Abstract
A profound change in thinking about the etiologies of many neurodegenerative diseases has far-reaching implications for developing therapeutics.
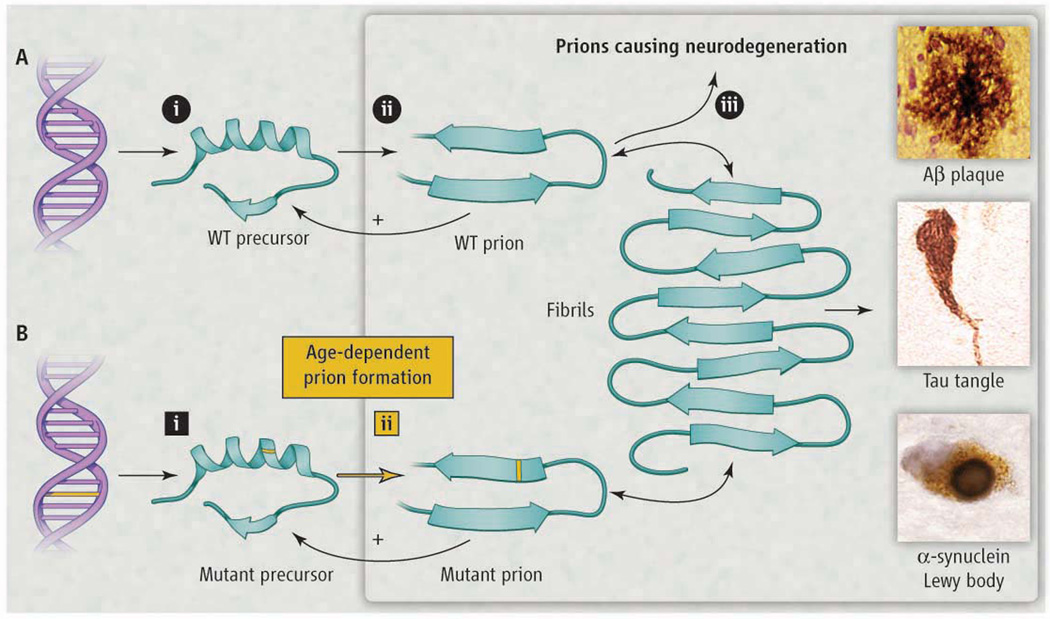
Many neurodegenerative diseases including Creutzfeldt-Jakob disease (CJD), Alzheimer’s, Parkinson’s, and amyotrophic lateral sclerosis (ALS) share two remarkable characteristics. The first is that more than 80% of cases are sporadic. The second is that although many of the disease-specific mutant proteins are expressed in embryogenesis, the inherited forms of these neurodegenerative diseases are late-onset. This suggests that some event occurs with aging that renders a disease-specific protein pathogenic. Over 20 years ago, I argued that this event involves a stochastic refolding of the etiologic protein into a misfolded infectious state known as a prion. While small numbers of prions could be cleared by protein degradation pathways, accumulation above a certain threshold over time would enable the prions to self-propagate (see the figure), resulting in central nervous system (CNS) dysfunction (1). In the past decade, there has been renewed interest in the possibility that the proteins causing neurodegeneration are all prions, which would profoundly influence the development of diagnostics and effective therapies.
Figure. Modeling Neurodegeneration Caused by Prions.
(A) Prions multiply through self-propagating cycles of post-translational modification; generally, an increase in β-sheet content accompanies prion formation. Pathogenic prions are most toxic as oligomers and less toxic after polymerization into amyloid fibrils. Depending on the protein, the fibrils coalesce into amyloid plaques, neurofibrillary tangles, or intracellular inclusions such as Lewy or Pick bodies. Drug targets for the development of therapeutics (octagons): 1) lowering precursor protein, 2) inhibiting prion formation, and 3) enhancing prion clearance. (B) Lateonset heritable neurodegeneration argues for two discrete events (squares): 1) mutation and 2) prion formation.
Many diverse explanations for the late onset of neurodegenerative diseases have been offered, including oxidative modifications of DNA, lipids, and/or proteins; somatic mutations; modified innate immunity; exogenous toxins; RNA-DNA differences; chaperone malfunction and haploinsufficiency. An alternative unifying explanation is that a diverse group of proteins can form prions, but most of the time, transformation of precursor proteins into prions represents a dead-end pathway where small numbers of prions are cleared via protein degradation pathways.
Fungal prions have been invaluable in defining the spectrum of prions. While yeast prions are not infectious in the sense of being released into the culture medium and infecting other yeast, they are transmissible from mother to daughter cells and thus, can readily multiply. Interestingly, many of the mutant proteins causing heritable neurodegenerative diseases are found in insoluble disease-specific aggregates known as amyloid deposits, such as plaques, neurofibrillary tangles (NFTs), and Lewy bodies (Figure 1, Table 1). Similarly, most fungal prions have a high β-sheet content and can polymerize into amyloid fibrils. That said, it is important to distinguish between prions and amyloids: prions need not polymerize into amyloid fibrils and can undergo self-propagation as oligomers. The self-propagation of an alternative conformational state is a key feature of all prions.
Table 1.
Evidence for prions causing many different neurodegenerative diseases
| Prion diseases | Precursor proteins |
Prion forms | Protein deposits |
Self-propagation in mammals | Self-propagation in cultured cells |
References |
|---|---|---|---|---|---|---|
| CJD/scrapie | PrPC | PrPSc | PrP plaques | inoc apes, monkeys, wt mice & Tg mice | N2a, GT1 | (1–13) |
| Alzheimer’s | APP | Aβ | Aβ plaques | inoc marmosets & Tg(ΔAPP) mice | (14–24) | |
| Tauopathies (FTD, PSP, Pick’s, CTE) | tau | tau aggregates | NFTs, Pick bodies | inoc Tg(HuTau), inoc Tg(HuTau,P301S) & inducible Tg(HuTau, ΔK280) mice | C17.2, HEK293 | (25–32) |
| Parkinson’s | α-synuclein | α-synuclein aggregates | Lewy bodies | Lewy bodies in grafts & inoc Tg(HuSNCA,H53T) mice | Primary mouse hippocampal neurons | (33–39) |
| fALS | ΔSOD1, ΔTDP43 | ΔSOD1 aggregates | Bunina bodies | N2a, HEK | (40–43) | |
| Huntington’s | ΔHtt | ΔHtt aggregates | Nuclear inclusions | Cos7 | (44–47) |
Abbreviations: CJD, Creutzfeldt-Jakob disease; PrP, prion protein; inoc, intracerebral inoculation; wt, wild-type; Tg, transgenic; APP, amyloid precursor protein; Aβ, amyloid beta; FTD, frontotemporal dementia; PSP, progressive supranuclear palsy; CTE, chronic traumatic encephalopathy; NFTs, neurofibrillary tangles; fALS, familial amyotrophic lateral sclerosis; ΔSOD1, mutant superoxide dismutase; ΔHtt, mutant huntingtin.
In Alzheimer’s disease (AD) for example, which is characterized by the deposition of Aβ amyloid plaques (Figure 1), Ridley and Baker performed a set of heroic experiments in which they inoculated human AD brain homogenates intracerebrally into marmosets. The marmosets developed Aβ amyloid plaques with incubation periods exceeding 3.5 years (2), demonstrating for the first time that the disease is transmissible and thus supporting the existence of a disease-causing prion. Similar results have been shown by Walker and Jucker, and others using transgenic AD mice (3, 4). Importantly, the disease agent has been identified as consisting solely of Aβ prions using synthetic Aβ peptides (5).
The tauopathies are a group of neurodegenerative diseases characterized by tau protein aggregation. Mutant tau has also been shown to be transmissible using transgenic mice (6), with tau aggregates being observed one year after inoculation. In addition, an aggregated segment of the tau protein initiated tau prion formation after being introduced into cultured cells (7). Among the tauopathies, the frontotemporal dementias (FTDs) are particularly interesting as they sit at the interface between psychiatry and neurology. Often, psychiatrists see FTD patients for years before recognizing subtle but progressive deterioration and referring them to neurologists. Aggregates of tau prions in the frontal lobes can produce inappropriate social interactions, depression, and diminished executive function as well as insomnia; later, drug abuse, alcoholism and suicide may occur. The discovery that some contact-sport athletes, as well as soldiers from the Iraq and Afghanistan wars, develop post-traumatic FTDs with psychiatric symptoms, often called post-traumatic stress disorders or PTSD initially, has begun to clarify how diverse neurological insults can all produce NFTs composed of tau prions (8, 9). Some of the variations in the clinical presentations of the tauopathies may be due to different prion strains, which represent distinct conformations (10).
Classical scrapie prions in ovines and rodents have been shown to spread throughout the peripheral nervous system and CNS. Heiko Braak and colleagues demonstrated the spreading of Aβ amyloid plaques and NFTs in AD from the entorhinal cortex to many regions of the cerebrum (11). Presumably, the Aβ prions spread through the extracellular space while tau prions seem more likely to move between neurons trans-synaptically (12). Recent studies have traced the spread of tau prions using fMRI intrinsic connectivity analysis in several tauopathies (13).
Parkinson’s disease is characterized by the accumulation of α-synuclein into so-called Lewy bodies in neurons. The finding of Lewy bodies in grafted fetal brain cells a decade after transplantation into Parkinson’s patients raised the possibility that α-synuclein proteins can also become prions that were synthesized in these grafted cells (14). The surface of Lewy bodies is covered with fibrils composed of β-sheet-rich, α-synuclein proteins (Figure 1). The normal form of α-synuclein seems to be either unstructured or high in α-helical structure, but like other prion proteins, α-synuclein can adopt a β-sheet-rich conformation. Though unproven, it seems likely that β-sheet-rich α-synuclein prions crossed from the transplanted patient’s own neurons into the grafted cells and induced a change in the structure of α-synuclein (15). Once established, this process became self-propagating, as with all pathogenic prions. Further evidence for α-synuclein prions comes from studies with recombinant α-synuclein assembled into fibrils that induced α-synuclein prions to multiply in both cultured cells and transgenic mice (16, 17).
Increasing evidence argues that prions cause some forms of ALS and may feature in the pathogenesis of Huntington’s disease. More than 60 different mutations in superoxide dismutase (SOD1) have been found to cause familial ALS. Aggregates of mutant human SOD1 have been shown to be self-propagating in cultured cells and as such are prions (18, 19). Studies of expanded polyglutamine repeats in a huntingtin protein fragment demonstrated self-propagation of spontaneous aggregates in cultured cells, i.e., they are prions (20). Huntingtin prions explain why people with 5 to 10 additional glutamines do not become ill until they are 40 to 60 years of age even though the mutant protein is synthesized in embryogenesis.
Not all animal prions cause disease. Some mammalian prions such as cytoplasmic polyadenylation element binding protein (CPEB), mitochondrial antiviral-signaling protein (MAVS), and T-cell-restricted intracellular antigen 1 (TIA-1) perform important cellular functions (21–23) including regulating gene transcription and the immune response. Unexpectedly, the biologically active forms of CPEB and MAVS are the oligomeric prion states and not the monomeric precursor proteins.
The convergence of studies demonstrating prions in the pathogenesis of common neurodegenerative maladies has been remarkable (Table 1). Many mysteries are now explicable within the framework of the prion concept. Most important, strategies for developing informative molecular diagnostics and effective therapeutics for these elusive disorders emerge from our knowledge of prions (see the figure). Early diagnosis will require reporters such as PET ligands to identify prions long before symptoms appear. Meaningful treatments are likely to require cocktails of drugs that diminish the precursor protein, interfere with conversion of precursors into prions, and/or enhance the clearance of prions.
References and Notes
- 1.Prusiner SB. Annu. Rev. Microbiol. 1989;43:345. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- 2.Ridley RM, Baker HF, Windle CP, Cummings RM. J. Neural Transm. 2006;113:1243. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- 3.Meyer-Luehmann M, et al. Science. 2006;313:1781. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 4.Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. Mol. Psychiatry. doi: 10.1038/mp.2011.120. (In press). [DOI] [PubMed] [Google Scholar]
- 5.Stöhr J, et al. Proc. Natl. Acad. Sci. U.S.A. (In press). [Google Scholar]
- 6.Clavaguera F, et al. Nat. Cell Biol. 2009;11:909. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Frost B, Jacks RL, Diamond MI. J. Biol. Chem. 2009;284:12845. doi: 10.1074/jbc.M808759200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McKee AC, et al. J. Neuropathol. Exp. Neurol. 2009;68:709. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omalu B, et al. Neurosurg. Focus. 2011;31:E3. doi: 10.3171/2011.9.FOCUS11178. [DOI] [PubMed] [Google Scholar]
- 10.Colby DW, Prusiner SB. Nat. Rev. Microbiol. 2011;9:771. doi: 10.1038/nrmicro2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braak H, Braak E. Acta Neuropathol. (Berl.) 1991;82:239. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 12.de Calignon A, et al. Neuron. 2012;73:685. doi: 10.1016/j.neuron.2011.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seeley WW, Crawford RK, Zhou J, Miller BL, Greicius MD. Neuron. 2009;62:42. doi: 10.1016/j.neuron.2009.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li JY, et al. Nat. Med. 2008;14:501. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 15.Olanow CW, Prusiner SB. Proc. Natl. Acad. Sci. USA. 2009;106:12571. doi: 10.1073/pnas.0906759106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mougenot AL, et al. Neurobiol. Aging. (In press). [Google Scholar]
- 17.Luk KC, et al. J. Exp. Med. (In press). [Google Scholar]
- 18.Münch C, O'Brien J, Bertolotti A. Proc. Natl. Acad. Sci. USA. 2011;108:3548. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grad LI, et al. Proc. Natl. Acad. Sci. USA. 2011;108:16398. [Google Scholar]
- 20.Ren PH, et al. Nat. Cell Biol. 2009;11:219. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Si K, Choi YB, White-Grindley E, Majumdar A, Kandel ER. Cell. 2010;140:421. doi: 10.1016/j.cell.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 22.Hou F, et al. Cell. 2011;146:448. doi: 10.1016/j.cell.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gilks N, et al. Mol. Biol. Cell. 2004;15:5383. doi: 10.1091/mbc.E04-08-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1.Eklund CM, Kennedy RC, Hadlow WJ. J. Infect. Dis. 1967;117:15. doi: 10.1093/infdis/117.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Alper T, Cramp WA, Haig DA, Clarke MC. Nature. 1967;214:764. doi: 10.1038/214764a0. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson AG, Meikle VMH, Fraser H. J. Comp. Pathol. 1968;78:293. doi: 10.1016/0021-9975(68)90005-4. [DOI] [PubMed] [Google Scholar]
- 4.Kimberlin RH. Sci. Prog. 1976;63:461. [PubMed] [Google Scholar]
- 5.Gibbs CJ, Jr, et al. Science. 1968;161:388. doi: 10.1126/science.161.3839.388. [DOI] [PubMed] [Google Scholar]
- 6.Prusiner SB, et al. Cell. 1983;35:349. doi: 10.1016/0092-8674(83)90168-x. [DOI] [PubMed] [Google Scholar]
- 7.Prusiner SB. N. Engl. J. Med. 1984;310:661. doi: 10.1056/NEJM198403083101021. [DOI] [PubMed] [Google Scholar]
- 8.Oesch B, et al. Cell. 1985;40:735. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- 9.Race RE, Fadness LH, Chesebro B. J. Gen. Virol. 1987;68:1391. doi: 10.1099/0022-1317-68-5-1391. [DOI] [PubMed] [Google Scholar]
- 10.Butler DA, et al. J. Virol. 1988;62:1558. doi: 10.1128/jvi.62.5.1558-1564.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Legname G, et al. Science. 2004;305:673. doi: 10.1126/science.1100195. [DOI] [PubMed] [Google Scholar]
- 12.Colby DW, et al. Proc. Natl. Acad. Sci. USA. 2009;106:20417. doi: 10.1073/pnas.0910350106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamgüney G, et al. Proc. Natl. Acad. Sci. USA. 2009;106:15002. doi: 10.1073/pnas.0907339106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glenner GG, Wong CW. Biochem. Biophys. Res. Commun. 1984;120:885. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 15.Masters CL, et al. Proc. Natl. Acad. Sci. U.S.A. 1985;82:4245. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang J, et al. Nature. 1987;325:733. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 17.Kane MD, et al. J. Neurosci. 2000;20:3606. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridley RM, Baker HF, Windle CP, Cummings RM. J. Neural Transm. 2006;113:1243. doi: 10.1007/s00702-005-0385-2. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Luehmann M, et al. Science. 2006;313:1781. doi: 10.1126/science.1131864. [DOI] [PubMed] [Google Scholar]
- 20.Eisele YS, et al. Science. 2010;330:980. doi: 10.1126/science.1194516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watts JC, et al. Proc. Natl. Acad. Sci. USA. 2011;108:2528. [Google Scholar]
- 22.Morales R, Duran-Aniotz C, Castilla J, Estrada LD, Soto C. Mol. Psychiatry. 2011 doi: 10.1038/mp.2011.120. [DOI] [PubMed] [Google Scholar]
- 23.Rosen RF, et al. J. Neurochem. 2012;120:660. doi: 10.1111/j.1471-4159.2011.07551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stöhr J, et al. Proc. Natl. Acad. Sci. U.S.A. 2011 [Google Scholar]
- 25.Corsellis JA, Bruton CJ, Freeman-Browne D. Psychol. Med. 1973;3:270. doi: 10.1017/s0033291700049588. [DOI] [PubMed] [Google Scholar]
- 26.Brion J-P, Passareiro H, Nunez J, Flament-Durand J. Arch. Biol. 1985;95:229. [Google Scholar]
- 27.Hutton M, et al. Nature. 1998;393:702. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 28.Omalu BI, et al. Neurosurgery. 2005;57:128. doi: 10.1227/01.neu.0000163407.92769.ed. [DOI] [PubMed] [Google Scholar]
- 29.Frost B, Ollesch J, Wille H, Diamond MI. J. Biol. Chem. 2009;284:3546. doi: 10.1074/jbc.M805627200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clavaguera F, et al. Nat. Cell Biol. 2009;11:909. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sydow A, Mandelkow EM. Neurodegener. Dis. 2010;7:28. doi: 10.1159/000283479. [DOI] [PubMed] [Google Scholar]
- 32.Guo JL, Lee VM-Y. J. Biol. Chem. 2011;286:15317. doi: 10.1074/jbc.M110.209296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polymeropoulos MH, et al. Science. 1997;276:2045. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 34.Spillantini MG, et al. Nature. 1997;388:839. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 35.Li JY, et al. Nat. Med. 2008;14:501. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 36.Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Nat. Med. 2008;14:504. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- 37.Volpicelli-Daley LA, et al. Neuron. 2011;72:57. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mougenot AL, et al. Neurobiol. Aging. 2011 [Google Scholar]
- 39.Luk KC, et al. J. Exp. Med. 2012;209:975. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosen DR, et al. Nature. 1993;362:59. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 41.Münch C, O'Brien J, Bertolotti A. Proc. Natl. Acad. Sci. USA. 2011;108:3548. doi: 10.1073/pnas.1017275108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grad LI, et al. Proc. Natl. Acad. Sci. USA. 2011;108:16398. [Google Scholar]
- 43.Udan M, Baloh RH. Prion. 2011;5:1. doi: 10.4161/pri.5.1.14265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.The Huntington's Disease Collaborative Research Group. Cell. 1993;72:971. doi: 10.1016/0092-8674(93)90585-e. [DOI] [PubMed] [Google Scholar]
- 45.Sapp E, et al. Ann. Neurol. 1997;42:604. doi: 10.1002/ana.410420411. [DOI] [PubMed] [Google Scholar]
- 46.Ren PH, et al. Nat. Cell Biol. 2009;11:219. doi: 10.1038/ncb1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee JM, et al. Neurology. 2012;78:690. doi: 10.1212/WNL.0b013e318249f683. [DOI] [PMC free article] [PubMed] [Google Scholar]