Abstract
Introduction
The objective of this paper is to systematically review the existing evidence of the effectiveness and safety profile of a long-acting inhaled muscarinic antagonist as add-on therapy in patients with asthma that is uncontrolled despite inhaled corticosteroid (ICS) use.
Methods
With the assistance of two experienced research librarians, we searched Ovid MEDLINE/PubMed (1946 to September 12, 2013), the Cochrane Library review, and the TRIP database. The key search terms were “tiotropium and asthma.” The search was limited to human data published in English. Included in the systematic review were all randomized controlled trials that evaluated the efficacy of tiotropium in patients with asthma. The clinical trials had to be at least 4 weeks in duration and to provide adequate information on clinically appropriate end points in asthma care (eg, change in lung function, exacerbation rates, and/or ICS dosing). Data on patient characteristics, study design, outcome measures, concomitant asthma medication, and adverse events were extracted from the full text of each included individual study. Marked heterogeneity of study design precluded statistical pooling of results for a meta-analysis. Consequently, only descriptive summaries of outcomes are provided.
Results
Our database search retrieved 149 citations. We found five randomized controlled trials in humans that met our criteria for inclusion in the systematic review. We also found two open-label uncontrolled trials that were considered in the discussion. Each of the five included studies met the Consolidated Standards of Reporting Trials criteria for a well-designed randomized trial.
Discussion
The five clinical studies included in this systematic review focused on evaluating the efficacy of tiotropium as add-on therapy to ICS or ICS in combination with a long-acting inhaled β2-agonist (LABA) in patients with uncontrolled moderate to severe persistent asthma. Tiotropium maintained lung function when ICSs were tapered and when an LABA was discontinued. Tiotropium improved lung function when added to ICS alone or ICS–LABA combination therapy. In the only trial to have compared the addition of tiotropium with doubling the dose of ICS, tiotropium provided significantly superior results. In trials in which the addition of tiotropium was compared with salmeterol, the beneficial effects of these two bronchodilators were similar. No safety concerns were found with use of tiotropium as add-on therapy.
Conclusion
Tiotropium may have a beneficial role in moderate to severe persistent asthma despite use of an ICS or ICS and LABA. Use of tiotropium as add-on therapy poses no safety concerns.
Keywords: tiotropium, asthma, lung, inflammation, inhaled corticosteroid, LABA, LAMA
Introduction
Asthma is a chronic inflammatory airway disease.1 Airway inflammation in asthma is characterized by bronchial wall infiltration by eosinophils, activated mast cells, and T lymphocytes. Cytokines from T helper 2 cells and leukotrienes play key roles in mediating airway inflammation. Also seen are goblet cell hyperplasia, mucus gland hypertrophy, and airway mucus accumulation. Increased airway smooth muscle mass and reversible bronchoconstriction are other important features of asthma. Airway inflammation, mucus accumulation, and bronchospasm account for the symptoms of shortness of breath, wheezing, cough, and chest tightness in asthma.1 Understanding asthma pathophysiology is an important issue for health care practitioners in the US because asthma prevalence increased from 7.3% in 2001 to 8.4% in 2010. In 2010, an estimated 25.7 million Americans had asthma – 18.7 million adults aged 18 years and over and 7.0 million children aged 0–17 years.2
The ultimate goal of asthma management is to minimize symptoms, prevent acute exacerbations, and avoid adverse medication effects. The pathophysiologic features of asthma explain why current US and international guidelines recommend the use of an inhaled corticosteroid (ICS) and a short-acting inhaled β2-agonist (SABA) for treatment of persistent asthma.3,4 ICSs can control airway inflammation,5,6 and SABAs can manage acute symptoms related to bronchospasm. To reduce the risk of drug-related side effects, current asthma management guidelines recommend a stepwise treatment approach to achieving optimal asthma control, with more aggressive ICS dosing reserved for patients with more severe disease.3,4 However, prospective studies have confirmed that a significant proportion of asthma patients do not achieve asthma control, even with maximal approved doses of ICS.7 In patients who do not respond to ICS treatment alone, asthma guidelines recommend addition of another long-term controller, such as a long-acting inhaled β2-agonist (LABA), a leukotriene-modifying agent (LMA), theophylline, and/or an anti-immunoglobulin E monoclonal antibody. Despite the addition of agents like LABAs and LMAs to ICSs, many asthma patients still remain both symptomatic and obstructed.8 There is clearly a need for alternative medications with different modes of action in asthma management. One intriguing option as an add-on therapy for asthma patients not responding to ICSs is an anticholinergic.
Tiotropium bromide is a long-acting inhaled muscarinic antagonist (LAMA). It has high potency as a selective antagonist at the muscarinic acetylcholine (Ach) receptors. Tiotropium rapidly dissociates from M2 Ach receptors but slowly dissociates from M3 Ach receptors.9 Consequently, tiotropium provides 24-hour bronchodilation as maintenance therapy in moderate to severe chronic obstructive pulmonary disease (COPD).10,11 Tiotropium has also been shown to reduce goblet cell metaplasia and airway smooth muscle mass in allergen-challenged mice and guinea pigs.12,13 Early evidence of clinical benefit with LAMA therapy in asthma was identified in COPD patients with airway hyper-responsiveness and concomitant asthma.14–16
The objective of this paper is to systematically review the existing evidence of the use of tiotropium in patients with uncontrolled asthma and to provide recommendations for its potential role in the treatment of asthma. We sought to answer the question what is the existing evidence of the effectiveness and safety profile of an LAMA as add-on therapy in patients with asthma that is uncontrolled despite ICS use?
Materials and methods
Search strategy and study selection
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria when performing this study.17 With the assistance of two experienced research librarians, we searched Ovid MEDLINE/PubMed (1946 to September 12, 2013), the Cochrane Library review, and the TRIP database. The key search terms were “tiotropium and asthma.” The search was limited to human data published in English.
Abstracts of articles identified in the search were reviewed independently by two of the authors. Articles meeting pre-identified inclusion and exclusion criteria were selected for evaluation in this systematic review. Disagreements about inclusion of individual articles were resolved by consensus or review by a third author. Included in the systematic review were all randomized controlled trials that evaluated the efficacy of tiotropium in patients with asthma. The clinical trials had to be at least 4 weeks in duration and to provide adequate information on clinically appropriate end points in asthma care (eg, change in lung function, exacerbation rates, and/or ICS dosing). Excluded were unpublished and ongoing research, animal studies, reviews, case reports, case series, editorials, and studies in COPD patients (with or without asthma). The references of published articles identified in the search were examined for additional studies appropriate for inclusion in the review.
Data extraction and quality assessment
Data on patient characteristics, study design, outcome measures, concomitant asthma medication, and adverse events were extracted from the full text of each included individual study. The methodologic quality of the trials was evaluated using criteria reported in the Consolidated Standards of Reporting Trials (CONSORT) Statement.18 Marked heterogeneity of study design precluded statistical pooling of results for a meta-analysis. Consequently, only descriptive summaries of outcomes are provided.
Results
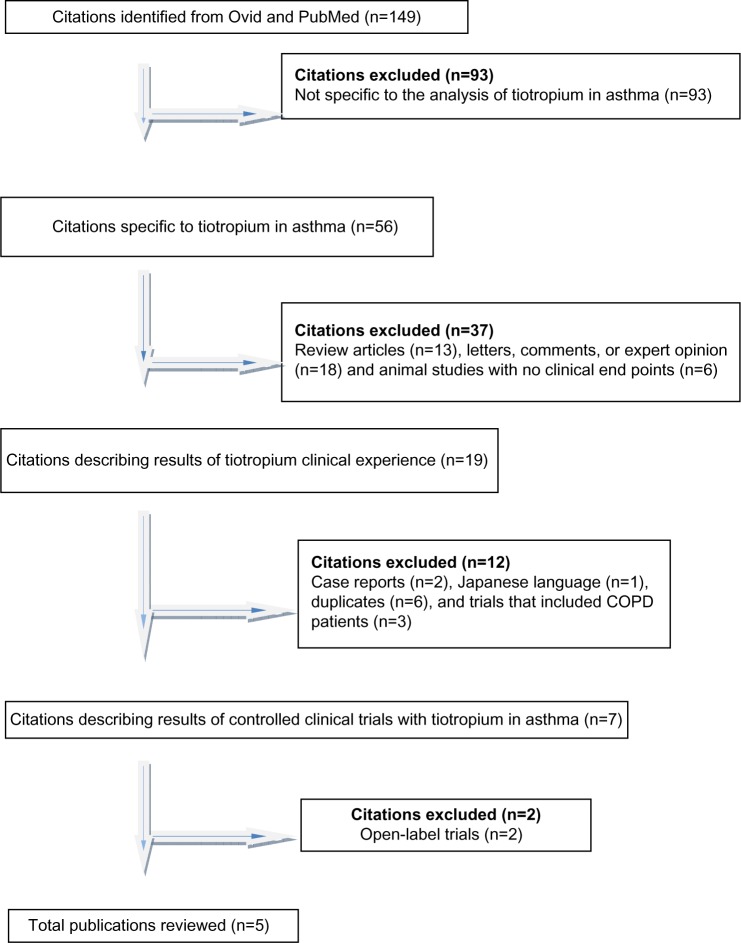
Our database search retrieved 149 citations of which 93 were excluded because they did not meet the inclusion criterion of being specific to the effect of tiotropium in asthma (Figure 1). An additional 37 citations were excluded because they did not describe results of clinical trials in humans. Twelve other citations were not included. Two were single-patient case reports, one was in Japanese, six had duplicate information, and three studies did not make a distinction between asthma and COPD.
Figure 1.
Selection process of citations.
Abbreviation: COPD, chronic obstructive pulmonary disease.
We found five randomized controlled trials in humans that met our criteria for inclusion in the systematic review.19–23 One open-label study without a control was identified in the database search.24 This study was not included in the systematic review but was considered in the discussion. Following review of the references of the selected papers, an additional open-label trial without a control group was identified.25 This study was not included in the systematic review but was considered in the discussion. Summary descriptions of the five publications that met inclusion criteria are provided in Tables 1 and 2. Detailed descriptions of the five selected studies are provided as follows.
Table 1.
Design summary of the reviewed articles
| Study | Inclusion criteria | Design | Duration | Size | Intervention | Overall conclusion |
|---|---|---|---|---|---|---|
| Bateman et al22 | Inadequately controlled moderate persistent asthma: B16-Arg/Arg patients FEV1 <90% for patients on ICS–LABA or <80% for patients on ICS Reversibility (> 12% and 200 mL ≤10 pack-year smoking |
Randomized, double-blind, double-dummy, placebo-controlled, parallel-group | 16 weeks | 388 | Initial 4 weeks: usual ICS dose (400–1,000 μg daily) and 50 μg salmeterol twice daily (run-in) Subsequent 16 weeks: 5 μg tiotropium daily in evening 50 μg salmeterol twice a day Placebo |
Tiotropium superior to placebo and noninferior to salmeterol in moderate persistent asthma with B16-Arg/Arg genotype |
| Fardon et al19 | Severe persistent asthma: FEV1 <65% predicted FVC <80% predicted Reversibility >5% Nonsmokers |
Randomized, double-blind, placebo-controlled, crossover | 4 weeks | 18 | Initial 4 weeks: fluticasone 1,000 mcg daily (run-in) Subsequent 4-week crossover periods: Fluticasone 500 μg all patients Salmeterol and placebo Salmeterol and tiotropium |
Tiotropium may offer small benefit when added to salmeterol in stepping down ICS with no effect on the quality of life or subjective symptoms |
| Kerstjens et al21 | Uncontrolled severe persistent asthma: FEV, <80% predicted despite high-dose ICS and LABA ACQ ≥ 1.5 < 10 pack-year smoking |
Randomized, double-blind, placebo-controlled, crossover | 8 weeks | 107 | Initial 2 weeks: usual dose of ICS and LABA (screen) Subsequent 8-week crossover periods: Tiotropium 5 mcg in morning Tiotropium 10 mcg in morning Placebo |
Tiotropium significantly improved lung function when added to ICS – LABA |
| Kerstjens et al23 | Inadequately controlled severe persistent asthma: FEV1 ≤80% ACQ ≥1.5 despite high-dose ICS and LABA < 10 pack-year smoking One exacerbation requiring oral CS in previous year |
Randomized, double-blind, placebo-controlled, parallel-group | 48 weeks | 912 | Initial 4 weeks: usual ICS (≥800 μg) and LABA (run-in) Subsequent 48 weeks: Tiotropium 5 μg daily in morning Placebo group |
Tiotropium improved lung function and reduced exacerbations when added to ICS–LABA |
| Peters et al20 | Inadequately controlled severe persistent asthma: FEV1 <70% or symptoms >6 days/week or nocturnal symptoms >twice/week < 10 pack-year smoking |
Randomized, three-way double-blind, triple-dummy, crossover | 14 weeks | 210 | Initial 4 weeks: ICS 160 μg daily (run-in) Subsequent 14-week crossover periods: Tiotropium 18 μg in morning Salmeterol 50 μg twice daily ICS 320 μg daily (double-dose ICS) |
Tiotropium noninferior to salmeterol and superior to double-dose ICS regarding symptoms and lung function |
Abbreviations: ACQ, Asthma Control Questionnaire; CS, corticosteroid; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; LABA, long-acting inhaled β2-agonist.
Table 2.
Results summary of the reviewed articles
| Study | Primary outcome | Secondary outcome | Mode of delivery | Safety |
|---|---|---|---|---|
| Bateman et al22 | Change in mean weekly predose PEF: Tiotropium: −3.9±4.87 L/min Salmeterol: −3.2±4.64 L/min Placebo: −24.6±4.84 L/min. Tiotropium was superior to placebo (P<0.05) but was noninferior to salmeterol (estimated difference −0.78 L/min, 95% CI: −13.096 to 11.53, P=0.002). |
Mean weekly PEF and FEV1 worse in the placebo group; similar between salmeterol and tiotropium (P<0.01). Rescue medication use, limitation of activities, and daytime asthma symptoms maintained in active treatment groups but worsened in the placebo group. Reduction of rescue medication use statistically significant only in the salmeterol group. No evidence of a treatment response in quality of life measures. Number of asthma-free days slightly increased in the tiotropium and salmeterol groups, more than placebo. |
Respimat | Adverse events reported by 41.3%, 39.8%, and 41.8% of patients in the placebo, tiotropium, and salmeterol groups, respectively. Individual adverse event reporting generally similar for the three treatment groups. Asthma exacerbations reported in similar rates for the three treatment groups. No cardiovascular events were reported. |
| Fardon et al19 | Not stated but change in FEV1 and AM PEF emphasized. Significant improvements in AM PEF with salmeterol–ICS (41.5 L/min, P<0.01) and salmeterol–ICS-tiotropium (55.3 L/min, P<0.0l). Significant improvements in FEV1 with salmeterol–ICS-tiotropium (0.17 L, P<0.05) but not salmeterol–ICS. No differences between the two treatments in change in AM PEF or FEV1 |
Salmeterol slightly superior regarding asthma-free days than tiotropium (1.84 vs 1.34, respectively, 95% CI: −0.955 to −0.069). RV and TLC were not altered by any treatment. Addition of tiotropium to salmeterol–ICS reduced exhaled NO by 2.86 ppb (P<0.05). No changes in quality of life or symptoms. |
Metered dose inhaler | Not discussed directly. |
| Kerstjens et al21 | Peak FEV, within 3 hours after dosing at end of 8-week treatment. 5 μg tiotropium: peak FEV1 significantly higher than placebo (139 mL difference, 95% CI: 96–181 mL). 10μg tiotropium: peak FEV1 significantly higher than placebo (170 mL difference, 95% CI: 128–213 mL). There were no significant differences between active treatments. |
Significant differences between 5 μg tiotropium and placebo for trough FEV1 (difference of 86 mL, 95% CI: 41–132 mL) and between 10 μg tiotropium and placebo (difference 113 mL, 95% CI: 67–59 mL). Both treatment doses of tiotropium were superior to placebo in peak FVC, trough FVC, and area under the curve for FVC during the first 3 hours, and weekly AM and PM PEF. The 10 μg dose of tiotropium resulted in significantly better AM and PM PEF than the lower dose. No significant differences in asthma symptoms, use of rescue medications, and change in Mini AQLQ with either dose of tiotropium or placebo. |
Respimat | Adverse events in 40%, 42%, and 50% of patients receiving placebo, 5 μg tiotropium, and 10 μg tiotropium, respectively. Dry mouth reported by more patients in the 10 μg tiotropium dose group. Severe adverse events in 5 patients (all nonlife-threatening and nondrug related). No blood pressure, pulse rate, ECG, or laboratory test abnormalities could be attributed to the study drug or dose. |
| Kerstjens et al23 | Coprimary end points were peak FEV, difference in tiotropium vs placebo after 3 hours of administration and trough FEV1 response at week 24. In both trials there was a superior response to tiotropium compared with placebo for peak FEV, (trial 1 difference of 86±34 mL, P=0.01; trial 2 difference of 154±32 mL, P<0.00l) and trough FEV1 (trial 1 difference of 88±3 1 mL, P=0.01; trial 2 difference of 110±30 mL, P<0.001). |
Time to first severe asthma exacerbation increased in the tiotropium group by 56 days, with overall 21% reduction in exacerbations (P=0.03). Small but inconsistent changes with tiotropium compared with placebo in ACQ-7, AQLQ, symptom-free days recorded in the electronic diary, and use of rescue medications. |
Respimat | Adverse events reported in 73.5% and 80.3% of patients in the tiotropium and placebo groups, respectively. Serious adverse events reported in 8.1% of patients in the tiotropium group and 8.8% in the placebo group. The only individual adverse event reported in more than 2% of patients and more frequently in the tiotropium group than placebo was allergic rhinitis. Drug-related cardiac events were infrequent (0.4% in the tiotropium group and 0.2% in the placebo group). Blood pressure, pulse rate, and ECG abnormalities were balanced between the two groups. Three life-threatening events occurred in the tiotropium group (2 asthma exacerbations and 1 cerebral infarction) but none with placebo. |
| Peters et al20 | AM PEF: tiotropium was superior to double dose of ICS (mean difference 25.8 L/min, P<0.00l). Tiotropium was noninferior to salmeterol (difference of 6.4 L/min, P=0.26). | Tiotropium was superior to double dose of ICS for PM PEF (difference of 35.3 L/min, P<0.001), FEV, (difference of 0.10 L, P=0.004), and daily symptom scores (difference −0.1 1 points, P<0.001). Tiotropium was noninferior to salmeterol in other lung function and symptom assessments except for prebronchidilator FEV1 (tiotropium superior with difference of 0.11 L, P=0.003). |
HandiHaler | Individual adverse events not reported. Asthma exacerbations occurred in 9 patients on tiotropium, 16 patients on double-dose ICS, and 5 patients on salmeterol. There were 12 serious adverse events, 3 in the tiotropium group, 4 in the double-dose ICS group, and 4 in the salmeterol group. |
Abbreviations: ACQ, Asthma Control Questionnaire; AM, morning; AQLQ, Asthma Quality of Life Questionnaire; CI, confidence interval; ECG, electrocardiogram; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; ICS, inhaled corticosteroid; NO, nitric oxide; PEF, peak expiratory flow; PM, evening; RV, residual volume; TLC, total lung capacity.
Fardon et al19 evaluated the ICS sparing ability of tiotropium in patients with severe persistent asthma. Inclusion criteria for this clinical trial required that study patients be adults with a clinically established diagnosis of asthma and with >15% reversibility after an SABA or a short-acting inhaled muscarinic antagonist (SAMA). Inclusion criteria also required a forced expiratory volume in 1 second (FEV1) of ≤65%. Patients were excluded if they had had a recent exacerbation or had smoked at any time during their life. The study was a double-blind, randomized, placebo-controlled, crossover study. Patients were changed from their usual asthma medications to 1,000 μg of fluticasone daily for a 4-week run-in period. Their dose of fluticasone was then halved to 500 μg/day and an LABA was added (125/25 μg per puff and two puffs twice daily). At that point, patients entered a crossover phase in which they were randomized, first, to the addition of either tiotropium bromide (18 μg administered once daily from a metered dose inhaler) or a matched placebo before, secondly, receiving the other treatment. The crossover phases lasted 4 weeks and there was no washout period between study phases. A specific primary outcome measure was not identified in this study. Twenty-six lifelong nonsmoking severe asthmatics with a mean FEV1 of 1.49 L (51% predicted) entered the run-in phase, but only 18 patients completed the study. Four weeks after patients were switched from fluticasone 1,000 μg/day to the lower dose of ICS with an LABA for the run-in period, there were significant increases in morning (AM) and evening (PM) peak expiratory flow (PEF). Four weeks after switching to the combination of an ICS–LABA with tiotropium there were significant improvements in FEV1 (0.17 L; 95% confidence interval [CI]: 0.01–0.32; P<0.05) and forced vital capacity (FVC) (0.24 L, 95% CI: 0.05–0.43; P<0.05). The ICS–LABA–tiotropium combination also resulted in reduced exhaled nitric oxide levels by 2.86 ppb (95% CI: 0.12–5.6; P<0.05). The increases in lung function with the triple combination of ICS–LABA–tiotropium were not significantly different from the changes found with the ICS–LABA combination, though. Although there were small improvements in subjective symptoms of quality of life, measured by the Mini Asthma Quality of Life Questionnaire (Mini AQLQ) after both crossover phases, the changes were not significant. The small sample size and the fact that eight of the participants dropped out due to an asthma exacerbation were mentioned as possible reasons for the failure of the study to detect improvement in quality of life. The authors also evaluated acute changes in lung function after single doses of an SABA with an SAMA. There was a significant bronchodilator benefit after the inhalation of albuterol and ipratropium, but the response to short-acting inhaled bronchodilators did not predict the response to tiotropium. Safety information was not provided in this study.
Peters et al20 compared the benefit of tiotropium as add-on therapy to an ICS with that obtained by either adding an LABA or doubling the ICS dose in patients with uncontrolled persistent asthma. Patients were included if they were nonsmokers (less than 10 pack-years per lifetime), were aged 18 years or older, had an FEV1 >40% of the predicted value, and had a diagnosis of asthma confirmed by bronchodilator reversibility or bronchial hyper-responsiveness. The study was a three-way, double-blind, triple-dummy, crossover trial. Patients entered a 4-week run-in period during which they were treated with 160 μg of beclomethasone daily. They were entered into the trial if they had either an FEV1 ≤70% or daytime symptoms for 6 or more days per week or night-time symptoms 2 or more nights per week during the final 2 weeks of the run-in period. The three crossover periods involved adding tiotropium (18 μg every AM from a dry powder inhaler), adding salmeterol (50 μg twice daily by dry powder inhaler), or doubling the ICS to 320 μg daily. Each treatment period lasted 14 weeks with a 2-week washout phase between treatment periods. The identified primary outcome measure in this study was the AM PEF, and the primary hypothesis was that the addition of tiotropium to ICS would provide greater benefit than doubling the dose of ICS. Two hundred and ten severe asthma patients with a mean FEV1 of 2.31 L (71.5% predicted) met the entry criteria and were randomized. Thirty-six participants dropped out during the treatment phase due to withdrawal of consent, loss to follow-up, drug-related adverse events, or miscellaneous reasons. The use of tiotropium resulted in a superior primary outcome compared with doubling the dose of an ICS. There was a greater improvement in the primary outcome measure AM PEF with tiotropium compared with a double dose of ICS, with a mean difference of 25.8 L/min (P<0.001). Tiotropium also showed superiority in most secondary outcomes. PM PEF was larger, with a difference of 35.5 L/min (P<0.001); the proportion of asthma control days was greater, with a difference of 0.079 (P=0.01); the FEV1 before bronchodilation was larger, with a difference of 0.10 L (P=0.004); and daily asthma symptom scores were lower, with a difference of −0.11 points (P<0.001). In the secondary outcome analysis, addition of tiotropium to an ICS was found to be noninferior to salmeterol, as measured by the AM PEF. Although the prebronchodilator and postbronchodilator FEV1 responses favored tiotropium, tiotropium was noninferior to salmeterol in other results, including PM PEF, asthma daily symptom scores, and sputum eosinophils. Interestingly, a small FEV1 decrease in patients receiving albuterol in addition to the standing dose of salmeterol suggested a tachyphylaxis effect for β-agonists that was not found in the tiotropium group. Not increasing the dose of ICS by more than a factor of two was proposed as a potential weakness of the trial, together with the short study duration (14 weeks) and the small sample size. Although long-term safety issues were not assessed in this trial, numerically more asthma exacerbations occurred during the double-dose ICS treatment period than during the tiotropium treatment period (n=16 versus [vs] n=9), with the fewest occurring with salmeterol treatment (n=5).
Kerstjens et al21 sought to compare the efficacy and safety of different doses of tiotropium in the Respimat® inhaler (Boehringer Ingelheim Pharma GmbH and Co, KG, Ingelheim, Germany) against placebo as add-on therapy in patients with uncontrolled severe persistent asthma. Patients included in this trial were aged 18 years and older and had at least a 5-year history of asthma. They were required to have a postbronchodilator FEV1 ≤80% and to have an Asthma Control Questionnaire (ACQ) score of ≥1.5 despite treatment with high-dose ICS and an LABA. Interestingly, patients were allowed to remain on a stable dose of theophylline, LMA, and oral corticosteroid throughout the study. Patients were excluded if they had a smoking history of ≥10 pack-years or a diagnosis of COPD. The authors conducted a randomized, double-blind, crossover study with three 8-week treatment periods. After a 2-week run-in period, eligible patients were randomized to receive each of three treatments in a random sequence for 8 weeks in a crossover design (5 μg or 10 μg of tiotropium or matching placebo administered as two actuations once daily in the AM from the Respimat inhaler). There was no washout phase between treatment periods. Throughout the 24-week treatment period, participants recorded PEF and FEV1 values twice daily at home using the Asthma Monitor AM2+ and responded to daily questions in the electronic diary component of this device. The primary end point was peak FEV1 (obtained within 3 hours of dosing) at the end of each treatment period. Of the 107 randomized patients, 100 completed all treatment periods. Peak FEV1 was significantly higher with 5 μg of tiotropium (difference 139 mL; 95% CI: 96–181 mL) and 10 μg (difference 170 mL; 95% CI: 128–213 mL) than with placebo (both P<0.0001). Trough FEV1 at the end of the dosing interval was also higher with both doses of tiotropium than with placebo (difference with 5 μg of 86 mL [95% CI: 41–132]; difference with 10 μg of 113 mL [95% CI: 67–159 mL], both P<0.0004). Although there were numerically greater FEV1 responses with the 10 μg dose of tiotropium than with the 5 μg dose compared with placebo for both peak and trough FEV1, the differences in outcome measures between the two active doses were not significant. Across groups, no significant difference was noted in asthma-related health status, rescue medication use, or symptoms measured in the electronic diary. There was a trend for more adverse events to be reported with the 10 μg tiotropium treatment group than with the placebo group. Patients receiving the highest dose of tiotropium specifically reported more complaints about dry mouth than the placebo group (6.8% vs 1.0%). Serious adverse events and asthma exacerbations were few and balanced among the treatment groups.
Bateman et al22 compared the efficacy and safety of tiotropium as add-on therapy to patients with moderate persistent asthma not adequately controlled by ICS therapy alone against salmeterol and placebo. As a novel twist of this study, the authors focused their study on a specific group of asthma patients with the B16-Arg/Arg genotype. Patients with a history of asthma with the B16-Arg/Arg genotype and receiving maintenance therapy with ICS of 400–1,000 μg/day of budesonide or equivalent were eligible for enrollment. The main inclusion criteria were reversible airway obstruction (improvement in FEV1 of at least 12% and 200 mL after sequential inhalation of 80 μg of ipratropium bromide and 400 μg of salbutamol) and prebronchodilator FEV1 of 90% of predicted value or less for patients previously receiving a daily dose of at least 100 μg of salmeterol or 18 μg of formoterol and an ICS of 80% of predicted value or less for patients previously receiving ICSs only. The main exclusion criteria were smoking history (>10 pack-years), COPD, serious concomitant illnesses, and concomitant respiratory medications. They conducted a double-blind, double-dummy, placebo-controlled, parallel-group trial. There was a 4-week run-in period during which patients received 50 μg of twice-daily salmeterol administered through a metered dose inhaler along with their ICS. After the run-in period, patients were randomized to 16 weeks of treatment with tiotropium (two puffs of 2.5 μg daily in the PM through the Respimat inhaler), salmeterol metered dose inhaler (two puffs of 25 μg twice daily plus tiotropium-matching placebo), or placebo (with both a tiotropium and salmeterol matching placebo). The primary end point was change in weekly AM (predose) PEF from the last week of run-in period to the last week of treatment. A total of 530 patients were eligible for the trial and 388 were randomized. At baseline almost all patients were receiving ICSs. The majority of patients in each treatment group were also receiving an LABA. The prebronchodilator FEV1 was between 68% and 70% for the three treatment groups. There was, on average, between a 24% and 28% increase in FEV1 after ipratropium and albuterol. The AM PEF was maintained with tiotropium (−3.9±4.87 L/min change from run-in) and salmeterol (−3.2±4.64 L/min change from run-in), but the AM PEF decreased with placebo (−24.6±4.84 L/min from run-in). Both active treatments provided significantly superior effects on AM PEF compared with placebo. The tiotropium effect was noninferior to salmeterol. Weekly FEV1 measures showed the same treatment effects as the AM PEF. Surprisingly, little evidence of treatment effect with tiotropium and salmeterol was found in asthma symptoms, rescue medication use, and the Mini AQLQ. In general, adverse event reporting was comparable across treatment groups. There were numerically more asthma exacerbations with salmeterol treatment (n=4) than with either placebo (n=1) or tiotropium (n=0).
In two large replicate studies, Kerstjens et al23 compared the effect of adding 5 μg of tiotropium each AM to placebo on lung function and exacerbations in poorly controlled asthma patients already receiving ICS and LABA. Included patients were aged between 18 years and 75 years and had a 5-year or longer asthma history. Other inclusion criteria consisted of an ACQ score of 1.5 or higher, a postbronchodilator FEV1 ≤80% predicted, and a postbronchodilator FVC ≤70% predicted despite daily therapy with high-dose ICS and LABA. Included patients also had at least one asthma exacerbation in the preceding year that required treatment with systemic corticosteroids. Main exclusion criteria consisted of a pre-existent diagnosis of COPD, serious coexisting illness, and concurrent use of anticholinergic bronchodilators. Patients with a ≥10 pack-years smoking history and who had smoked within the past year were also excluded. The study was a randomized, double-blind, placebo-controlled, parallel-group design. After a 4-week screening period, patients were randomized to treatment with either tiotropium (5 μg daily) or placebo, both administered by the Respimat Soft Mist Inhaler in the AM for 48 weeks, which made it the longest trial carried out on this subject. The primary outcomes were the peak FEV1 (measured within 3 hours of treatment) and trough FEV1 at week 24 expressed as change from baseline. In the two studies, 912 eligible patients were randomized. At 24 weeks, the mean change in the peak FEV1 from baseline was greater with tiotropium than with placebo in the two trials: a difference of 86±34 mL in trial 1 (P=0.01) and 154±32 mL in trial 2 (P<0.001). Similar significant differences in favor of tiotropium treatment were found with the trough FEV1. The effects of tiotropium continued throughout the entire 48 weeks of the study. In addition, the addition of tiotropium increased the time to the first severe exacerbation (282 days vs 226 days) with an overall reduction of 21% in the risk of a severe exacerbation (hazard ratio 0.79; P=0.03). Consistent differences between tiotropium and placebo treatment on the ACQ, the AQLQ symptom-free days, and rescue medication use were not found, however. Adverse event reporting was slightly lower with tiotropium than with placebo. No differences were found between the groups in cardiac adverse events and serious adverse events. Treatment with tiotropium was associated with a slightly higher complaint rate of dry mouth.
We performed an assessment of the methodologic quality of the five studies, and we assessed the consistency and adherence of the five trials to the CONSORT. We concluded that each study met the CONSORT criteria for a well-designed randomized trial.18
Discussion
Tiotropium is an inhaled long-acting anticholinergic currently approved in the US for once-daily maintenance treatment of bronchospasm associated with COPD.26 It has been proven to be an effective bronchodilator and to reduce the frequency of COPD exacerbations.10,11 Despite the well-studied pharmacology of tiotropium, its role in asthma remains unproven. The drug is currently not approved for non-COPD obstructive airway disease. In this systematic review we found that adding tiotropium to pre-existing therapy with only an ICS or an ICS–LABA combination resulted in significant improvements in lung function. There was also evidence from one study suggesting a reduction in exacerbations in patients with uncontrolled severe asthma. These observations are supported by findings in open-label, uncontrolled studies. The findings are consistent with the known effect of a muscarinic antagonist and its mechanism of action in the context of asthma pathophysiology.
The five clinical studies included in this systematic review focused on evaluating the efficacy of tiotropium as add-on therapy to ICS or ICS in combination with an LABA in patients with uncontrolled moderate to severe persistent asthma. Tiotropium maintained lung function when ICSs were tapered and when an LABA was discontinued.19,22 Tiotropium improved lung function when added to an ICS alone or an ICS–LABA combination therapy.20–23 In the only trial to have compared the addition of tiotropium with doubling the dose of ICS, tiotropium provided significantly superior results.20 In trials in which the addition of tiotropium was compared with salmeterol, the beneficial effects of these two bronchodilators was similar.20,22 Although the improvements in lung function appeared to be clinically meaningful, these clinical trials failed to show that tiotropium provided consistent and clinically relevant improvements in respiratory symptoms, rescue medication use, or quality of life. Perhaps the failure to show symptomatic improvements was a function of study size. In the one report powered sufficiently to assess any change in the symptomatic burden of asthma, the addition of tiotropium resulted in an overall reduction of exacerbations and an increase in the time between them.23 Overall, the consistent improvement in lung function with the addition of tiotropium should probably lead to US Food and Drug Administration approval of tiotropium as add-on therapy for patients with uncontrolled moderate to severe persistent asthma already on ICSs. Unfortunately, the available information does not enable health care providers to determine whether an individual patient might respond more to tiotropium or an LABA as add-on therapy.27
Although excluded from the systematic review, the two open-label trials we found on this topic support the findings of the other five studies. Iwamoto et al24 added tiotropium to 17 patients with severe persistent asthma who were receiving high-dose ICSs. Many of these patients were also taking an LABA and/or oral corticosteroids. The study was an open-label, uncontrolled design. They found that use of tiotropium for 4 weeks significantly improved FEV1 (P=0.001). Of note, the percentage of eosinophils in the induced sputum of the participants was inversely correlated with the change in FEV1, whereas the neutrophil proportion was directly correlated with an increase in FEV1. This suggested that the noneosinophilic sputum profile may separate responders vs nonresponders to tiotropium therapy. A similar interest in distinguishing responders from nonresponders was reflected in the study conducted by Park et al.25 They studied 138 patients with severe persistent asthma who had reduced lung function despite high-dose ICSs and LABA. Tiotropium, administered as 18 μg daily from a dry powder inhaler, was added to this regimen. Lung function tests were evaluated every 4 weeks for a minimum of 12 weeks. Responders were defined as having a >15% (or >200 mL) improvement in FEV1, with a sustained effect lasting at least 8 successive weeks. Forty-six of the 138 patients (33.3%) responded to tiotropium treatment. Logistic regression analysis (controlled for age, sex, and smoking status) showed that Arg16Gly in ADRB2 (P=0.003, odds ratio 0.21, 95% CI: 0.07–0.59) in a minor allele-dominant model was significantly associated with the response to tiotropium.
Improved lung function (increased FEV1 and PEF) in asthma with tiotropium can be explained by its mechanism of action in the context of asthma pathophysiology. The major bronchi (the site of bronchoconstriction in asthma) have the densest population of cholinergic nerves in the respiratory tract. Bronchoconstriction is mainly mediated through cholinergic mechanisms in humans.27 Stimulation of cholinergic fibers leads to activation of M3 Ach receptors in the airway smooth muscle, which, in turn, causes bronchoconstriction and mucus secretion. Tiotropium is an effective M3 Ach receptor antagonist. Cholinergic stimulation may also have influences in addition to controlling airway smooth muscle contraction. Cholinergic pathways influence mucus secretion and possibly airway inflammation. It has been speculated that tiotropium might be able to affect airway remodeling in asthma through these mechanisms.28,29
Only four of the clinical trials reported complete information on adverse events related to tiotropium use. In general, similar total and individual reporting of adverse events was described for tiotropium and salmeterol compared with placebo. The only exception was that patients receiving higher doses of tiotropium (eg, 10 μg) tended to report dry mouth more frequently.21 The cardiovascular safety profile of tiotropium is of particular interest. In COPD patients, Verhamme et al30 found that tiotropium administered 5 μg daily by the Respimat Soft Mist Inhaler was associated with about a 30% increase in mortality compared with a comparable dose of tiotropium administered by a dry powder inhaler. The mortality association was strongest for cardiovascular and cerebrovascular death. However, it remains unclear whether this association is causal or due to residual confounding by COPD severity. A more recent study, also performed in COPD patients, found that tiotropium administered by the Respimat Soft Mist Inhaler at a dose of 5 μg or 2.5 μg had a safety profile similar to that of tiotropium given by dry powder inhaler at a dose of 18 μg.31 The 48-week studies reported by Kerstjens et al23 described no deaths and few cardiovascular safety events in asthma patients using tiotropium administered by the Respimat Soft Mist Inhaler.
There are strengths and limitations to this systematic review. Although the number of studies included was small, the studies were of high quality. They were randomized and controlled. All studies met the CONSORT criteria for reporting of randomized trials. There were limitations, though, in the five studies. One study was small, with only 18 completed patients.19 Two studies were short, only 4 and 8 weeks.19,21 Consequently, information available regarding sustainability of benefit is somewhat limited.23 The trials were a mixture of crossover and parallel-group design, making integration of efficacy results impractical. There may still be unresolved issues regarding the appropriate dosing of tiotropium in the treatment of asthma. Different inhalation devices (with different doses) were used to administer tiotropium, metered dose inhaler,19 dry powder inhaler,20 and the Respimat Soft Mist Inhaler.21–23 One study compared the effect of 5 μg of tiotropium from the Respimat vs 10 μg. The effects on lung function of the 10 μg tiotropium dose tended to be greater, but there was also a higher reporting rate of dry mouth with the larger dose. It is unclear whether the 10 μg tiotropium dose might provide additional benefits with long-term use, such as a further reduction in exacerbation rates. Although two of the studies using tiotropium in the Respimat had patients self-administer their dose in the AM, one of the studies required PM dosing.21–23 It is difficult to determine from these studies whether there might be greater benefits of PM vs AM dosing.
There was limited information available from these studies regarding the anti-inflammatory benefits of tiotropium in uncontrolled asthma. Fardon et al19 found that addition of tiotropium was associated with a significant reduction in exhaled nitric oxide levels, but Peters et al32 found that immunoglobulin E levels, sputum eosinophil counts, and exhaled nitric oxide levels were not predictive of a clinical response to tiotropium. Similarly, reversibility testing to either an SABA or an SAMA was not helpful in identifying patients who would have a beneficial tiotropium response.19 Because all studies required SABA reversibility as an inclusion criterion, it is unclear whether an uncontrolled asthma patient with moderate to severe persistent disease but without reversibility would have a beneficial response to tiotropium.
In summary, there may be a beneficial role for tiotropium in moderate to severe persistent asthma patients who are uncontrolled despite use of an ICS or an ICS and an LABA. However, it remains to be determined whether the spirometric changes will ultimately reciprocate in long-term symptom improvement. The currently available literature lays a sound foundation for further studies to be designed with particular focus on the asthma population. An important issue will be to determine whether patterns of genotypes, physiology, or inflammatory markers can be identified that would identify patients with the maximal potential benefit from treatment with tiotropium in addition to currently approved therapy.
Footnotes
Disclosure
Drs Befekadu and Onofrei report no conflicts of interest in this work. Dr Colice is a consultant/advisory board member/speaker for Teva, MedImmune, Pearl, Dey, Mylan, Forest, and Alitair, which are pharmaceutical companies developing treatments for asthma and COPD.
References
- 1.Fanta CH. Asthma. N Eng J Med. 2009;360:1002–1014. doi: 10.1056/NEJMra0804579. [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Bailey C, et al. Trends in Asthma Prevalence, Health Care Use, and Mortality in the United States, 2001–2010. [Accessed January 16, 2014]. Available from: http://www.cdc.gov/nchs/data/databriefs/db94.htm.
- 3.National Asthma Education and Prevention Program . Guidelines for the Diagnosis and Management of Asthma (EPR-3) Bethesda, MD: National Heart, Lung, and Blood Institute; 2007. [Accessed January 16, 2014]. Expert Panel Report III. NIH publication No 08-4051. Available from: http://www.nhlbi.nih.gov/guidelines/asthma/ [Google Scholar]
- 4.Global Initiative for Asthma Global Strategy for Asthma Management and Prevention. 2012. [Accessed January 16, 2014]. Update. Available from: http://www.ginasthma.org/documents/4.
- 5.Olivieri D, Chetta A, Del Donno M, et al. Effect of short-term treatment with low-dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma. Am J Respir Crit Care Med. 1997;155:1864–1871. doi: 10.1164/ajrccm.155.6.9196087. [DOI] [PubMed] [Google Scholar]
- 6.Trigg CJ, Manolitsas ND, Wang J, et al. Placebo-controlled immunopathologic study of four months of inhaled corticosteroids in asthma. Am J Respir Crit Care Med. 1994;150:17–22. doi: 10.1164/ajrccm.150.1.8025745. [DOI] [PubMed] [Google Scholar]
- 7.Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? Am J Resp Crit Care Med. 2004;170:836–844. doi: 10.1164/rccm.200401-033OC. [DOI] [PubMed] [Google Scholar]
- 8.Price D, Musgrave SD, Shepstone L, et al. Leukotriene antagonists as first-line or add-on asthma-controller therapy. N Engl J Med. 2011;364:1695–1707. doi: 10.1056/NEJMoa1010846. [DOI] [PubMed] [Google Scholar]
- 9.Scullion JE. The development of anticholinergics in the management of COPD. Int J COPD. 2007;2:33–40. doi: 10.2147/copd.2007.2.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359:1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 11.Vogelmeier C, Hederer B, Glaab T, et al. Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N Engl J Med. 2011;364:1093–1103. doi: 10.1056/NEJMoa1008378. [DOI] [PubMed] [Google Scholar]
- 12.Gosens R, Bos IS, Zaagsma J, Meurs H. Protective effects of tiotropium bromide in the progression of airway smooth muscle remodeling. Am J Respir Crit Care Med. 2005;171:1096–1102. doi: 10.1164/rccm.200409-1249OC. [DOI] [PubMed] [Google Scholar]
- 13.Kang JY, Rhee CK, Kim JS, et al. Effect of tiotropium bromide on airway remodeling in a chronic asthma model. Ann Allergy Asthma Immunol. 2012;109:29–35. doi: 10.1016/j.anai.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Terzano C, Petroianni A, Ricci A, et al. Early protective effects of tiotropium bromide in patients with airways hyperresponsiveness. Eur Rev Med Pharmacol Sci. 2004;8:259–264. [PubMed] [Google Scholar]
- 15.Magnussen H, Bugnas B, van Noord J, et al. Improvements with tiotropium in COPD patients with concomitant asthma. Respir Med. 2008;102:50–56. doi: 10.1016/j.rmed.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Yoshida M, Nakano T, Fukuyama S, et al. Effects of tiotropium on lung function in severe asthmatics with or without emphysematous changes. Pulm Pharmacol Ther. 2013;26:159–166. doi: 10.1016/j.pupt.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. Ann Intern Med. 2009;151:264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 18.Schulz KF, Altman DG, Moher D, et al. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomized trials. Ann Intern Med. 2010;152:726–732. doi: 10.7326/0003-4819-152-11-201006010-00232. [DOI] [PubMed] [Google Scholar]
- 19.Fardon T, Haggart K, Lee DK, Lipworth BJ. A proof of concept study to evaluate stepping down the dose of fluticasone in combination with salmeterol and tiotripium in severe persistent asthma. Respir Med. 2007;101:1218–1228. doi: 10.1016/j.rmed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. NEMJ. 2010;363:1715–1726. doi: 10.1056/NEJMoa1008770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerstjens HAM, Disse B, Schroder-Babo W, et al. Tiotropium improves lung function in patients with severe uncontrolled asthma: a randomized controlled trial. J Allergy Clin Immunol. 2011;128:308–314. doi: 10.1016/j.jaci.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 22.Bateman ED, Kornamann O, Schmidt P, et al. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. J Allergy Clin Immunol. 2011;128:315–322. doi: 10.1016/j.jaci.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Kerstjens HA, Engle M, Dahl R, et al. Tiotropium in asthma poorly controlled with standard combination therapy. NEMJ. 2012;367:1257–1259. doi: 10.1056/NEJMoa1208606. [DOI] [PubMed] [Google Scholar]
- 24.Iwamoto H, Yokoyama A, Shiota N, et al. Tiotropium bromide is effective for severe asthma with noneosinophilic phenotype. Eur Respir J. 2008;31:1379–1382. doi: 10.1183/09031936.00014108. [DOI] [PubMed] [Google Scholar]
- 25.Park HW, Yang MS, Park CS, et al. Additive role of tiotropium in severe asthmatics and Arg16Gly in ADRB2 as a potential marker to predict response. Allergy. 2009;64:778–783. doi: 10.1111/j.1398-9995.2008.01876.x. [DOI] [PubMed] [Google Scholar]
- 26.Spiriva (tiotropium) Ridgefield. CT: Boehringer Ingleheim Pharmacetuticals, Inc.; Mar, 2012. Product information. [Google Scholar]
- 27.Verner HA. Status asthmaticus in children: a review. Chest. 2001;119:1913–1929. doi: 10.1378/chest.119.6.1913. [DOI] [PubMed] [Google Scholar]
- 28.Gosens R, Zaagsma J, Meurs H, Halayko AJ. Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res. 2006;7:73. doi: 10.1186/1465-9921-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bateman ED, Rennard S, Barnes PJ, et al. Alternative mechanisms for tiotropium. Pulm Pharmacol Ther. 2009;22:533–542. doi: 10.1016/j.pupt.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 30.Verhamme KM, Afonso A, Romio S, et al. Use of tiotropium Respimat Soft Mist Inhaler versus HandiHaler and mortality in patients with COPD. Eur Respir J. 2013;42:606–615. doi: 10.1183/09031936.00005813. [DOI] [PubMed] [Google Scholar]
- 31.Wise R, Anzueto A, Cotton D, et al. Tiotropium Respimat inhaler and the risk of death in COPD. N Engl J Med. 2013;369:1491–1501. doi: 10.1056/NEJMoa1303342. [DOI] [PubMed] [Google Scholar]
- 32.Peters SP, Bleecker ER, Kunselman SJ, et al. Predictors of response to tiotropium versus salmeterol in asthmatic adults. J Allergy Clin Immunol. 2013;132:1068–1074. doi: 10.1016/j.jaci.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]