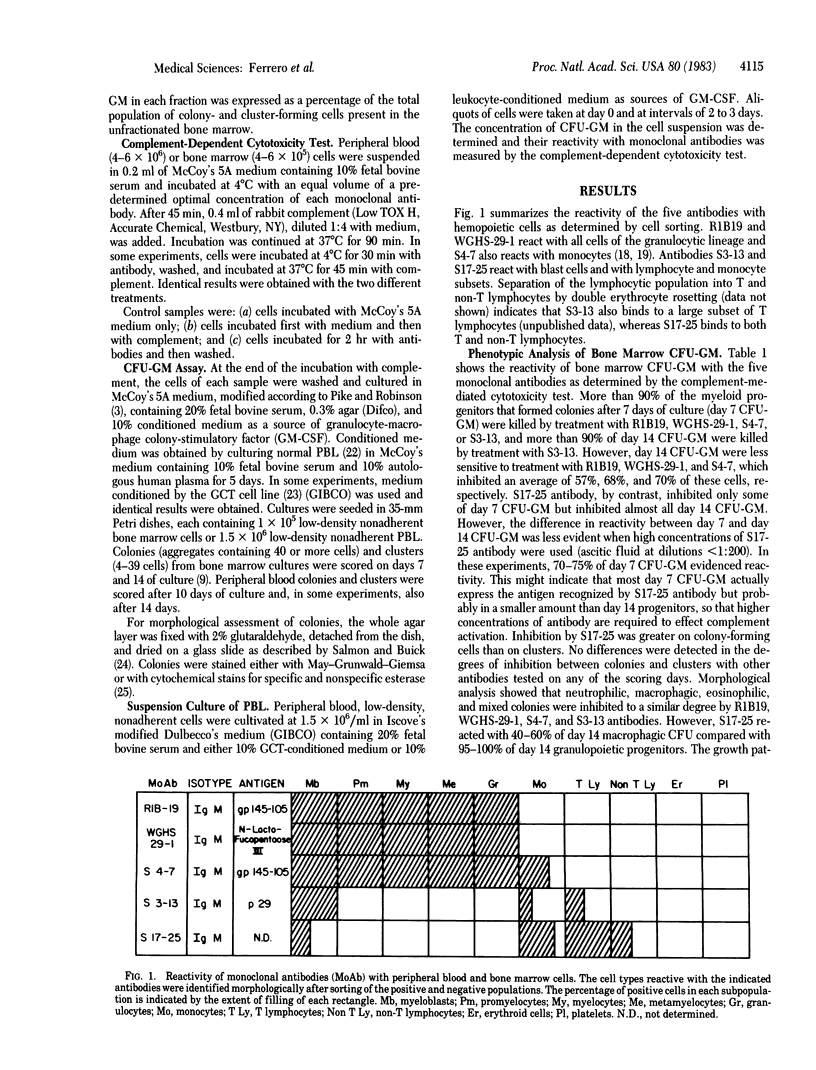
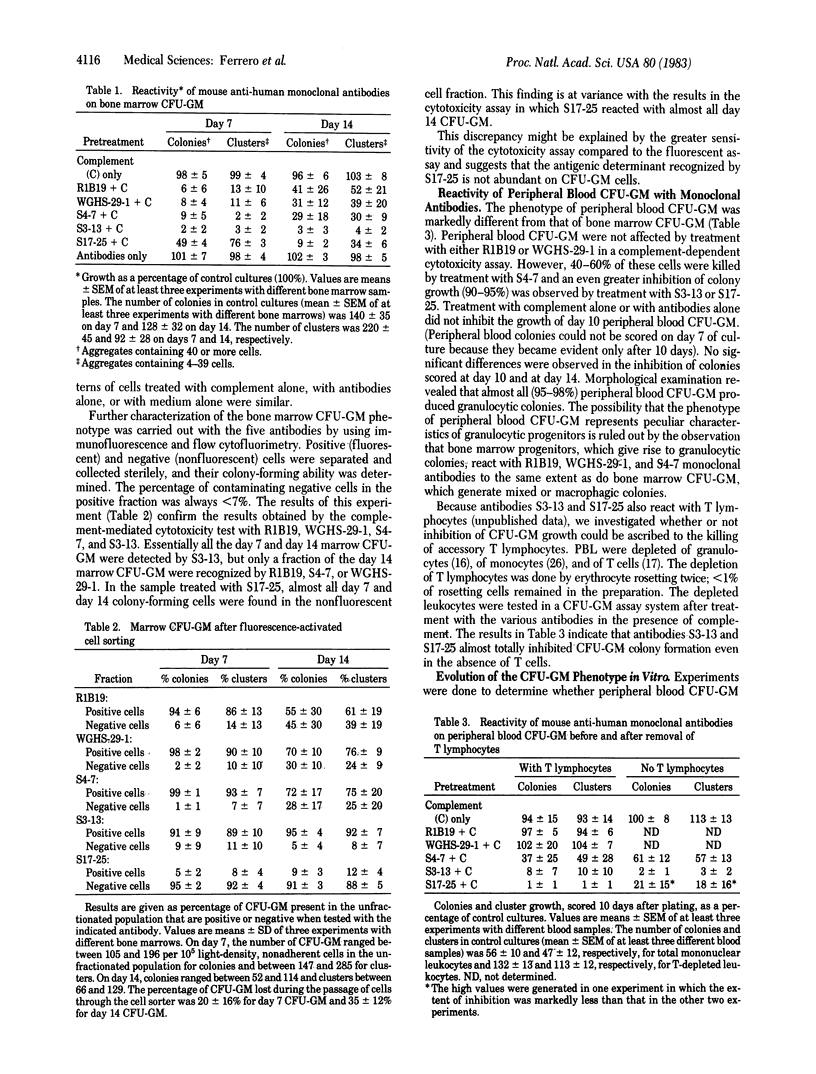
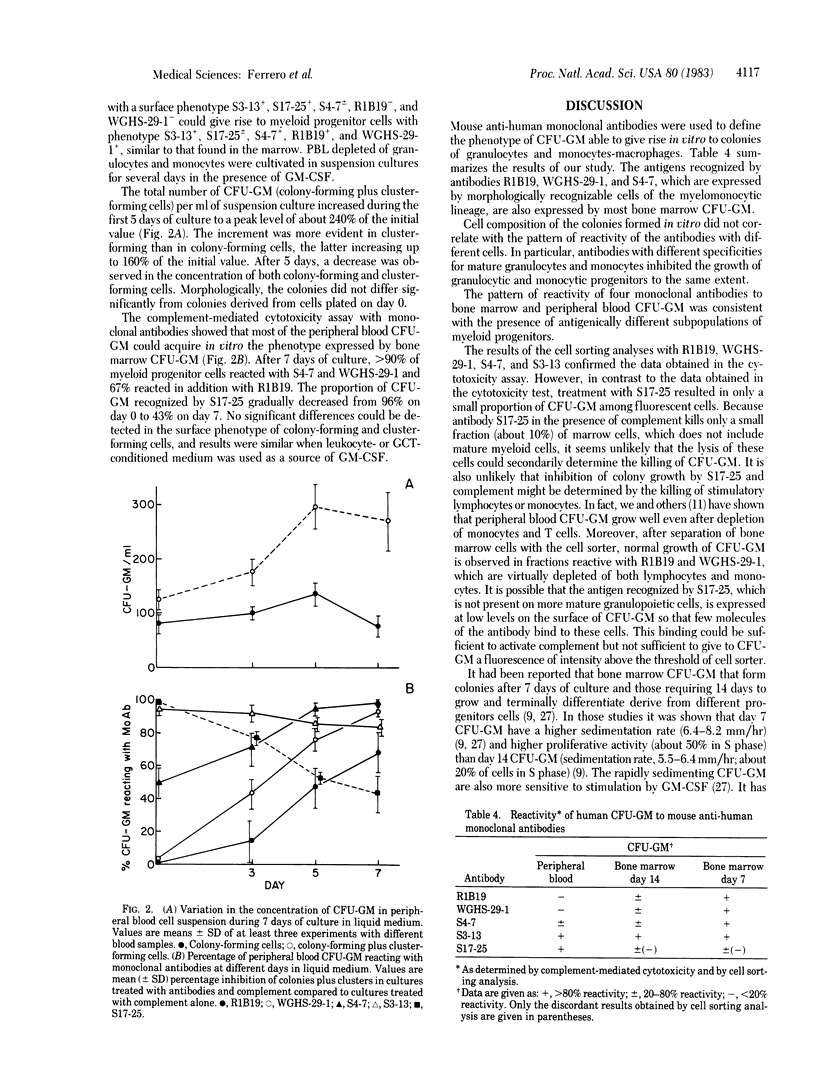
Abstract
Two types of progenitor cells of the human granulocytic and monocytic lineages (CFU-GM) can be distinguished by using mouse monoclonal antibodies against human hemopoietic cells. Type 1 CFU-GM contribute all of the peripheral blood CFU-GM as well as a small fraction of bone marrow CFU-GM and express surface antigens recognized by "anti-lymphomonocytic" monoclonal antibodies S3-13 and S17-25 but not the antigens recognized by R1B19 and WGHS-29-1 (two monoclonal antibodies that react with all the cells of the granulocytic lineage). Type 2 CFU-GM are present only in the marrow and react with S3-13, R1B19, and WGHS-29-1. Partial reactivity with S17-25 was observed only in the complement-dependent cytotoxicity test. In vitro culture of type 1 CFU-GM in liquid medium in the presence of granulocyte-macrophage colony-stimulatory factor (GM-CSF) generates colony-forming cells that have the surface phenotype of type 2 CFU-GM. This finding supports the idea of two different stages of maturation of myelomonocytic progenitor cells represented by type 1 and type 2 CFU-GM.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Brockhaus M., Magnani J. L., Herlyn M., Blaszczyk M., Steplewski Z., Koprowski H., Ginsburg V. Monoclonal antibodies directed against the sugar sequence of lacto-N-fucopentaose III are obtained from mice immunized with human tumors. Arch Biochem Biophys. 1982 Sep;217(2):647–651. doi: 10.1016/0003-9861(82)90546-x. [DOI] [PubMed] [Google Scholar]
- Chervenick P. A., Boggs D. R. Bone marrow colonies: stimulation in vitro by supernatant from incubated human blood cells. Science. 1970 Aug 14;169(3946):691–692. doi: 10.1126/science.169.3946.691. [DOI] [PubMed] [Google Scholar]
- Di Persio J. F., Brennan J. K., Lichtman M. A., Speiser B. L. Human cell lines that elaborate colon-stimulating activity for the marrow cells of man and other species. Blood. 1978 Mar;51(3):507–519. [PubMed] [Google Scholar]
- Fitchen J. H., Ferrone S., Quaranta V., Molinaro G. A., Cline M. J. Monoclonal antibodies to HLA-A,B, and Ia-like antigens inhibit colony formation by human myeloid progenitor cells. J Immunol. 1980 Nov;125(5):2004–2008. [PubMed] [Google Scholar]
- Gooi H. C., Feizi T., Kapadia A., Knowles B. B., Solter D., Evans M. J. Stage-specific embryonic antigen involves alpha 1 goes to 3 fucosylated type 2 blood group chains. Nature. 1981 Jul 9;292(5819):156–158. doi: 10.1038/292156a0. [DOI] [PubMed] [Google Scholar]
- Inoue S., Ottenbreit M. J. Heterogeneity of human colony-forming cells. Blood. 1978 Feb;51(2):195–206. [PubMed] [Google Scholar]
- Jacobsen N., Broxmeyer H. E., Grossbard E., Moore M. A. Colony-forming units in diffusion chambers (CFU-d) and colony-forming units in agar culture (CFU-c) obtained from normal human bone marrow: a possible parent-progeny relationship. Cell Tissue Kinet. 1979 Mar;12(2):213–226. doi: 10.1111/j.1365-2184.1979.tb00127.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen N., Broxmeyer H. E., Grossbard E., Moore M. A. Diversity of human granulopoietic precursor cells: separation of cells that form colonies in diffusion chambers (CFU-d) from populations of colony-forming cells in vitro (CFU-c) by velocity sedimentation. Blood. 1978 Jul;52(1):221–232. [PubMed] [Google Scholar]
- Johnson G. R., Dresch C., Metcalf D. Heterogeneity in human neutrophil, macrophage and eosinophil progenitor cells demonstrated by velocity sedimentation separation. Blood. 1977 Nov;50(5):823–831. [PubMed] [Google Scholar]
- Kaplan M. E., Clark C. An improved rosetting assay for detection of human T lymphocytes. J Immunol Methods. 1974 Jul;5(2):131–135. doi: 10.1016/0022-1759(74)90003-9. [DOI] [PubMed] [Google Scholar]
- Levine M. N., Fay J. W., Jones N. H., Metzgar R. S., Haynes B. F. Phenotypic characterization of human bone marrow granulocyte-macrophage forming progenitor cells. Blood. 1981 Nov;58(5):1047–1049. [PubMed] [Google Scholar]
- Metcalf D., MacDonald H. R. Heterogeneity of in vitro colony- and cluster-forming cells in the mouse marrow: segregation by velocity sedimentation. J Cell Physiol. 1975 Jun;85(3):643–654. doi: 10.1002/jcp.1040850317. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Williams N., Metcalf D. In vitro colony formation by normal and leukemic human hematopoietic cells: characterization of the colony-forming cells. J Natl Cancer Inst. 1973 Mar;50(3):603–623. doi: 10.1093/jnci/50.3.603. [DOI] [PubMed] [Google Scholar]
- Moore M. A., Williams N., Metcalf D. Purification and characterisation of the in vitro colony forming cell in monkey hemopoietic tissue. J Cell Physiol. 1972 Apr;79(2):283–292. doi: 10.1002/jcp.1040790213. [DOI] [PubMed] [Google Scholar]
- Perussia B., Trinchieri G., Lebman D., Jankiewicz J., Lange B., Rovera G. Monoclonal antibodies that detect differentiation surface antigens on human myelomonocytic cells. Blood. 1982 Feb;59(2):382–392. [PubMed] [Google Scholar]
- Pike B. L., Robinson W. A. Human bone marrow colony growth in agar-gel. J Cell Physiol. 1970 Aug;76(1):77–84. doi: 10.1002/jcp.1040760111. [DOI] [PubMed] [Google Scholar]
- Pluznik D. H., Sachs L. The cloning of normal "mast" cells in tissue culture. J Cell Physiol. 1965 Dec;66(3):319–324. doi: 10.1002/jcp.1030660309. [DOI] [PubMed] [Google Scholar]
- Rovera G., Ferrero D., Pagliardi G. L., Vartikar J., Pessano S., Bottero L., Abraham S., Lebman D. Induction of differentiation of human myeloid leukemias by phorbol diesters: phenotypic changes and mode of action. Ann N Y Acad Sci. 1982 Dec 10;397:211–220. doi: 10.1111/j.1749-6632.1982.tb43428.x. [DOI] [PubMed] [Google Scholar]
- Salmon S. E., Buick R. N. Preparation of permanent slides of intact soft-agar colony cultures of hematopoietic and tumor stem cells. Cancer Res. 1979 Mar;39(3):1133–1136. [PubMed] [Google Scholar]
- Winchester R. J., Ross G. D., Jarowski C. I., Wang C. Y., Halper J., Broxmeyer H. E. Expression of Ia-like antigen molecules on human granulocytes during early phases of differentiation. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4012–4016. doi: 10.1073/pnas.74.9.4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. M., Siminovitch L., Till J. E., McCulloch E. A. Evidence for a relationship between mouse hemopoietic stem cells and cells forming colonies in culture. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1209–1215. doi: 10.1073/pnas.59.4.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam L. T., Li C. Y., Crosby W. H. Cytochemical identification of monocytes and granulocytes. Am J Clin Pathol. 1971 Mar;55(3):283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- Young N. S., Hwang-Chen S. P. Anti-K562 cell monoclonal antibodies recognize hematopoietic progenitors. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7073–7077. doi: 10.1073/pnas.78.11.7073. [DOI] [PMC free article] [PubMed] [Google Scholar]


