0.91 to 1.2) | 0.6 |
| 4 (best fit) | Referent | Referent | Referent | |||||
| 5 | 1.00 (0.88 to 1.13) | 1.0 | 1.01 (0.92 to 1.10) | 0.9 | 1.06 (0.89 to 1.26) | 0.5 | 1.06 (0.93 to 1.20) | 0.4 |
| 6 | 1.06 (0.94 to 1.19) | 0.4 | 1.03 (0.94 to 1.12) | 0.5 | 1.03 (0.86 to 1.24) | 0.7 | 1.06 (0.94 to 1.21) | 0.3 |
| 7 (most-oversized donor) | 1.08 (0.96 to 1.22) | 0.2 | 1.05 (0.96 to 1.15) | 0.3 | 0.95 (0.78 to 1.16) | 0.6 | 0.96 (0.84 to 1.11) | 0.6 |
| Sex Categories | ||||||||
| MR/MD | Referent | Referent | Referent | Referent | ||||
| MR/FD | 1.32 (1.22 to 1.43) | <0.001 | 1.21 (1.14 to 1.28) | <0.001 | 1.00 (0.85 to 1.17) | 1.0 | 0.95 (0.85 to 1.07) | 0.4 |
| FR/FD | 1.17 (1.06 to 1.29) | 0.002 | 1.14 (1.06 to 1.23) | 0.001 | 1.26 (1.08 to 1.47) | 0.003 | 1.18 (1.06 to 1.32) | 0.003 |
| FR/MD | 1.17 (1.06 to 1.30) | 0.002 | 1.17 (1.09 to 1.26) | <0.001 | 1.62 (1.36 to 1.92) | <0.001 | 1.44 (1.27 to 1.63) | <0.001 |
| FR/MD† | 1.00 (0.88 to 1.14) | 1.0 | 1.03 (0.94 to 1.13) | 0.5 | 1.28 (1.04 to 1.57) | 0.02 | 1.22 (1.05 to 1.41) | 0.008 |
Data are hazard ratios (HR) (confidence intervals [CI]) for predicted heart mass (pHM).
Additional variables in the models included recipient age, serum creatinine, total bilirubin, presence of diabetes, pulmonary vascular resistance index, hospitalized status, localization in intensive care unit, mechanical assistance through ventricular assist or extra-corporeal membranous oxygenation, inotrope use, primary indication for transplantation, donor age, donor cause of death, ischemic time, and transplantation era.
HR referent to female recipient/ female donor pairings.
Abbreviations as in Table 1.
In the multivariate model that included pHM by septile, the effect of male–recipient sex mismatch was no longer present at 1 year (HR: 1.00; p = 1.0) or at 5 years (HR: 0.95; p = 0.4). However, in the multivariate model, sex mismatch in female recipients became associated with worse survival at 1 year (HR: 1.28; p = 0.02) and at 5 years (HR: 1.22; p = 0.008). Multivariate models also demonstrated worse survival in sex-matched females compared with sex-matched males at 1 year (HR: 1.26; p = 0.003) and at 5 years (HR: 1.18; p = 0.003). This suggests a risk factor specific to female recipients that is independent of size mismatch and is not apparent on univariate analysis due to confounding effects of size mismatch that conceal the signal.
We assessed for interaction factors between size categories and sex. These were not significant. Analyses stratified by sex, however, were limited by under-representation of women in the most-undersized category (n = 167).
Treatment for acute rejection in the first year post-transplantation
Data indicating whether a patient had or had not been treated for acute rejection in the first year post-transplantation were available in 17,694 of patients (56%) in the cohort. Multivariate logistic regression assessing this cohort demonstrated a nonsignificant increased risk of treatment for rejection associated with undersizing (OR: 1.19; p = 0.07) and a reduced risk of treatment for rejection associated with oversizing (OR: 0.70; p < 0.001) (Table 3).
Table 3.
Rejection Treated in First Year, by Septiles of Difference in pHM
| Unadjusted Models (N = 17,694) |
Adjusted Models (N = 11,348)* |
|||
|---|---|---|---|---|
| OR (95% CI) |
p Value | OR (95% CI) |
p Value | |
| Quantiles | ||||
| 1 (undersized donor) | 1.33 (1.19 to 1.49) | <0.001 | 1.19 (0.99 to 1.44) | 0.07 |
| 2 | 1.04 (0.93 to 1.17) | 0.5 | 1.01 (0.86 to 1.18) | 0.9 |
| 3 | 1.02 (0.91 to 1.14) | 0.7 | 1.01 (0.87 to 1.18) | 0.9 |
| 4 (best fit) | Referent | Referent | ||
| 5 | 1.00 (0.89 to 1.11) | 1.0 | 0.90 (0.77 to 1.05) | 0.2 |
| 6 | 0.98 (0.88 to 1.10) | 0.7 | 0.91 (0.77 to 1.06) | 0.2 |
| 7 (oversized donor) | 0.99 (0.88 to 1.11) | 0.8 | 0.70 (0.59 to 0.84) | <0.001 |
| Sex Categories | ||||
| MR/MD | Referent | Referent | ||
| MR/FD | 1.20 (1.10 to 1.30) | <0.001 | 1.00 (0.86 to 1.16) | 1.0 |
| FR/FD | 1.43 (1.31 to 1.58) | <0.001 | 1.38 (1.20 to 1.59) | <0.001 |
| FR/MD | 1.51 (1.37 to 1.66) | <0.001 | 1.55 (1.33 to 1.82) | <0.001 |
| FR/MD† | 1.05 (0.93 to 1.19) | 0.4 | 1.13 (0.94 to 1.35) | 0.2 |
Data are odds ratios (OR) (CI) for difference in heart mass in patients with rejection treated in first year.
Additional variables in the models included: recipient age, serum creatinine, total bilirubin, presence of diabetes, pulmonary vascular resistance index, hospitalized status, localization in intensive care unit, mechanical assistance through ventricular assist or extra-corporeal membranous oxygenation, inotrope use, primary indication for transplantation, donor age, donor cause of death, ischemic time, and transplantation era.
Hazard ratio referent to female recipient/female donor pairings.
Abbreviations as in Table 1.
We applied an a priori alternate analytical approach consisting of combining quantiles similar to the centered quantile (p ≥ 0.2). Applying this methodology, we combined septiles 3 to 6 into a best-matched referent category (Online Appendix Table S4 to S7). This yielded similar results overall, with a more apparent association between increased risk of treatment for rejection with undersizing (OR: 1.25; p = 0.009) and reduced risk with oversizing (OR: 0.70; p < 0.001) (Online Table S7).
Female patients overall had a higher likelihood of treatment for rejection compared with male patients in both unadjusted (OR: 1.41; p < 0.001) as well as adjusted (OR: 1.45; p < 0.001) models. In unadjusted models, sex mismatch was associated with increased treatment for rejection in male, but not in female, recipients (Table 3). Similar to the effects on survival, the model adjusted to control for potential confounders including body weight differences did not change the associations between sex match and rejection, whereas the model controlling for the same potential confounders but adjusting for differences in pHM rather than body weight yielded substantially different findings (Online Table S3). These adjusted models failed to demonstrate increased risk of rejection associated with sex mismatch in male (OR: 1.00; p =1.0) or in female (OR: 1.13; p =0.2) recipients. With only 2,745 female patients in fully adjusted models, power was notably limited in female recipients.
Discussion
In this study we applied models of pHM to evaluate the effects of cardiac size matching in orthotopic heart transplantation. There were several important findings:
We confirmed previous reports suggesting the current system of heart size matching according to donor–recipient weight difference functions poorly to inform decisions of optimal organ allocation (2,3,12).
We found that contemporary models of pHM permit the identification of undersized pairings associated with increased risk. Specifically, a mismatch involving donor organs with a pHM greater than 10% to 15% below that of the recipient’s pHM was associated with markedly increase risk of mortality. We term this the “Grinch effect.”
We found that survival differences associated with donor–recipient sex mismatch are changed by, and largely attributable to, differences in pHM.
We found that the likelihood of treatment for acute rejection in the first year after transplantation is lower in oversized and higher in undersized organ pairings.
Although our data do not allow for determination of mechanism, a transplanted heart carries a number of physiologic disadvantages that could worsen with under-sizing (13,14). After transplantation, the heart exhibits chronotropic incompetence due to denervation as well as diastolic dysfunction. As such, augmentation of workload depends primarily upon increased stroke volume, which is facilitated chronically through increased filling pressures (12,13). These high filling pressures could have particularly pronounced effects in undersized hearts, which likely manifest a relatively greater degree of diastolic dysfunction. By virtue of having fewer myocardiocytes, undersized hearts are also capable of less work during systole, and so require a higher heart rate to maintain adequate cardiac output (12). Heart rate in the denervated graft is augmented through increased levels of catecholamines, which can be directly detrimental to the long-term function of the heart (15). Furthermore, tachycardia can potentially contribute to acute rejection and graft failure through a variety of proposed mechanisms (16). The lack of excess 30-day mortality associated with undersizing seems inconsistent with an abrupt perioperative effect and suggests a primary mechanism that manifests more gradually.
It is possible that undersized hearts undergo pathologic hypertrophy over a period of time and that this process may relate to the worse outcomes observed with undersizing. Mather et al. (12) performed an echocardiographic study in adult heart transplant recipients and demonstrated that undersized hearts did indeed exhibit hypertrophy after transplantation, whereas appropriately matched hearts did not. Pathological cardiac hypertrophy (that not attributable to exercise) is associated with a multitude of cellular and molecular derangements, including increased fibrosis as well as increased risk of arrhythmias (17). Arrhythmias are common in the post-transplantation setting and often herald a transplantation complication such as rejection (18,19). It is therefore possible that the higher rates of treatment for rejection that we observed to be associated with undersizing actually represent empirical treatment for rejection based on more frequent arrhythmias.
The observation that the excess mortality associated with sex mismatch in male recipients was not significant after controlling for differences in size may suggest that the increased risk results from physiological mechanisms. Otherwise stated, the sex-mismatch issue in male recipients appears strictly related to size mismatch alone, and an appropriately sized heart from a female donor performs as well as does a similarly sized heart from a male donor. Sex mismatch in female recipients is associated with worse survival that becomes apparent only in adjusted models. We failed to find a difference in the rates of treatment for rejection received in the first year to explain this observation, but detection of true rejection may have been limited by both power as well as nondifferential misclassification bias. Furthermore, the nature and severity of the rejection episodes were not captured. Female sex has been shown to be a risk factor for greater severity of rejection (20) as well as recurrent rejection episodes (21). Female recipients who receive male donor organs are newly exposed to male antigens encoded on the Y chromosome (22) and have been reported to have increased acute rejection (20,23,24) and increased coronary artery vasculopathy (25,26). Based on our findings, we cannot dismiss an immunologic mechanism to explain the differential outcomes associated with sex mismatch in female recipients.
The potential impact that our proposed sizing strategy would have on organ allocation warrants consideration. It is unlikely that a change in sizing allocation would result in a change in overall organ utilization rates, but rather would redistribute organs to donors most likely to derive the optimal benefit from them. This could result in expanded opportunities for transplantation in female patients through improved recognition of the true optimal sizing of many male donor organs that could otherwise be overlooked and not allocated due to concern for being undersized by conventional estimates based on weight differences. Furthermore, the allocation of female donor organs to male recipients could be accomplished without the excess risk associated with that sex mismatch.
Study limitations
Rejection was defined as a transplant recipient’s having been treated for rejection in the first year after transplantation. Although transplant programs typically follow a protocol of post-transplantation surveillance biopsies, patients who are doing poorly are subjected to more frequent biopsies and are occasionally treated empirically for rejection. This could potentially lead to misclassification bias. Combined with the reduced power associated with the more limited number of female recipients, this could lead to type II error (failure to identify a true association) in the case of female-recipient/male-donor combinations and type I error (identification of a false association) in the case of the association between sizing and rejection.
Conclusions
The difference in pHM between donors and recipients of orthotopic heart transplants is a risk factor for decreased survival. Organ allocation may be improved by avoiding donor organs undersized for their intended recipients.
Supplementary Material
Acknowledgments
The authors acknowledge Dr. Carl Shanholtz for his critical review of the manuscript.
This work was supported in part by Health Resources and Services Administration contract 234-2005-370011C. Dr. Reed is funded in part by the Alpha-1 Antitrypsin Foundation/Chest Foundation as well as the Flight Attendant Medical Research Institute. Dr. Netzer is funded in part by National Institutes of Health (NIH) 5K12RR023250-03. Dr. Mitchell is funded in part by the Department of Veterans Affairs and Veterans Affairs Medical Center Baltimore GRECC. Dr. Scharf is funded in part by National Heart Lung, and Blood Institute HL074441. All other authors report that they have no relationships relevant to the contents of this paper to disclose. All authors contributed to the study design and data interpretation.
Abbreviation and Acronym
- pHM
predicted heart mass
APPENDIX
For supplemental Tables, please see online version of this article.
REFERENCES
- 1.Stehlik J, Edwards LB, Kucheryavaya AY, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-eighth adult heart transplant report—2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Patel ND, Weiss ES, Nwakanma LU, et al. Impact of donor-to-recipient weight ratio on survival after heart transplantation: analysis of the United Network for Organ Sharing Database. Circulation. 2008;118(Suppl):S83–S88. doi: 10.1161/CIRCULATIONAHA.107.756866. [DOI] [PubMed] [Google Scholar]
- 3.Khush KK, Kubo JT, Desai M. Influence of donor and recipient sex mismatch on heart transplant outcomes: analysis of the International Society for Heart and Lung Transplantation Registry. J Heart Lung Transplant. 2012;31:459–466. doi: 10.1016/j.healun.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Khaldi A, Oyer PE, Robbins RC. Outcome analysis of donor gender in heart transplantation. J Heart Lung Transplant. 2006;25:461–468. doi: 10.1016/j.healun.2005.11.456. [DOI] [PubMed] [Google Scholar]
- 5.Eifert S, Kofler S, Nickel T, et al. Gender-based analysis of outcome after heart transplantation. Exp Clin Transplant. 2012;10:368–374. doi: 10.6002/ect.2011.0164. [DOI] [PubMed] [Google Scholar]
- 6.Kittleson MM, Shemin R, Patel JK, et al. Donor-recipient sex mismatch portends poor 10-year outcomes in a single-center experience. J Heart Lung Transplant. 2011;30:1018–1022. doi: 10.1016/j.healun.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant. 2006;25:634–637. doi: 10.1016/j.healun.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Stehlik J, Feldman DS, Brown RN, et al. Interactions among donor characteristics influence post-transplant survival: a multi-institutional analysis. J Heart Lung Transplant. 2010;29:291–298. doi: 10.1016/j.healun.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Weiss ES, Allen JG, Patel ND, et al. The impact of donor-recipient sex matching on survival after orthotopic heart transplantation: analysis of 18 000 transplants in the modern era. Circ Heart Fail. 2009;2:401–408. doi: 10.1161/CIRCHEARTFAILURE.108.844183. [DOI] [PubMed] [Google Scholar]
- 10.Bluemke DA, Kronmal RA, Lima JA, et al. The relationship of left ventricular mass and geometry to incident cardiovascular events: the MESA (Multi-Ethnic Study of Atherosclerosis) study. J Am Coll Cardiol. 2008;52:2148–2155. doi: 10.1016/j.jacc.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawut SM, Lima JA, Barr RG, et al. Sex and race differences in right ventricular structure and function: the multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mather PJ, Jeevanandam V, Eisen HJ, et al. Functional and morphologic adaptation of undersized donor hearts after heart transplantation. J Am Coll Cardiol. 1995;26:737–742. doi: 10.1016/0735-1097(95)00216-Q. [DOI] [PubMed] [Google Scholar]
- 13.Mettauer B, Levy F, Richard R, et al. Exercising with a denervated heart after cardiac transplantation. Ann Transplant. 2005;10:35–42. [PubMed] [Google Scholar]
- 14.Andreassen AK. Point: cardiac denervation does/does not play a major role in exercise limitation after heart transplantation. J Appl Physiol. 2008;104:559–560. doi: 10.1152/japplphysiol.00694.2007. [DOI] [PubMed] [Google Scholar]
- 15.Ferretti G, Marconi C, Achilli G, et al. The heart rate response to exercise and circulating catecholamines in heart transplant recipients. Pflugers Arch. 2002;443:370–376. doi: 10.1007/s004240100701. [DOI] [PubMed] [Google Scholar]
- 16.Critchley WR, Yonan N, Shaw SM, Fildes JE. Heart rate after cardiac transplantation—lessons from the tortoise and the shrew. Transplantation. 2013;95:259–265. doi: 10.1097/TP.0b013e31826bc42a. [DOI] [PubMed] [Google Scholar]
- 17.Bernardo BC, Weeks KL, Pretorius L, McMullen JR. Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies. Pharmacol Ther. 2010;128:191–227. doi: 10.1016/j.pharmthera.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Thajudeen A, Stecker EC, Shehata M, et al. Arrhythmias after heart transplantation: mechanisms and management. J Am Heart Assoc. 2012;1:e001461. doi: 10.1161/JAHA.112.001461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stecker EC, Strelich KR, Chugh SS, Crispell K, McAnulty JH. Arrhythmias after orthotopic heart transplantation. J Card Fail. 2005;11:464–472. doi: 10.1016/j.cardfail.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 20.Mills RM, Naftel DC, Kirklin JK, et al. on behalf of the Cardiac Transplant Research Database Group. Heart transplant rejection with hemodynamic compromise: a multiinstitutional study of the role of endomyocardial cellular infiltrate. J Heart Lung Transplant. 1997;16:813–821. [PubMed] [Google Scholar]
- 21.Kubo SH, Naftel DC, Mills RM, Jr, et al. on behalf of the Cardiac Transplant Research Database Group. Risk factors for late recurrent rejection after heart transplantation: a multiinstitutional, multivariable analysis. J Heart Lung Transplant. 1995;14:409–418. [PubMed] [Google Scholar]
- 22.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 23.Prendergast TW, Furukawa S, Beyer AJ, III, Browne BJ, Eisen HJ, Jeevanandam V. The role of gender in heart transplantation. Ann Thorac Surg. 1998;65:88–94. doi: 10.1016/s0003-4975(97)01105-3. [DOI] [PubMed] [Google Scholar]
- 24.Crandall BG, Renlund DG, O’Connell JB, et al. Increased cardiac allograft rejection in female heart transplant recipients. J Heart Transplant. 1988;7:419–423. [PubMed] [Google Scholar]
- 25.Mehra MR, Stapleton DD, Ventura HO, et al. Influence of donor and recipient gender on cardiac allograft vasculopathy. An intravascular ultrasound study. Circulation. 1994;90:II78–II82. [PubMed] [Google Scholar]
- 26.Erinc K, Yamani MH, Starling RC, et al. The influence of donor gender on allograft vasculopathy: evidence from intravascular ultrasound. Transplant Proc. 2004;36:3129–3131. doi: 10.1016/j.transproceed.2004.10.072. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.