Abstract
Trigeminal neuralgia (TN) is one of the most excruciating pain syndromes afflicting the orofacial region. Trigeminal neuralgia may be primary i.e. idiopathic or secondary, resulting from trauma or a CNS lesion. Considering the agonizing nature of the disease and TN being the commonest of the neural maladies affecting the orofacial region it is important for the oral physician to be aware of all available treatment options. This article makes an attempt to present a brief insight into the current treatment modalities that are on hand to treat this condition. From the perspective of the oral physician the pharmacotherapy constitutes the cornerstone in the management of TN. At the same time, it is also important to be aware and updated of the role of the oral surgeon and radiologist in the application of the array of interventional procedures available for treating TN.
Keywords: Trigeminal neuralgia treatment, Carbamazepine, Microvascular decompression, Sterotactic radiosurgery
Introduction
Trigeminal neuralgia (TN) is reportedly is one of the most excruciating pain syndromes afflicting the orofacial region. It is defined as sudden, usually unilateral, severe, brief, stabbing, lancinating, recurring pain in the distribution of one or more branches of fifth cranial nerve.1 Trigeminal neuralgia may be primary i.e. idiopathic or secondary, resulting from trauma or CNS lesion.2 The criteria for trigeminal neuralgia as defined by the International Headache Society3 are depicted in Table 1. 60% of patients complain of lancinating pain shooting from the corner of the mouth to the angle of the jaw, i.e.V3-mandibular division. 30% of patients present with the involvement with V2-maxillary division. Less than 5% of patients experience involvement of V1-ophthalmic branch.4
Table 1.
Criteria for trigeminal neuralgia by the international headache association.
| Disease description | Features |
|---|---|
| Classic trigeminal neuralgia |
|
| Symptomatic trigeminal neuralgia |
|
Recently, the ignition hypothesis has been suggested to explain the pathophysiology of TN. According to the hypothesis, trigeminal neuralgia results from specific abnormalities of trigeminal afferent neurons in the trigeminal root or ganglion. Injury renders axons and axotomized somata hyperexcitable. The hyperexcitable afferents, in turn, give rise to pain paroxysms as a result of synchronized discharge activity.5 The exact cause of this disease process remains uncertain. In most cases it is idiopathic. Table 2 lists the probable etiologic factors for trigeminal neuralgia.6 TN typically manifests as intermittent, paroxysmal, shock like pain, elicited by touching the “trigger points”, which radiates from that point, across the distribution of one or more branches of the trigeminal nerve. The trigger points are provocable by activities such as chewing, speaking, smiling, or washing the face. The pain rarely crosses the midline and the attacks never occur during sleep. Even though there is a refractory period between the attacks, some patients report a dull ache in between the attacks. During an attack, the patient grimaces with pain, clutches his hands over the affected side and holds or rubs his face, which may redden and the eyes may water until the attack lasts. The oral hygiene is poor as patient avoids brushing of teeth. Male patients avoid shaving in the fear of triggering an attack. In extreme cases, the patient will have a motionless face – ‘the frozen or mask like face’. It is very common for these patients to undergo indiscriminate dental extractions on the affected side without any relief from pain, because the pain may mimic that of odontogenic origin. 60% of patients complain of lancinating pain shooting from the corner of the mouth to the angle of the jaw, i.e. V3-mandibular division, 30% of patients present with the involvement with V2-maxillary division whereas less than 5% of patients experience involvement of V1-ophthalmic branch.1
Table 2.
The various etiologic factors for TN.
| Aetiology |
|---|
|
Materials and methods
An electronic literature search was employed to identify, select and utilize relevant studies and reviews published in the English language. The following terms were used in the search: trigeminal neuralgia treatment & trigeminal neuralgia management. Further, from the results obtained each of individual modalities of the interventional procedures was searched electronically to identify retrospective studies. Studies with the largest sample sizes were chosen to be discussed in the outcomes rendered by the procedures. The databases of Pubmed and Cochrane were used in this review.
Considering the agonizing nature of the disease and TN being the commonest of the neural maladies affecting the orofacial region it is important for the oral physician to be aware of all available treatment options. This article makes an attempt to present a brief insight into the current treatment modalities that are on hand to treat this condition. The treatment for trigeminal neuralgia may broadly be classified:
-
I.
Pharmacotherapy
-
II.
Interventional procedures
Pharmacotherapy of trigeminal neuralgia
The goal of pharmacotherapy is to reduce pain and accord symptomatic relief.
Carbamazepine
Traditionally, carbamazepine, an anticonvulsant medication, has been used as a first-line drug for the treatment of trigeminal neuralgia.7 A serum level should be determined 2–3 weeks after beginning therapy, and again every 1–3 months. A complete blood cell count and liver function tests should be done periodically on patients treated for longer periods. After the pain has been controlled for 6–8 weeks, the dosage should be decreased to the lowest level that maintains pain control. Some drugs, such as erythromycin, cimetidine, diltiazem hydrochloride, and terfenadine, can increase the plasma concentration of carbamazepine. Carbamazepine may also interact with other anticonvulsants and can decrease the effectiveness of oral contraceptives.8
Oxcarbazepine
Oxcarbazepine is the second drug of choice for trigeminal neuralgia. It is a 10-keto analogue of carbamazepine and is as effective as carbamazepine. It has fewer drug interactions and causes the release of fewer catabolic enzymes than does carbamazepine.8
Phenytoin
Phenytoin is also an anticonvulsant drug. If pain relief is not obtained after reaching adequate serum levels for 3 weeks, the drug should be discontinued because higher doses may lead to toxicity. The short-term efficacy rate is 60%; the efficacy rate decreases to 30% after 2 years.8
Lamotrigine
Lamotrigine is a relatively new anticonvulsant drug used in the treatment of partial and generalized seizures. It has an action similar to that of carbamazepine, but fewer side effects.8
Gabapentin
Gabapentin is becoming increasingly popular as a treatment option for trigeminal neuralgia and has a relatively benign adverse effect profile. Low-dose gabapentin proved effective when used as an adjunct to lamotrigine or carbamazepine.8
Topiramate
Topiramate is the newest drug to be evaluated for the treatment of trigeminal neuralgia. In one study, patients with multiple sclerosis and trigeminal neuralgia refractory to combination therapy with a variety of conventional treatments all became free of pain with topiramate therapy.
The Table 3 summarizes the mechanism of actions, dosages and adverse effects concerned with these agents8 whereas Table 4 enlists adjuvant drugs that have shown partial success in the pharmacotherapy of TN.7
Table 3.
Mechanism of action, dosages and adverse effects of drugs used to treat trigeminal neuralgia.
| Medication | Mechanisms of action | Dosage | Adverse effects |
|---|---|---|---|
| Carbamazepine | Slow recovery of voltage-gated sodium channels, modulates calcium channel activity, activates descending inhibitory modulation | 200–1200 mg daily in divided doses | Nausea, drowsiness, fatigue, dizziness, memory problems, diplopic, nystagmus, liver dysfunction, and haematosuppresion |
| Phenytoin | Promotes sodium efflux from neurons | 300–500 mg daily | Nystagmus, ataxia, slurred speech, decreased coordination, mental confusion |
| Oxcarbazepine | Same as carbamazepine | 300–1800 mg daily in 2 divided doses | Decreased blood sodium level, dizziness, fatigue, headache, tremors, drowsiness, diminished concentration, diplopia, and stammering |
| Lamotrigine | Decreases repetitive firing of sodium channels by slowing the recovery rate of voltage-gated channels | 100–150 mg daily in 2 divided doses; starting dosage, 25 mg every other day for 6–8 days, dosage is increased by 25–50 mg every 1–2 weeks | Sleepiness, dizziness, headache, vertigo, rash, Stevens–Johnson syndrome |
| Gabapentin | Blockage of voltage-gated calcium channels by binding to α2/Δ subunit | 1200–3600 mg daily in 3 or 4 divided doses | Fatigue, somnolence, dizziness, ataxia, nystagmus and tremor |
| Topiramate | Voltage-gated sodium channel blockage; potentiation of γ-aminobutyric acid activation receptor mechanisms | 200–300 mg daily in 2 divided doses | Fatigue, nervousness, tremors, weight loss and difficulty with concentration/attention |
Table 4.
Adjuvant drugs in the treatment of trigeminal neuralgia.
| Drug | Dosage | |
|---|---|---|
| Baclofen | Oral: initial dose: 5 mg orally 3 times a day for 3 days, then 10 mg orally 3 times a day for 3 days, then 15 mg orally 3 times a day for 3 days, then 20 mg orally 3 times a day. Maintenance dose: 40–80 mg/day in divided doses | |
| Pimozide | Dose of 1–2 mg a day in divided doses. The dose may be increased thereafter every other day. Most patients are maintained at less than 0.2 mg/kg per day, or 10 mg/day, whichever is less | |
| Tizanidine | 15–20 mg/day in divided doses | |
| Valproate sodium | 800–1600 mg/day in divided doses | |
| Racemic ketamine | 0.5 mg/kg/day in divided doses | |
| Methadone | 80–120 mg/day in divided doses | |
| Topical capsaicin cream | 0.25%, 0.75% ointment | |
| Proparacaine | 0.5% eye drops instilled on the affected side | |
Interventional procedures
Microvascular decompression
Microvascular decompression is a neurosurgical procedure used to treat trigeminal neuralgia. The goal of MVD is to decompress the trigeminal root from offending vessels, aiming at a permanent cure with no or little sensory deficit. Preoperative identification of neurovascular compression, therefore, has potentially important implications for patient selection, surgical planning, and outcomes. Ni et al9 recommended an enhanced three-dimensional fast spoiled gradient recalled MRI and three-dimensional magnetic resonance angiography with a 3.0-Tesla MRI system to detect the anatomic relationship of neural and vascular structures at the trigeminal root entry zone (TREZ) preoperatively. In 27 of 29 patients (93%), surgical findings were consistent with the imaging results. The posterior fossa is approached through a sub occipital craniotomy. After aspiration of the cerebrospinal fluid, the operator advances towards the nerve by gently retracting the superolateral margin of the cerebellum. The most common finding is a segment of the superior cerebellar artery compressing the nerve at the root entry zone (Fig. 1). Less frequently, the anterior inferior cerebellar artery or the superior petrosal veins are the cause of the compression. After the arachnoid is dissected and the vessel freed, piece of shredded Teflon felt is placed between the vessel and the nerve to separate them. Partial section of the root (nerve rhizotomy) is recommended if no vascular compression is found or the artery cannot be mobilized.10
Fig. 1.
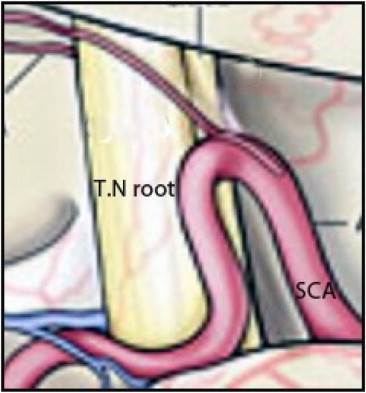
Vascular compression of the trigeminal nerve (T.N) root by the superior cerebellar artery (SCA).
Outcome: Kondo11 in his follow-up series suggests a high level of initial success with MVD, most patients (87–98%) experiencing immediate pain relief. At 1–2 yr the incidence of complete pain relief was 75–80%. After 8–10 yr, this proportion was reduced to 58–64%, with a further 4–12% suffering from minor recurrence only.
Radiofrequency gangliolysis
Essentially, it involves a selective partial “lesioning” of the affected ganglion or retrogasserian root. Thermocoagulation results in a selective loss of pain mediating thinly myelinated and non-myelinated fibres. The procedure is carried out in the intermittently anaesthetized patient under fluoroscopic control. The radiofrequency needle is inserted through the foramen ovale into Meckel's cave using bony landmarks. The relationship of the trigeminal rootlets to the foramen ovale is such that by stepwise advancement of the needle, the third, second and first divisions will be stimulated in succession. As soon as the needle has entered Meckel's cave, aspiration will yield CSF. Once the needle has travelled the pre-planned distance, the patient is allowed to awake, the stylet replaced by the electrode and stimulation of the nerve root carried out. The paraesthesia elicited must conform to the location of the neuralgia; otherwise the needle must be repositioned. Once appropriate seating of the needle has taken place, the patient is anaesthetized again for thermal lesioning. This is performed in cycles of 45–90 s at temperatures of 60–90 °C. After each lesioning the patient is awakened and manual sensory testing of the face carried out. Additional thermal lesionings are performed until clear hypoalgesia has ensued.10
Outcome: Scrivani et al12 in their retrospective study of 215 patients, reported that the vast majority experienced immediate pain relief (92%). Recurrence of TN pain occurred in 27% at a mean follow-up of 32 months.
Glycerol gangliolysis
The method was introduced by Hakansson13 after a fortuitous discovery, during the development of a stereotactic technique for gamma radiation, that glycerol mixed with tantalum powder not only visualized the trigeminal cistern but also abolished pain in patients with trigeminal neuralgia. The procedure can be done under local anaesthesia in fully awake patients, although mild sedation is usually used. The needle is inserted into the trigeminal cistern through the foramen ovale using similar trajectories as in radiofrequency lesioning. Fluoroscopic control is mandatory but the use of radio opaque contrast (cisternography) to visualize the cistern is optional. Once the needle is optimally placed, the patient is brought into a sitting position and a small test dose of sterile anhydrous glycerol is injected. This is followed by small dose increments up to a total of 0.1–0.4 ml, depending on the divisions involved. Patients are usually able to perceive the effect of the injection as a tingling or burning sensation in the affected divisions. Patients should remain in the sitting position for 2 h after the injections.10
Outcome: although pain relief is usually immediate; it may take up to 7 days to occur in some patients. Authors13 report initial pain relief in over 80% of their patients but long-term results are highly variable. At 12 months, reported recurrence rates vary from 10 to 53%. This method is generally well tolerated and mortality is negligible.
Balloon compression
It is advocated that compression of the ganglion produced similar results as the decompression of the nerve root. This procedure is performed under general anaesthesia. Using fluoroscopic control, a guide needle is inserted into the foramen ovale, but not beyond it. Through the needle, the Fogarty catheter is advanced until its tip lies in Meckel's cave and the balloon is slowly inflated with 0.5–1.0 ml of contrast dye until it occupies the cave, ensuring adequate compression (Fig. 2). Total compression time varies from 1 to 6 min. The patient usually only requires an overnight stay.10
Fig. 2.
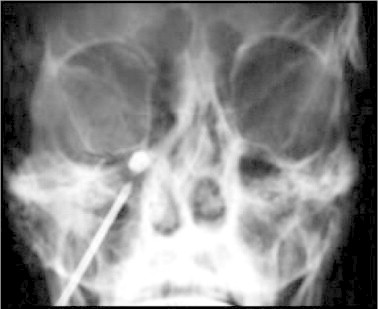
Balloon compression – AP view of skull showing inflated balloon in position.
Outcome: Kauzounias et al14 studied the factors that influence outcome of percutaneous balloon compression in the treatment of trigeminal neuralgia. They concluded that the balloon shape is a parameter with a very strong impact on outcome, and balloon volume should be adjusted to this parameter. Persistent elliptical balloon shapes should raise consideration of aborting the procedure. There were no differences in outcomes between 60 s and longer compression times. The number of previous operations did not correlate with pain relief, but seemed to increase the risk of complications. Patients with multiple sclerosis seemed to obtain similar benefit from the procedure as do patients with classic trigeminal neuralgia. Recurrence is reported in 6–14% cases in the first year. Very few studies employing long-term follow-up are available. In Abdennebi's15 series, one-third needed re-operation during a mean follow-up of 4.3 years. Lichtor and Mullan16 obtained slightly better results in that recurrence was seen in 20% of patients at 5 yr follow-up, and 28% of a small number of patients followed even longer (up to 10 yr).
Stereotactic radiosurgery
Lars Leksell developed a radiosurgical tool, the ‘Gamma Knife’, to treat functional brain disorders as an alternative to open intracranial procedures. This method seems a valuable addition to the existing treatments, as it is very patient friendly and safe. The Gamma Knife is a focused array of 201 intercepting beams of gamma radiation, produced by separate cobalt sources. A stereotactic frame is first secured to the patient's head, followed by MRI to identify the trigeminal nerve. Radiosurgery is carried out with the patient in the supine position, with the head placed under the collimator helmet (Fig. 3). Local anaesthesia is used for securing the frame and irradiation is frequently carried out under mild oral or i.v. sedation. The dose used is 70–90 Gy.10
Fig. 3.
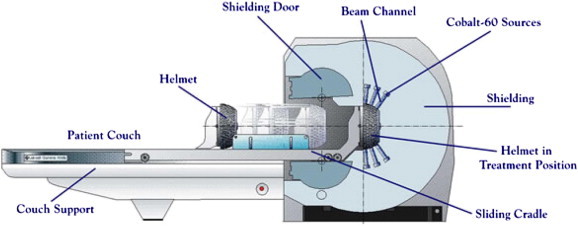
Schematic representation of stereotactic radiosurgery unit.
Outcome: Han et al17 studied the long-term outcome of gamma knife radiosurgery for treatment of typical trigeminal neuralgia in 62 patients. They found that the actuarial recurrence-free survival rate was 84.8%, 76.1%, 69.6%, 63.0%, and 45.8% at 1, 2, 3, 4, and 5 years after radiosurgery, respectively. Park et al18 compared gamma knife radiosurgery as a first and a second treatment for the management of medically refractory idiopathic trigeminal neuralgia (TN). No significant differences in radiation dose, time to initial response, recurrence or pain relief were observed between its use as a primary and a secondary procedure for idiopathic TN. The occurrence of new onset after the procedure was the same for the two groups, but overall facial sensory changes were higher in the secondary group.
Peripheral neurectomy
It is a, safe and simple procedure especially in the elderly and preferred over injection techniques. Neurectomies are performed through an incision made at the eyebrow (supra-orbital nerve) or intra-orally (infra-orbital, alveolar and lingual nerves). All branches are divided and avulsed under magnification. The relevant foramen is blocked by bone wax, wooden sticks or silicone plugs. The remnant of the nerve may also be cauterized. Despite these efforts, remnants may be found on re-exploration and dealing with them in the same way is said to lead to pain relief.10
Outcome: Danish investigators found that, during a mean follow-up of 7 yr, 78% of patients who had undergone neurectomy experienced a recurrence. One-half of the patients had their first recurrence within a month. In their series, neurectomy (as well as alcohol block) compared unfavourably with radiofrequency lesioning.19
Cryotherapy
Cryotherapy is a surgical technique in which a peripheral branch of the three major divisions of the trigeminal nerve is exposed and frozen by direct application of a cryoprobe with a tip temperature from −50 to −70 °C. The patient requires i.v. sedation or general anaesthesia.10
Outcome: Although well tolerated by patients, the results are modest. Of 145 patients who underwent 1–11 sessions of cryotherapy (56% had more than one session), the effect lasted less than 6 months in one-half and at 12 months, only 27% were pain-free. Almost two-thirds had to remain on their previous medication. Although improvement in individual nerves was better, from the pain point of view, cryosurgery falls short of results obtained either with procedures aimed at the ganglion or the root.20
Alcohol block
Peripheral and ganglionic alcohol blocks have been used since the beginning of the last century but have fallen into disrepute mainly because of capricious results and reports of adverse effects. Alcohol injections must be administered directly into the nerve. The injections are painful and often cause local oedema.10
Outcome: In a retrospective analysis of 45 patients treated with one or several alcohol blocks, 84% of patients had a recurrence of pain during the mean follow-up of 8 yr. One-half had a recurrence within a month.19
Local laser irradiation and laser puncture
This technique consists of local laser irradiation of the orofacial region followed by laser puncture of selected points on the face. The technique has been recommended by B. Popov et al. They recommended a low power 12 mw Helium–Neon laser of wavelength 6328 Å units and a laser beam density of 150–170 mw/cm2 for irradiation. The treatment is first given intra-orally following the path of the nerve branch, for 1–2 min. Then extra-orally, the so called “bio-active points” are laser punctured. The treatment time is 10 min per day for 10–12 days.21
Outcome: it was found that remissions achieved with this procedure are considerably longer than those achieved with other local methods.21
Electroacupuncture
Kubler et al22 assessed the effectiveness of electroacupuncture in the treatment of trigeminal neuralgia. A full course treatment consists of 10 sessions performed every second day. The technique consists of insertion of acupuncture needles 0.5 cm into the skin of the face. 4–6 needles are placed during each session. After insertion each needle is connected to a generator for electric stimulation. Impulses with low frequency (10 Hz) are applied for 30 min. Intensity of stimulation should be such that it should be felt by the patient but should not cause pain.
Outcome: the technique is recommended as an adjunct and not for monotherapy.22
Table 5 highlights some of the complications encountered in the interventional procedures for TN.10
Table 5.
Complications associated with interventional treatment modalities for TN.
| Procedure | Reported complications |
|---|---|
| Microvascular decompression | Cerebellar injury, eighth nerve injury and CSF leak |
| Radiofrequency gangliolysis | Dysaesthesia, corneal anaesthesia meningitis, carotid-cavernous fistula, intracranial haemorrhage, cranial nerve deficits |
| Glycerol gangliolysis | Meningitis, dysaesthesia, cranial nerve palsies, local haematomas, activation of herpes labialis, permanent masseter weakness, keratitis |
| Ballon compression | Dysaesthesia and masseter muscle weakness |
| Stereotactic radiosurgery | Facial paraesthesia |
| Peripheral neurectomy | Eye problems, dysaesthesia |
| Cryotherapy | Post-operative local infection, frank dysaesthesia |
| Alcohol block | Dysaesthesia, facial nerve palsy, loss of vision, cutaneous necrosis, development of bony sequestrum, diplopia |
| Local laser irradiation and laser puncture | None reported |
| Electroacupuncture | None reported |
Discussion
As regards the pharmacotherapy of TN, many of the drug trials in trigeminal neuralgia were conducted prior to the CONSORT guidelines for reporting of trials and have reporting flaws, making it difficult to compare studies. As more drugs become available in the market, it becomes important that these are subject to high quality randomized controlled trials and it may be more important to take a mechanism-based approach, rather than a disease-based approach to trial design, which would increase the size of trials as many are underpowered and hence fail to show an effect.23 Although many new drugs have come on the market carbamazepine remains the most effective drug, despite its poor tolerability, and is used by many neurologists and neurosurgeons as a diagnostic drug for this condition. However, it needs to be used carefully, especially in the elderly, in whom the side can outweigh the benefits. Oxcarbazepine has been shown to be effective and it is considered the second line of treatment for trigeminal neuralgia. Its side effect profile and reduced drug interactions make it especially useful in a population that is already on a range of drugs for other systemic conditions. As oxcarbazepine is pharmacologically similar to carbamazepine, it is important to remember that patients who are allergic to carbamazepine may also be allergic to oxcarbazepine. Lamotrigine, pimozide and baclofen are possibly effective for controlling pain in patients with trigeminal neuralgia, but there are problems associated with the use of these drugs. Lamotrigine is not recommended for rapidly progressive trigeminal neuralgia as too rapid a dose escalation leads to skin rashes. Pimozide's toxicity has virtually eliminated its use. Baclofen may be effective, but its long-term efficacy has not been evaluated. It may be a useful drug to use in combination with carbamazepine, or in patients who have an allergy to carbamazepine and or oxcarbazepine. Due to lack of high quality data, there is insufficient evidence to support or refute the efficacy of topical capsaicin, clonazepam, gabapentin, phenytoin, pregabalin, mexiletine and valproate for controlling pain in patients with trigeminal neuralgia.
Amongst the surgical and invasive procedures Barker et al24 performed a retrospective study on 1185 patients with trigeminal neuralgia who underwent microvascular decompression of the trigeminal nerve root over a 20-year period to assess the long-term outcome of the procedure. They concluded that microvascular decompression is a safe and effective treatment for trigeminal neuralgia, with a high rate of long-term success. Lopez et al25 performed a systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Studies on randomized controlled trials of retrogasserian percutaneous radiofrequency thermocoagulation, glycerol rhizolysis, balloon compression of the gasserian ganglion, and stereotactic radiosurgery were included in their review. They concluded that radiofrequency thermocoagulation offered higher rates of complete pain relief, compared with glycerol rhizolysis and stereotactic radiosurgery, although it demonstrated the greatest number of complications.
MVD can be effective in patients with a history of failed percutaneous procedures. Radiosurgery can be utilized to treat those that have not responded to other surgical modalities. In cases of recurrence following MVD, surgical re-exploration and neuroablative procedures have been recommended.26 For the treatment of atypical TN combination therapy has been tried successfully. Nguyen et al27 described the successful treatment of atypical V2 TN refractive to medical management, with pulsed radiofrequency treatment, a sphenopalatine block series, and low-dose methadone.
To sum up it can be suggested that carbamazepine (stronger evidence) or oxcarbazepine (better tolerability) should be offered as first-line treatment for pain control. For patients with TN refractory to medical therapy early surgical therapy may be considered. Gasserian ganglion percutaneous techniques, gamma knife and microvascular decompression may be considered as surgical options. Microvascular decompression may be considered over other surgical techniques to provide the longest duration of pain freedom.28
Conclusion
The natural history of trigeminal neuralgia is cyclical with periods of complete or partial remission, but as yet it is impossible to predict these. There are no studies that have attempted to determine the best long-term policy with regard to the continual or intermittent use of medication. The use of carbamazepine remains the first choice in the pharmacological treatment for classical TN, and oxcarbazepine, baclofen or lamotrigine as second choice. Although all the surgical procedures are inherently supported by low-level evidence, the results in thousands of patients indicate that the surgical treatments for trigeminal neuralgia are efficacious and acceptably safe. An evidence-based direct comparison between the different surgical procedures is so far impossible. Fig. 4 provides an algorithm for an evidence-based management of classical trigeminal neuralgia.
Fig. 4.
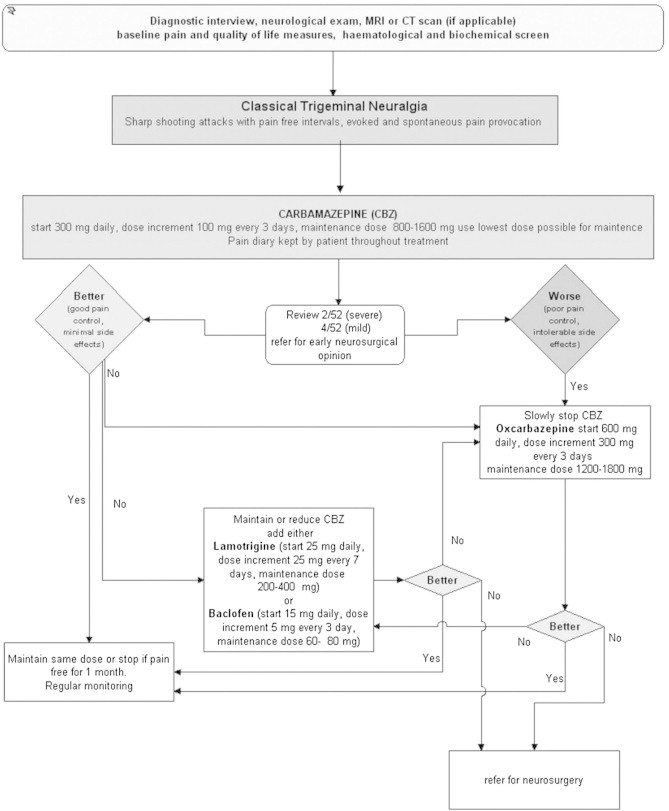
Algorithm of evidence-based medical management of classical trigeminal neuralgia.
From the perspective of the oral physician the pharmacotherapy constitutes the cornerstone in the management of TN. Individualized treatment regimens either monotherapy or a combination of drugs may be utilized as per patients' response and needs. At the same time it is important to be aware and updated of the role of the oral surgeon and radiologist in the application of the array of interventional procedures for TN, that require preoperative and intra-operative imaging.
Conflicts of interest
All authors have none to declare.
References
- 1.Malik N.A. 1st ed. Jaypee Brothers Medical Publishers (p) Ltd; New Delhi: 2002. Trigeminal Neuralgia and It's Management in Textbook of Oral and Maxillofacial Surgery. 633–649. [Google Scholar]
- 2.Lynch M.A., Brightman V.J., Greenberg M.S. Chronic oral sensory disorders: pain and abnormalities of taste. In: Cohen G.S., Brightman V.J., editors. Burket's Oral Medicine: Diagnosis and Treatment. 9th ed. Wilkins; Philadelphia: 1994. p. 334. [Google Scholar]
- 3.Headache Classification Subcommittee of the International Headache Society The international classification of headache disorders: 2nd ed. Cephalalgia. 2004;24(suppl 1):9–160. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 4.Okeson J.P. Neuralgias of the orofacial region. In: Harmon Lindsay., editor. Bell's Orofacial Pains: The Clinical Management of Orofacial Pain. 6th ed. Quintessence Publishing Co Inc; Illinois: 2005. p. 114. [Google Scholar]
- 5.Devor M., Amir R., Rappaport Z.H. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18:4–13. doi: 10.1097/00002508-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Love S., Coakham B.H. Trigeminal neuralgia – pathology and pathogenesis. Brain. 2001;124:2347–2360. doi: 10.1093/brain/124.12.2347. [DOI] [PubMed] [Google Scholar]
- 7.Delzell J.E., Grelle A.R. Trigeminal neuralgia – new treatment options for a well-known cause of facial pain. Arch Fam Med. 1999;8:264–268. doi: 10.1001/archfami.8.3.264. [DOI] [PubMed] [Google Scholar]
- 8.Siddiqui M.N., Siddiqui S., Ranasinghe S.J., Furgang A.F. Pain management: trigeminal neuralgia. Hosp Physician. 2000;1:64–70. [Google Scholar]
- 9.Ni S., Su W., Li X. Enhanced three-dimensional fast spoiled gradient recalled MRI combined with magnetic resonance angiography for preoperative assessment of patients with trigeminal neuralgia. J Clin Neurosci. 2009;16(12):1555–1559. doi: 10.1016/j.jocn.2009.02.028. [DOI] [PubMed] [Google Scholar]
- 10.Nurmikko T.J., Elderidge P.R. Trigeminal neuralgia – pathophysiology, diagnosis and current treatment. Br J Anaesth. 2001;87:117–132. doi: 10.1093/bja/87.1.117. [DOI] [PubMed] [Google Scholar]
- 11.Kondo A. Follow-up results of microvascular decompression in trigeminal neuralgia and hemifacial spasm. Neurosurgery. 1997;40:46–52. doi: 10.1097/00006123-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Scrivani S.J., Keith D.A., Mathews E.S., Kaban L.B. Percutaneous stereotactic differential radiofrequency thermal rhizotomy for the treatment of trigeminal neuralgia. J Oral Maxillofac Surg. 1999;57:104–111. doi: 10.1016/s0278-2391(99)90218-5. [DOI] [PubMed] [Google Scholar]
- 13.Hakansson S. Trigeminal neuralgia treated by the injection into the trigeminal cistern. Neurosurgery. 1981;9:638–646. doi: 10.1227/00006123-198112000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Kouzounias K., Schechtmann G., Lind G., Winter J., Linderoth B. Factors that influence outcome of percutaneous balloon compression in the treatment of trigeminal neuralgia. Neurosurg. 2010;67(4):925–934. doi: 10.1227/NEU.0b013e3181eb5230. [DOI] [PubMed] [Google Scholar]
- 15.Abdennebi B., Mahfouf L., Nedjahi T. Long-term results of percutaneous compression of the gasserian ganglion in trigeminal neuralgia. Stereotact Funct Neurosurg. 1997;68:190–195. doi: 10.1159/000099922. [DOI] [PubMed] [Google Scholar]
- 16.Lichtor T., Mullan J.F. A 10-year follow-up review of percutaneous microcompression of the trigeminal ganglion. J Neurosurg. 1990;72:49–54. doi: 10.3171/jns.1990.72.1.0049. [DOI] [PubMed] [Google Scholar]
- 17.Han J.H., Kim D.G., Chung H.T. Long-term outcome of gamma knife radiosurgery for treatment of typical trigeminal neuralgia. Int J Rad Oncol Bio Phy. 2009;75(3):822–827. doi: 10.1016/j.ijrobp.2008.11.046. [DOI] [PubMed] [Google Scholar]
- 18.Park Y.S., Kim J.P., Chang W.S., Kim H.Y., Park Y.G., Chang J.W. Gamma knife radiosurgery for idiopathic trigeminal neuralgia as primary vs. secondary treatment option. Clin Neurol Neurosurg. 2011;113(6):447–452. doi: 10.1016/j.clineuro.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Oturai A.B., Jensen K., Erikson J., Marsden F. Neurosurgery for trigeminal neuralgia: comparison of alcohol block, neurectomy and radiofrequency coagulation. Clin J Pain. 1996;12:311–315. doi: 10.1097/00002508-199612000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Zakrzewska J.M., Nally F.F. The role of cryotherapy (cryoanalgesia) in the management of paroxysmal trigeminal neuralgia: a six year experience. Br J Oral Maxillofac Surg. 1988;26:18–25. doi: 10.1016/0266-4356(88)90145-3. [DOI] [PubMed] [Google Scholar]
- 21.Stefanoff V. Treatment of trigeminal neuralgia (TN) with local laser irradiation and laser puncture. Int J Oral Maxillofac Surg. 1990;19:379–380. [Google Scholar]
- 22.Kubler A., Paweia T., Nroz G., Janusz W., Wnukiewicz C. Electroacupuncture in the treatment of trigeminal neuralgia. Int J Oral Maxillofac Surg. 1996;25:329. [Google Scholar]
- 23.Dworkin R.H., Turk D.C., Farrar J.T. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 24.Barker F.G., Janetta P.J., Bisonette D.J., Larkins M.V., Jho D.H. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077–1082. doi: 10.1056/NEJM199604253341701. [DOI] [PubMed] [Google Scholar]
- 25.Lopez B.C., Hamlyn P.J., Zakrzewska J.M. Systematic review of ablative neurosurgical techniques for the treatment of trigeminal neuralgia. Neurosurgery. 2004;54(4):973–982. doi: 10.1227/01.neu.0000114867.98896.f0. [DOI] [PubMed] [Google Scholar]
- 26.Han I., Shin D., Chang J. Effect of various surgical modalities in recurrent or persistent trigeminal neuralgia. Stereotact Funct Neurosurg. 2010;88(3):156–162. doi: 10.1159/000303530. [DOI] [PubMed] [Google Scholar]
- 27.Nguyen M., Wilkes D. Pulsed radiofrequency V2 treatment and intranasal sphenopalatine ganglion block: a combination therapy for atypical trigeminal neuralgia. Pain Pract. 2010;10(4):370–374. doi: 10.1111/j.1533-2500.2010.00382.x. [DOI] [PubMed] [Google Scholar]
- 28.Cruccu G., Gronseth G., Alksne J. AAN-EFNS guidelines on trigeminal neuralgia management. Eur J Neurol. 2008;15(10):1013–1028. doi: 10.1111/j.1468-1331.2008.02185.x. [DOI] [PubMed] [Google Scholar]


