Abstract
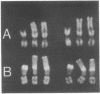
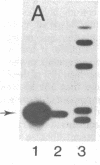
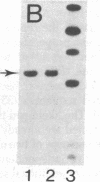
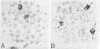
Human neuroblastoma IMR-32 cells have large homogeneously staining regions (HSRs), primarily in the short arms of chromosome 1. We have constructed a recombinant phage library that is enriched for DNA present in the HSR of this chromosome by using fluorescence-activated flow sorting for initial chromosome purification. Eleven distinct cloned DNA segments were identified that showed significantly greater hybridization to IMR-32 genomic DNA, detected by Southern blotting, than to normal human genomic DNA. These sequences have also been localized to the HSR of chromosome 1 by in situ hybridization. Based on an approximate 50-fold sequence amplification for each cloned segment and a total HSR size of 150,000 kilobases, the amplified unit in the HSR is estimated to be 3,000 kilobases. Sequences homologous to all cloned HSR DNA segments were mapped to human chromosome 2 by using human-mouse hybrid cells. Further work using in situ hybridization demonstrated that cloned HSR segments were localized in the short arm of chromosome 2 in both normal and IMR-32 cells. Thus, the amplification of these sequences in IMR-32 cells may have involved transposition from chromosome 2 to chromosome I.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balaban-Malenbaum G., Gilbert F. Double minute chromosomes and the homogeneously staining regions in chromosomes of a human neuroblastoma cell line. Science. 1977 Nov 18;198(4318):739–741. doi: 10.1126/science.71759. [DOI] [PubMed] [Google Scholar]
- Balaban-Malenbaum G., Grove G., Gilbert F. Increased DNA content of HSR-marker chromosomes of human neuroblastoma cells. Exp Cell Res. 1979 Mar 15;119(2):419–423. doi: 10.1016/0014-4827(79)90376-8. [DOI] [PubMed] [Google Scholar]
- Barker P. E., Hsu T. C. Double minutes in human carcinoma cell lines, with special reference to breast tumors. J Natl Cancer Inst. 1979 Feb;62(2):257–262. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Biedler J. L., Roffler-Tarlov S., Schachner M., Freedman L. S. Multiple neurotransmitter synthesis by human neuroblastoma cell lines and clones. Cancer Res. 1978 Nov;38(11 Pt 1):3751–3757. [PubMed] [Google Scholar]
- Biedler J. L., Spengler B. A. Metaphase chromosome anomaly: association with drug resistance and cell-specific products. Science. 1976 Jan 16;191(4223):185–187. doi: 10.1126/science.942798. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Botstein D., White R. L., Skolnick M., Davis R. W. Construction of a genetic linkage map in man using restriction fragment length polymorphisms. Am J Hum Genet. 1980 May;32(3):314–331. [PMC free article] [PubMed] [Google Scholar]
- Brison O., Ardeshir F., Stark G. R. General method for cloning amplified DNA by differential screening with genomic probes. Mol Cell Biol. 1982 May;2(5):578–587. doi: 10.1128/mcb.2.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodeur G. M., Green A. A., Hayes F. A., Williams K. J., Williams D. L., Tsiatis A. A. Cytogenetic features of human neuroblastomas and cell lines. Cancer Res. 1981 Nov;41(11 Pt 1):4678–4686. [PubMed] [Google Scholar]
- Bruns G. A., Mintz B. J., Leary A. C., Regina V. M., Gerald P. S. Human lysosomal genes: arylsulfatase A and beta-galactosidase. Biochem Genet. 1979 Dec;17(11-12):1031–1059. doi: 10.1007/BF00504344. [DOI] [PubMed] [Google Scholar]
- George D. L., Powers V. E. Amplified DNA sequences in Y1 mouse adrenal tumor cells: association with double minutes and localization to a homogeneously staining chromosomal region. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1597–1601. doi: 10.1073/pnas.79.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George D. L., Powers V. E. Cloning of DNA from double minutes of Y1 mouse adrenocortical tumor cells: evidence for gene amplification. Cell. 1981 Apr;24(1):117–123. doi: 10.1016/0092-8674(81)90507-9. [DOI] [PubMed] [Google Scholar]
- Gilbert F., Balaban G., Breg W. R., Gallie B., Reid T., Nichols W. Homogeneously staining region in a retinoblastoma cell line: relevance to tumor initiation and progression. J Natl Cancer Inst. 1981 Aug;67(2):301–306. [PubMed] [Google Scholar]
- Gilbert F., Balaban G., Moorhead P., Bianchi D., Schlesinger H. Abnormalities of chromosome 1p in human neuroblastoma tumors and cell lines. Cancer Genet Cytogenet. 1982 Sep;7(1):33–42. doi: 10.1016/0165-4608(82)90105-4. [DOI] [PubMed] [Google Scholar]
- Harper M. E., Ullrich A., Saunders G. F. Localization of the human insulin gene to the distal end of the short arm of chromosome 11. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4458–4460. doi: 10.1073/pnas.78.7.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapuściński J., Skoczylas B. Simple and rapid fluorimetric method for DNA microassay. Anal Biochem. 1977 Nov;83(1):252–257. doi: 10.1016/0003-2697(77)90533-4. [DOI] [PubMed] [Google Scholar]
- Krumlauf R., Jeanpierre M., Young B. D. Construction and characterization of genomic libraries from specific human chromosomes. Proc Natl Acad Sci U S A. 1982 May;79(9):2971–2975. doi: 10.1073/pnas.79.9.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Tantravahi U., Eisenhard M., Latt S. A. Regional localization on the human X of DNA segments cloned from flow sorted chromosomes. Nucleic Acids Res. 1982 Mar 11;10(5):1557–1578. doi: 10.1093/nar/10.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Wohlleb J. C. Optical studies of the interaction of 33258 Hoechst with DNA, chromatin, and metaphase chromosomes. Chromosoma. 1975 Nov 11;52(4):297–316. doi: 10.1007/BF00364015. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Mark J. Chromosomal characteristics of neurogenic tumours in adults. Hereditas. 1971;68(1):61–100. doi: 10.1111/j.1601-5223.1971.tb02388.x. [DOI] [PubMed] [Google Scholar]
- Otto F. J., Oldiges H., Göhde W., Barlogie B., Schumann J. Flow cytogenetics of uncloned and cloned Chinese hamster cells. Cytogenet Cell Genet. 1980;27(1):52–56. doi: 10.1159/000131464. [DOI] [PubMed] [Google Scholar]
- Perucho M., Goldfarb M., Shimizu K., Lama C., Fogh J., Wigler M. Human-tumor-derived cell lines contain common and different transforming genes. Cell. 1981 Dec;27(3 Pt 2):467–476. doi: 10.1016/0092-8674(81)90388-3. [DOI] [PubMed] [Google Scholar]
- Quinn L. A., Moore G. E., Morgan R. T., Woods L. K. Cell lines from human colon carcinoma with unusual cell products, double minutes, and homogeneously staining regions. Cancer Res. 1979 Dec;39(12):4914–4924. [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sahar E., Latt S. A. Energy transfer and binding competition between dyes used to enhance staining differentiation in metaphase chromosomes. Chromosoma. 1980;79(1):1–28. doi: 10.1007/BF00328469. [DOI] [PubMed] [Google Scholar]
- Shimizu K., Goldfarb M., Perucho M., Wigler M. Isolation and preliminary characterization of the transforming gene of a human neuroblastoma cell line. Proc Natl Acad Sci U S A. 1983 Jan;80(2):383–387. doi: 10.1073/pnas.80.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tumilowicz J. J., Nichols W. W., Cholon J. J., Greene A. E. Definition of a continuous human cell line derived from neuroblastoma. Cancer Res. 1970 Aug;30(8):2110–2118. [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]