Abstract
Dryland ecosystems account for ~27% of global soil organic carbon (C) reserves, yet it is largely unknown how climate change will impact C cycling and storage in these areas. In drylands, soil C concentrates at the surface, making it particularly sensitive to the activity of organisms inhabiting the soil uppermost levels, such as communities dominated by lichens, mosses, bacteria and fungi (biocrusts). We conducted a full factorial warming and rainfall exclusion experiment at two semiarid sites in Spain to show how an average increase of air temperature of 2–3°C promoted a drastic reduction in biocrust cover (~ 44% in four years). Warming significantly increased soil CO2 efflux, and reduced soil net CO2 uptake, in biocrust-dominated microsites. Losses of biocrust cover with warming through time were paralleled by increases in recalcitrant C sources, such as aromatic compounds, and in the abundance of fungi relative to bacteria. The dramatic reduction in biocrust cover with warming will lessen the capacity of drylands to sequester atmospheric CO2. This decrease may act synergistically with other warming-induced effects, such as the increase in soil CO2 efflux and the changes in microbial communities, to alter C cycling in drylands, and to reduce soil C stocks in the mid to long term.
Keywords: drylands, lichens, biological soil crusts, carbon cycling, soil CO2 efflux, soil net CO2 exchange, climate change, fungi, bacteria
Introduction
Arid, semiarid and dry-subhumid ecosystems (drylands) occupy 41% of the terrestrial surface, and account for ~25% of global soil organic carbon (C) reserves (Safriel & Adeel, 2005). However, key processes related to the C cycle, such as soil CO2 efflux and net ecosystem CO2 exchange, have been poorly studied in drylands in comparison to other biomes (Ciais et al., 2011; Bond-Lamberty & Thomson, 2010; Maestre et al., 2012a). Climate models forecast average (median) warming values ranging from 3.2°C to 3.7°C, and important alterations in rainfall amounts and patterns, for drylands worldwide by the late XXI century (Solomon et al., 2007). These climatic changes are predicted to have large effects on dryland biodiversity (Maestre et al., 2012a), which plays relevant roles in supporting multiple ecosystem functions related to the C cycle (Safriel & Adeel, 2005; Maestre et al., 2012b). While the importance of biodiversity for C cycling and storage in terrestrial ecosystems is well-known (Strassburg et al., 2010; Cardinale et al., 2012; Maestre et al., 2012b), it is less certain how possible alterations in biotic communities induced by climate change will directly impact these processes (but see Zhou et al., 2012; Hartley et al., 2012).
Soil C largely concentrates at the surface in drylands (Ciais et al., 2011; Thomas, 2012), making it particularly sensitive to the activity of organisms inhabiting the soil uppermost levels, such as communities dominated by lichens, mosses, bacteria and fungi (biocrusts). Biocrusts are a key biotic component of drylands worldwide (Belnap & Lange, 2003), and largely regulate the C cycle in the ecosystems where they are present. These communities fix large amounts of atmospheric CO2 (over 2.6 Pg of C per year globally; Elbert et al., 2012), regulate the temporal dynamics of soil CO2 efflux and net CO2 uptake (Castillo-Monroy et al., 2011; Wilske et al. 2008, 2009), and affect the activity of soil enzymes such as β-glucosidase (Bowker et al., 2011; Miralles et al., 2013). Biocrusts also influence other processes important for C cycling and storage, such as N fixation (Belnap, 2002; Elbert et al., 2012), nitrification (Castillo-Monroy et al., 2010; Delgado-Baquerizo et al., 2010) and runoff-infiltration (Chamizo et al., 2012; Zaady et al., 2013) rates. Climate change is expected to negatively impact the photosynthetic activity of soil lichens (Maphangwa et al., 2012) and mosses (Grote et al., 2010), ultimately reducing their growth and dominance within biocrusts (Zelikova et al., 2012; Reed et al., 2012; Escolar et al., 2012). Reductions in the abundance of other biocrust constituents, such as cyanobacteria, with changes in rainfall patterns have also been reported (Johnson et al., 2012). Recent studies have shown that the replacement of mosses by cyanobacteria promoted by rainfall alterations led to substantial alterations in nitrogen cycling and soil fertility in the Southwestern US (Reed et al., 2012; Zelikova et al., 2012). These findings illustrate how climate-change induced alterations in the composition and abundance of biocrusts can determine ecosystem responses to changes in temperature and rainfall patterns, highlighting the need to account for biocrusts when assessing climate change impacts in drylands.
While the importance of biocrusts for the global C cycle is being recognized (Elbert et al., 2012), few studies have explicitly evaluated how climate change-induced impacts on biocrusts will affect C cycling and storage in drylands (Maestre et al., 2010; Zelikova et al., 2012). Here we report results from a full factorial field experiment conducted at two semiarid sites in Spain, where we independently increased air temperature by open top chambers (2–3°C increase), and reduced precipitation using rainout shelters (~35% reduction), in microsites with low and high biocrust cover. Using this experimental design, we aimed to test the effects of climate change on biocrusts, and to assess how such effects impact multiple soil variables that inform us about fundamental aspects of the C cycle (CO2 efflux, net CO2 exchange, activity of β-glucosidase, organic C, phenols, aromatic compounds, and hexoses). Quantifying soil CO2 fluxes is fundamental to understand whether a given ecosystem acts as a source or sink of atmospheric C (Rustad et al., 2000). The enzyme β-glucosidase plays an active role in the decomposition of organic matter by catalyzing the hydrolysis of labile cellulose and other carbohydrates (Eivazi & Tabatabai, 1988). The other C variables studied are important to quantify the different soil C pools and their decomposability (Rovira & Vallejo, 2002; Miralles et al., 2013). We tested the following hypotheses: i) expected increases in temperature and reductions in rainfall amounts will diminish the growth of visible biocrust constituents (lichens and mosses) because their photosynthetic activity is highly dependent on ambient moisture and dew events (Belnap et al., 2004; Lange et al., 2006; del Prado & Sancho, 2007; Green et al., 2011), which can be reduced with these climatic changes (Maphangwa et al., 2012); ii) the increases in temperature will alter the composition of microbial communities, favoring fungi over bacteria (Zhang et al., 2005; Castro et al., 2010); and iii) the degree of biocrust development will modulate C cycle and microbial responses to climate change. Such an effect is expected because processes such as soil CO2 efflux, net CO2 exchange and the activity of β-glucosidase, are regulated by both environmental factors and biocrust development (Yeager et al., 2004; Housman et al., 2006; Castillo-Monroy et al., 2011; Miralles et al., 2013).
Materials and methods
Study area and experimental design
This study was conducted in two sites located in central (Aranjuez, 40°02′ N – 3° 32′W; 590 m a.s.l.), and south-eastern (Sorbas, 37° 05′N – 2° 04′W; 397 m a.s.l.) Spain (Fig. S1). Their climate is semiarid Mediterranean, with dry and hot summers and mean annual temperature values of 15°C (Aranjuez) and 17°C (Sorbas). Mean annual rainfall values are 349 mm (Aranjuez) and 274 mm (Sorbas), and precipitation events mostly occur in autumn/winter and spring. Soils are derived from gypsum, have pH values ~7 (Table S1), and are classified as Gypsiric Leptosols (IUSS Working Group WRB, 2006). Perennial plant cover is below 40%, and is dominated by grasses such as Stipa tenacissima and small shrubs such as Helianthemum squamatum and Gypsophila struthium. At both sites, the areas located between perennial plants are colonized by a well-developed biocrust community dominated by lichens such as Diploschistes diacapsis, Squamarina lentigera and Psora decipiens (see Table S2 for a species checklist).
At each site, we established a fully factorial experimental design with three factors, each with two levels: biocrust cover (poorly developed biocrust communities with cover < 20% vs. well-developed biocrust communities with cover > 50%), warming (control vs. temperature increase) and rainfall exclusion (RE, control vs. rainfall reduction). Ten and eight replicates per combination of treatments were established in Aranjuez and Sorbas, resulting in a total of 80 and 64 experimental plots, respectively. We kept a minimum separation distance of 1 m between plots to minimize the risk of sampling non-independent areas. In Aranjuez, the open top chambers and rainfall shelters were setup in July and November 2008, respectively. In Sorbas, the full experiment was set up in May 2010.
The warming treatment aimed to simulate the average of predictions derived from six Atmosphere-Ocean General Circulation Models for the second half of the 21st century (2040-2070) in central and south-eastern Spain (De Castro et al., 2005). To achieve a temperature increase within this range, we used open top chambers (OTCs) of hexagonal design with sloping sides of 40 cm × 50 cm × 32 cm (see Fig. S2 for details). We used methacrylate to build our OTCs because this material does not substantially alter the characteristics of the light spectrum, and because it is commonly used in warming experiments (e.g., Hollister & Weber, 2000), including some conducted with biocrust-forming lichens (Maphanga et al., 2012). The methacrylate sheets used in our experiment transmit ~92% of visible light, have a reflection of incoming radiation of 4%, and pass on ~85% of incoming energy (information provided by the manufacturer; Decorplax S. L., Humanes, Spain). Direct measurements in our experiment revealed that these sheets filtered up to 15% of UV radiation (data not shown).
While predicted changes in rainfall for our study area are subject to a high degree of uncertainty, most climate models foresee important reductions in the total amount of rainfall received during spring and fall (between 10% and 50%; Escolar et al., 2012). To simulate these conditions, we set up passive rainfall shelters (described in Fig. S2). These shelters did not modify the frequency of rainfall events, which has been shown to strongly affect biocrust functioning and dynamics in other dryland regions (Reed et al., 2012), but effectively reduced the total amount of rainfall reaching the soil surface (average reduction of 33% and 36% in Aranjuez and Sorbas, respectively).
Air and surface soil (0-2 cm) temperatures, and soil moisture (0-5 cm depth) were continuously monitored in all treatments and sites using replicated automated sensors (HOBO® U23 Pro v2 Temp/RH and TMC20-HD sensors, Onset Corp., Pocasset, MA, USA, and EC-5 soil moisture sensors, Decagon Devices Inc., Pullman, WA, USA, respectively). Rainfall was also monitored using an on-site meteorological station (Onset Corp.).
Monitoring of biocrust dynamics
Within each plot we inserted 5 cm into the soil a PVC collar (20 cm diameter, 8 cm height) for measuring CO2 fluxes (see below), and for monitoring crust composition and cover (Fig. S2). The total cover of the biocrust community was estimated in each PVC collar at the beginning of the experiment and then at different time intervals (13, 32 and 46 months in Aranjuez, 19 and 31 months in Sorbas) using high resolution photographs. From these photographs, we estimated the proportion of each PVC collar covered by lichens and mosses by mapping their area with the software GIMP (http://www.gimp.org/) and ImageJ (http://rsb.info.nih.gov/ij/). Cover estimates obtained with this method were highly related to those gathered directly in the field (Fig. S3).
Measurements of soil CO2 efflux and net CO2 uptake
The soil CO2 efflux rate of the whole soil column, which include both the biocrust living on its surface and the entire soil community associated to them, was measured in situ every one to four months in all the PVC collars with a closed dynamic system (Li-8100 Automated Soil CO2 Flux System, Li-COR, Lincoln, NB, USA). The opaque chamber used for these measurements had a volume of 4843 cm3, and covered an area of 317.8 cm2. Because of the low CO2 efflux rates typically observed in areas such as those studied here (Castillo-Monroy et al., 2011; Rey et al., 2011), each measurement period was 120 s to ensure reliable measurements. In every survey, half of the replicates were measured in one day (between 10:00 and 13:00 h local time, GMT +1), and the other half were measured on the next day. The chamber used in these measurements does not allow any radiation to reach biocrusts, and under these conditions we expect C fixation, if any, to be minimal. Thus, we also measured the net CO2 exchange (i.e. the difference between photosynthesis and soil CO2 efflux) with an open dynamic system (Li-6400XT infrared gas analyzer, Li-COR). We used for these measurements a custom transparent chamber with a volume of 2385 cm3, designed and calibrated by two of us (M. Ladrón de Guevara & R. Lázaro). System airflow of 800 μmol·s−1 and additional ventilation of 0.7 m·s−1 were used to obtain an adequate air mixing within the chamber. These measurements were conducted every two months between September 2010 and February 2012 on 4-8 plots per combination of treatments randomly selected at each sampling period. Preliminary daily curves conducted at both study sites (results not shown) show peak photosynthetic activity during dawn periods, a response observed also with biocrust-forming lichens in other semiarid sites from SE Spain (del Prado & Sancho, 2007; Pintado et al., 2010) and elsewhere (e.g., Veste et al., 2001; Lange et al., 2006). Thus, net CO2 exchange measurements were conducted at dawn, starting when the collars receiving direct light, in an interval of two hours. Half of the replicates were measured in one day, and the other half were measured on the next day, which always had similar weather conditions (cloudless sky).
Soil sampling and laboratory analyses
Soil samples (0-1 cm depth) from all the plots were collected at both study sites at the beginning of the experiment, and then 46 months later from five plots per combination of treatments randomly selected in the Aranjuez site. Samples were collected outside the PVC collars in all cases, to avoid perturbations in the measurements of CO2 fluxes. In the laboratory, visible biocrust components were carefully removed from the soil, which was sieved (2 mm mesh) and separated into two fractions. One fraction was immediately frozen at −80°C for quantifying the amount of fungi and bacteria present in our samples, the other was air-dried for one month for analyses of variables of the C cycle (organic C, phenols, aromatic compounds, hexoses, and the activity of β-glucosidase).
Soil DNA was extracted from 0.5 g of defrosted soil samples using the Powersoil® DNA Isolation Kit (Mo Bio Laboratories, Carlsbad, CA, USA) according to the instructions provided by the manufacturer. The extracted DNA had a high quality, with ratios of A260/A230 and A260/A280 above 1.5 and 1.8, respectively. We performed quantitative PCR (qPCR) reactions in triplicate using 96-well plates on an ABI 7300 Real-Time PCR (Applied Biosystems, Foster City, CA, USA). The bacterial 16S and fungal 18S rRNA genes were amplified with the Eub 338-Eub 518 and ITS 1-5.8S primer sets, respectively, following Evans & Wallenstein (2011). To obtain the bacterial and fungal standards for qPCR analyses, we used DNA extracted from composite soil samples. The qPCR products were cloned in parallel into Escherichia coli using a TOPO® TA Cloning® Kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Plasmid DNA was extracted with a Plasmid Mini Kit (Invitrogen); the inserts were sequenced using the generic primers set M13F and M13R, which region is included in this plasmid, to check that fungal and bacterial amplicons were correctly inserted in their respective plasmids. The results were compared to known fungal and bacterial genes in the Genbank database (http://www.ncbi.nlm.nih.gov) using the BLAST application. BLAST analysis showed that the sequences obtained were > 99% similar to known fungal and bacterial genes. During the testing phase, we generated melting curves for each run to verify product specificity by increasing the temperature from 55°C to 95°C. Additionally, and to further check for the integrity of the fragments obtained, we evaluated the length of the inserted bacterial and fungal amplicons in their respective plasmids by conducting additional qPCR analyses with the fungal, bacterial and M13 primers followed by electrophoresis in agarose gels.
Organic C was determined by colorimetry after oxidation with a mixture of potassium dichromate and sulfuric acid (Anderson & Ingramm, 1993). Phenols, aromatic compounds and hexoses were measured from K2SO4 0.5 M soil extracts in a ratio 1:5 at 725, 254 and 625 nm, respectively (Chantigny et al., 2006). Soil extracts were shaken in an orbital shaker at 200 rpm for 1 h at 20°C and filtered to pass a 0.45-μm Millipore filter. The filtered extract was kept at 2°C until colorimetric analyses, which were conducted within the 24 h following the extraction according to Chantigny et al. (2006). The activity of β-glucosidase was measured as described in Maestre et al. (2012b).
Statistical analyses
Visual inspection of the data and preliminary analyses showed that biocrust cover had important interactive effects with warming and/or rainfall exclusion (RE) on many of the response variables measured. Thus, analyses were conducted separately for plots with low and high biocrust cover. Soil CO2 efflux and biocrust cover data were analyzed using a three-way (warming, RE and Time) ANOVA, with repeated measures of one of the factors (Time). As the assumption of multisample sphericity was not met, the Huynh-Feldt adjusted degrees of freedom were used for within-subjects tests (Quinn & Keough, 2002). In the case of soil CO2 efflux, only the sampling dates with data from all the treatment combinations were included in the ANOVAs. As diverse subsets of samples were measured for net CO2 exchange at different times, the effects of warming and RE on this variable were evaluated at each sampling date by using a two-way ANOVA. To estimate how warming and RE affected soil C variables throughout the duration of the experiment in Aranjuez, we calculated the absolute effect size (Ae) as C46 – C0, where C0 and C46 are the value of a given variable at the beginning of the experiment and 46 months later, respectively. Due to the low DNA concentration present in some of our soil samples, we were not able to successfully analyze either fungi or bacteria for all of them. This reduced substantially the number of Ae values of the fungal: bacterial ratio. Therefore, and to avoid losing replicates for our analyses, we directly analyzed this ratio at the beginning of the experiment and 46 months after, rather than its Ae. We evaluated the effects of warming and RE on the fungal: bacterial ratio and Ae data using a two-way ANOVA. To test whether changes in soil variables were linked to changes in biocrust cover throughout the course of the experiment, linear and non-linear (quadratic, logarithmic, power and exponential) regression analyses were used to examine the relationships between the Ae in soil variables (raw data in the case of the fungal: bacterial ratio) and the Ae in biocrust cover. When significant relationships were found, the function that minimized the second-order Akaike information criterion (Sugiura, 1978) was chosen. In ANOVA analyses, warming and RE were considered fixed factors. Prior to these analyses, data were tested for ANOVA/regression assumptions, and were sqrt-, arcsin- or log-transformed when necessary. All the analyses were performed using SPSS 15.0 software (SPSS Inc., Chicago, IL, USA).
Results
Treatment effects on environmental variables
Throughout the study period, the warming treatment increased air temperature by 2.7°C and 1.5°C in Aranjuez and Sorbas, respectively (Fig. S4). It also increased surface soil temperature by 3.0°C and 2.3°C on average in Aranjuez and Sorbas, respectively (Fig. S5). Warming effects were maximized during summer (June-September), where soil temperatures where increased by warming up to 7°C in some days (Fig. S5). Rainfall shelters did not substantially alter air/soil temperature, as average differences between RE and both control and warming treatments throughout the study period were below 0.4°C in all cases (Figs. S4 and S5). Surface soil moisture closely followed the rainfall events registered, and was reduced by rainfall shelters on average by 4% and 1% in Aranjuez and Sorbas, respectively (Fig. S6). The reduction of soil moisture by shelters was mainly noticeable during rainfall events (Fig. S6). The dynamics of relative air humidity varied among the two study sites, as the number of days with periods of relative air humidity (RH) = 100% was higher in Sorbas than in Aranjuez (Fig. S7). Warming reduced the duration of such periods at both sites (average reduction of 51 and 26 minutes·day−1 in Aranjuez and Sorbas, respectively; Fig. S7).
Biocrust dynamics
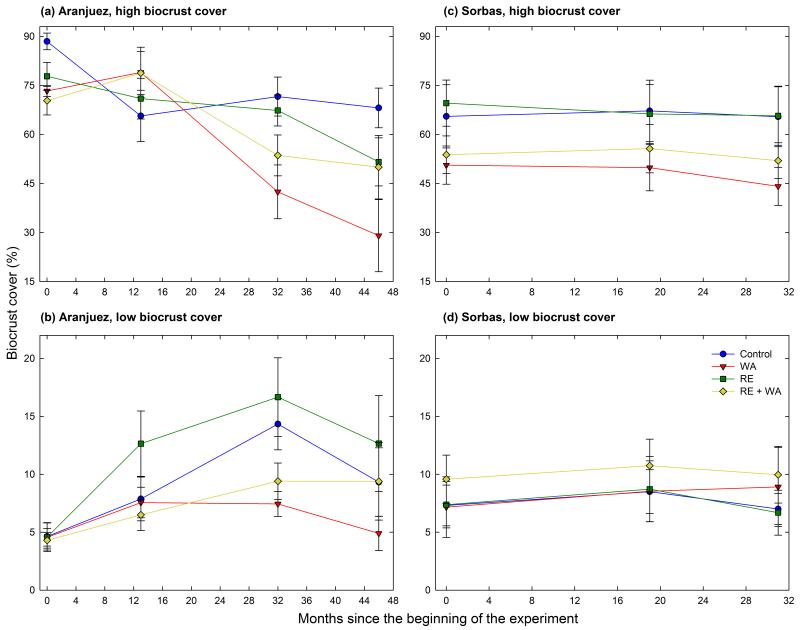
The dynamics of biocrust cover varied depending on the site and initial cover considered (Fig. 1). In Aranjuez, high biocrust cover plots lost cover 46 months after the beginning of the experiment in all the treatments evaluated (Fig. 1a). These losses were clearly accelerated with warming (Within-subjects tests: FTime × warming = 7.73, df = 2.8, 99.4, P < 0.001), particularly when this treatment was applied alone (Within-subjects tests: FTime × warming × RE = 2.84, df = 2.7, 99.4, P = 0.046). Rainfall exclusion did not affect changes in cover through time, regardless the initial biocrust cover (Within-subjects tests: FTime × RE < 0.82, P > 0.481 in all cases). The dynamics of low biocrust cover plots were the opposite, as they increased their cover in both control and RE treatments by ~6%, but only by ~3% in plots subjected to warming (Fig. 1b, Within-subjects tests: FTime × warming = 2.25, df = 2.5, 90.6, P = 0.098; FTime × warming × RE = 0.67, df = 2.5, 90.6, P = 0.546). In Sorbas, biocrust cover remained more stable during the first 31 months of the experiment (Fig. 1c,d). At this site, neither warming nor RE affected temporal changes in biocrust cover (Within-subjects tests: F < 0.68, P > 0.488 in all cases).
Figure 1. Temporal changes in biocrust cover (mosses and lichens) in the Aranjuez (a, b) and Sorbas (c, d) experimental sites.
Data are means ± SE (n = 10 and 8 for Aranjuez and Sorbas, respectively). WA = warming, and RE = rainfall exclusion.
Treatment effects on soil CO2 fluxes
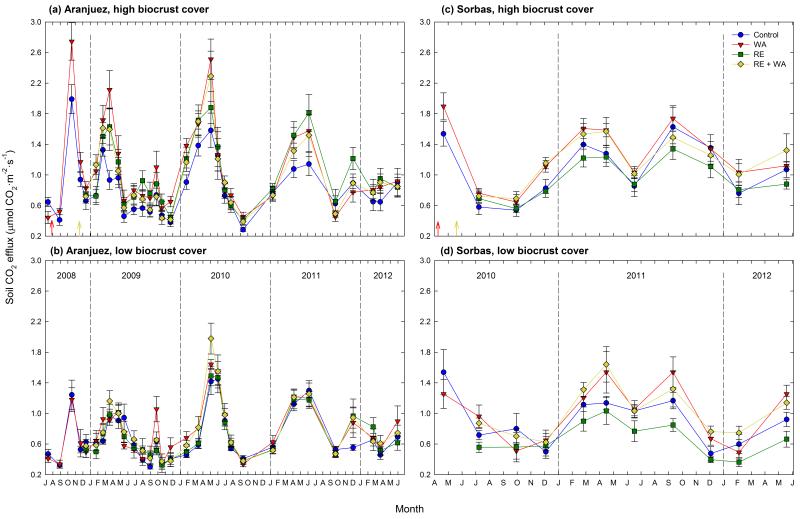
We found substantial within- and between-year variation in soil CO2 efflux at both study sites, which varied from 0.29 to 2.75 μmol·m−2·s−1, and from 0.36 to 1.89 μmol·m−2·s−1 in Aranjuez and Sorbas, respectively (Fig. 2). Overall, warming tended to either increase or have no effect on soil CO2 efflux rates at both sites, whereas few direct effects of RE were observed. In Aranjuez, a significant warming × RE interaction was observed in plots with high biocrust cover (Fig. 2a; Between-subjects tests: F1,36 = 4.34, P = 0.044). In these areas, soil CO2 efflux increased with warming (Between-subjects tests: F1,18 = 9.97, P = 0.005), an effect that was not evident when rainfall was also excluded (Between-subjects tests: F1,18 = 0.19, P = 0.672). No significant effects of warming and RE on this variable were found in areas with low biocrust cover (Fig. 2b; Between-subjects tests: F1,36 < 1.40, P > 0.248 in all cases). In Sorbas, the increase in soil CO2 efflux with warming was observed regardless the initial biocrust cover (Fig. 2c,d; Between-subjects tests: F1,28 > 9.61, P < 0.010 in all cases), and no significant effects of RE or warming × RE interactions were found (Between-subjects tests: F1,18 < 1.75, P > 0.197 in all cases).
Figure 2. Temporal variation of soil CO2 efflux in the Aranjuez (a, b) and Sorbas (c, d) experimental sites.
Red and light yellow arrows indicate the dates when the warming (WA) and rainfall exclusion (RE) treatments were installed, respectively. Data are means ± SE (n = 10 and 8 for Aranjuez and Sorbas, respectively).
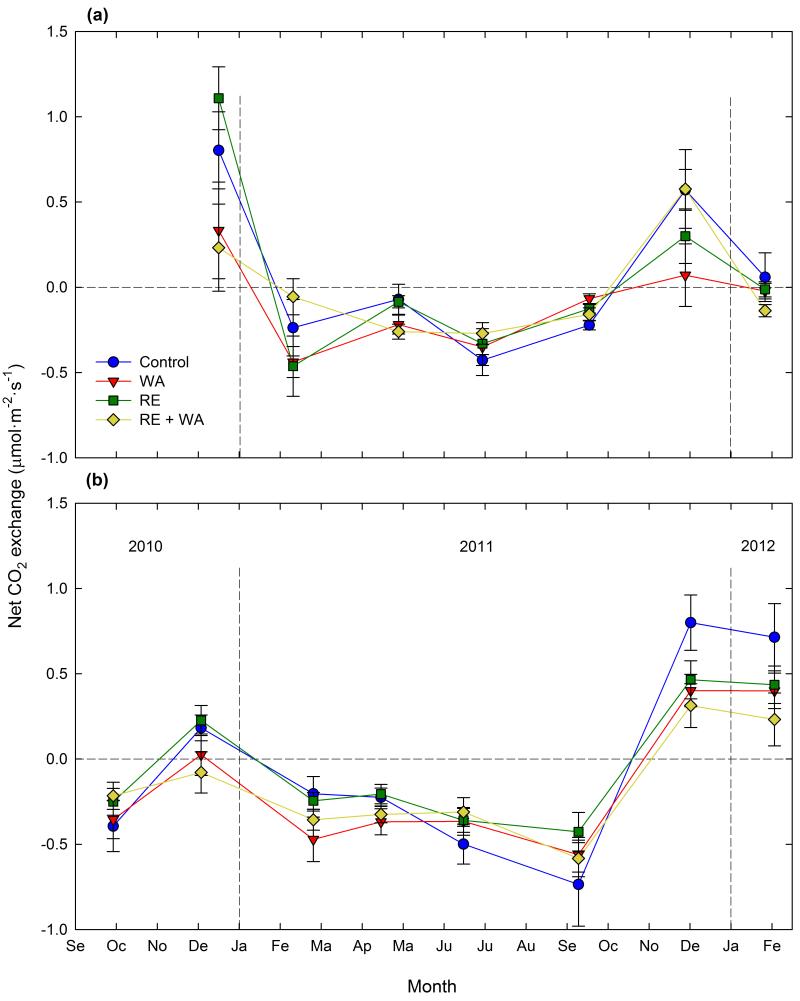
Net CO2 fixation in high biocrust cover areas was only observed during winter months, and was significantly reduced by warming at both study sites during these surveys (Fig. 3a,b; P < 0.045, Table S3). No significant effects of RE were observed at any of the sites (P > 0.110 in all cases, Table S3), albeit significant warming × RE interactions were found in Aranjuez during three of the sampling periods (Fig. 3a; P < 0.045, Table S3). Separate analyses for each RE level showed that in November 2011, when net CO2 uptake was observed, reductions in such uptake with warming were observed only when rainfall was not excluded (Fig. 3a).
Figure 3. Temporal variation of net CO2 exchange in high biocrust cover plots in the Aranjuez (a) and Sorbas (b) experimental sites.
Data are means ± SE (n = 4-8). WA = warming, and RE = rainfall exclusion.
Treatment effects on soil C variables, bacteria and fungi
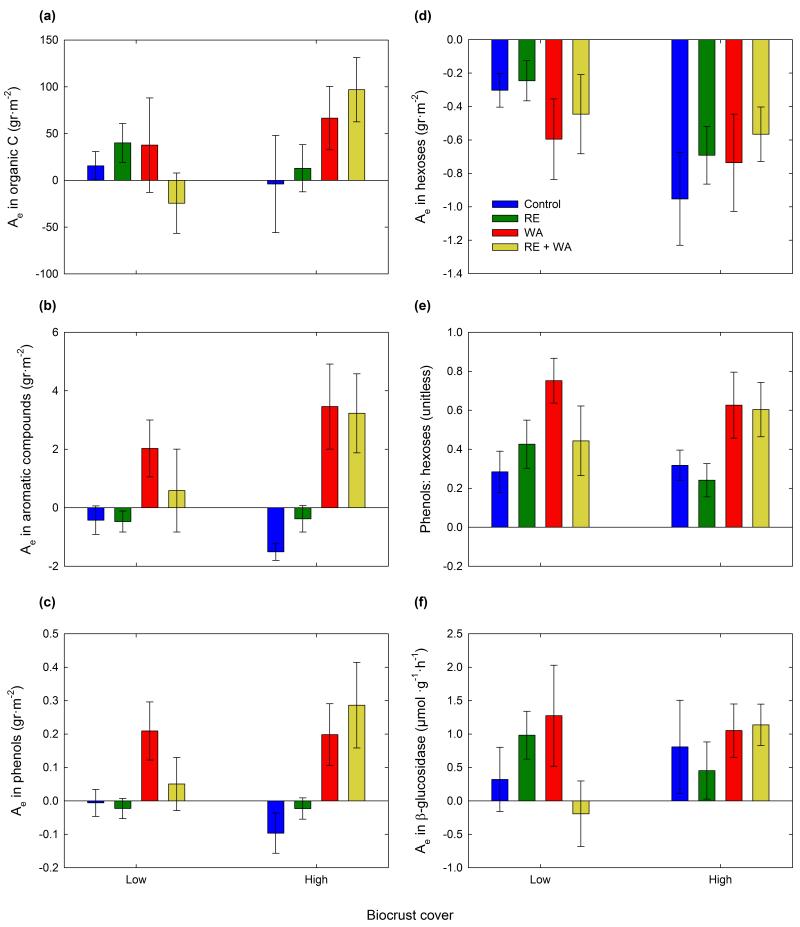
In Aranjuez, we found a clear trend of increasing soil organic C with warming in plots with high biocrust cover (Fig. 4a, F1,16 = 4.21, P = 0.057). This response may have been driven by the significant increase observed in recalcitrant C sources, such as phenols and aromatic compounds (Fig. 4b,c; F1,16 > 12.30, P < 0.005 in both cases). Increases were not observed in more labile C fractions, such as hexoses, regardless the initial biocrust cover (Fig. 4d, F1,16 < 1.80, P > 0.200 in all cases). As a consequence, warming increased the ratio phenols: hexoses through time in plots with high biocrust cover (Fig. 4e, F1,16 = 7.32, P = 0.016). Changes in the activity of β-glucosidase were not affected by warming (Fig. 4f, F1,16 < 0.95, P > 0.345 in all cases). Rainfall exclusion did not influence any of the variables measured (F1,16 < 1.89, P > 0.185 in all cases).
Figure 4. Changes (Ae) in organic C (a), aromatic compounds (b), phenols (c), hexoses (d), phenols: hexoses ratio (e) and β-glucosidase (f) during the first 46 months of the experiment at the Aranjuez experimental site.
Data are means ± SE (n = 5). WA = warming, and RE = rainfall exclusion. See Supplementary Table S1 for the raw data.
Warming promoted changes in microbial communities in Aranjuez, as the fungal: bacterial ratio increased during the course of the experiment (Fig. S8). Before the setting up of the experiment, this ratio did not significantly vary among the plots assigned to each treatment combination, regardless the initial biocrust cover (F < 1.85, P > 0.186 in all cases). 46 months later, the fungal: bacterial ratio increased with warming in both low (F1,13 = 14.23, P = 0.002) and high (F1,12 = 15.27, P = 0.002) biocrust cover plots, albeit the magnitude of the increase was substantially lower when both warming and RE treatments acted together (FWarming × RE > 5.44, P < 0.040 in all cases).
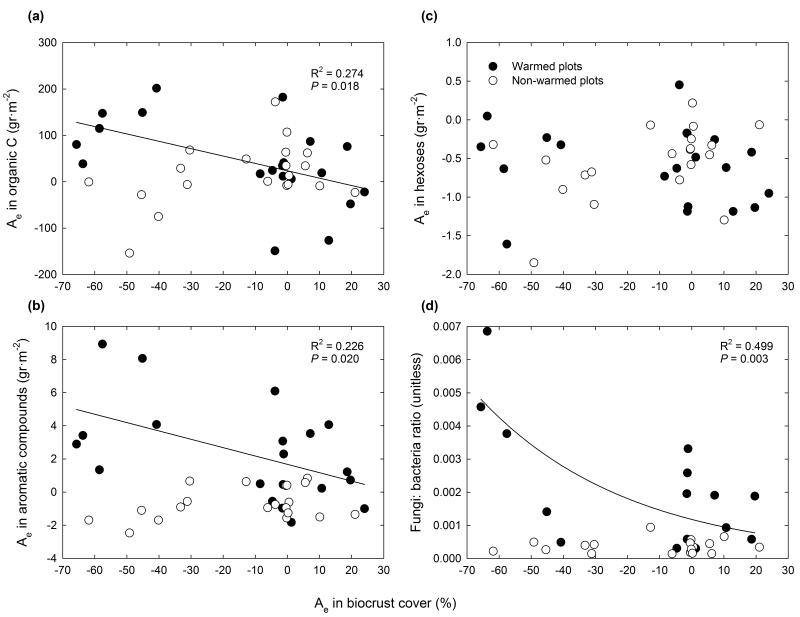
The observed increase in soil organic C with warming during the first 46 months of the experiment in Aranjuez was linked to the loss of biocrust cover, a relationship that was not found in the control and RE treatments (Fig. 5a). Similar results were found when evaluating the relationships between changes in biocrust cover and those in aromatic compounds (Fig. 5b), but not when more labile fractions, such as hexoses, were examined (Fig. 5c). Increases in the fungal: bacterial ratio were also observed in those plots that experienced reductions in biocrust cover (Fig. 5d).
Figure 5. Relationships between the absolute changes (Ae) in biocrust cover and those in organic C (a), aromatic compounds (b), and hexoses (c) during the first 46 months of the experiment at the Aranjuez experimental site, and between the relationship between the Ae in biocrust cover and the fungal: bacterial ratio at this site (d).
Solid lines are significant regressions fitted to the warmed plots. None of the regressions fitted to the non-warmed plots were significant.
Discussion
Understanding how biotic communities affect biogeochemical responses to altered climatic conditions is crucial to improve our ability to forecast the ecological consequences of climate change (Zhou et al., 2012; Hartley et al., 2012). While the potential for biotic feedbacks to climate change in drylands is large (Reed et al., 2012), no previous study has evaluated how the degree of biocrust development affects multiple C cycle responses to climate change. Our results indicate that a 2–3°C air/surface soil warming has important effects on different variables related to C cycling and storage, which are also largely modulated by biocrust development and by warming-induced changes in these communities. The impacts of increased temperatures in the biocrust and C cycle variables measured were in most cases independent of those of RE, which overall had little effects on the different variables measured.
Alteration of biocrust dynamics and net CO2 exchange in response to simulated climate change
Four years after the initiation of the experiment, warming dramatically reduced the joint cover of lichens and mosses in areas with well-developed biocrusts, and hampered the recovery of these organisms in those places devoid of them, in Aranjuez. We did not find significant treatment effects on biocrust cover in Sorbas, albeit some degree of reduction with warming could be appreciated 31 months after the beginning of the experiment (Fig. 1c). The differences found among sites may be due to different reasons. First, our experiment has been running for longer in Aranjuez than in Sorbas, and thus more time is likely needed to detect treatment effects on the biocrust communities studied in Sorbas. Second, and perhaps more importantly, our OTCs treatment increased air and soil temperatures more in Aranjuez than in Sorbas (Figs. S4 and S5), and this difference (1.2°C and 0.8°C of average increment in Aranjuez and Sorbas, respectively) may explain the reduced cover response to warming in Sorbas. Similarly, a study conducted with OTCs at two sites in South Africa (Maphangwa et al., 2012) found that the warming effect caused by this treatment was higher in an inland site compared to a coastal site, characterized by lower rainfall but higher water inputs from dew. To further investigate the mechanisms underlying the differential cover response observed between our study sites, additional physiological measurements, and a longer monitoring period, are necessary.
Biocrust-forming lichens are resistant to desiccation, and are well adapted to the high temperatures and low and unpredictable rainfall conditions characterizing drylands (Green et al., 2011). Our results, however, indicate that annual average increases in air temperature in the range of 2–3°C can trigger mortality events in these organisms. These findings are in the line of those reported by Belnap et al. (2006), who showed that a 6°C increase in maximum summer temperatures over eight years resulted in a significant decrease in lichen cover in the Colorado Plateau. The observed reductions in biocrust cover with warming contrast with those found in moss-dominated biocrusts from the Southwestern US, where altered rainfall regimes, rather than a 2–4°C warming, promoted widespread moss mortality (Reed et al., 2012; Zelikova et al., 2012). The mechanisms underlying the observed responses cannot be elucidated with our measurements. However, we speculate with the idea that they are caused by an increase in carbon losses because of higher CO2 efflux rates with warming (Reed et al., 2012), and by a reduction in carbon fixation caused by the effects of warming on variables such as soil temperature, moisture and relative air humidity (Figs. S5-S7). It is interesting to note that, over the course of the experiment, the space previously occupied by lichens in Aranjuez has not been colonized by other visible biocrust components (Fig. S9). Future studies are needed to elucidate whether this space is being colonized by cyanobacteria, as found in moss-dominated biocrusts of the Southwestern US (Zelikova et al., 2012).
Net soil CO2 uptake was only detected during late autumn and winter months at both study sites. These seasonal patterns resemble those found in biocrusts from sandy soils in the Negev Desert (Wilske et al., 2008), and agree with studies showing that biocrust-forming lichens are mainly photosynthetically active during winter in semiarid areas such as those studied here (del Prado & Sancho, 2007; Pintado et al., 2010). Warming had a significant negative effect on this variable during all seasons except in summer (Table S3). These findings agree with studies showing reductions in the photosynthetic capacity of these lichens under experimental temperature increases of 2–4°C (Maphangwa et al., 2012). Nocturnal moistening by fog or dew largely determines the photosynthetic activity and distribution patterns of biocrust-forming lichens in Mediterranean drylands (Veste et al., 2001; del Prado & Sancho, 2007). As found by previous studies conducted in South Africa (Maphangwa et al., 2012), warming substantially reduced the duration of suitable conditions for the formation of dew in our experiment (i.e., periods where air relative humidity if 100%; Fig. S7). This treatment also increased soil surface temperature (Fig. S5), and therefore its evapotranspiration, and reduced soil moisture (Fig. S6). These environmental effects of warming likely promoted a reduction in the photosynthetic activity of the biocrust communities studied (Veste et al., 2001; Lange et al., 2006; del Prado & Sancho, 2007).
Biocrust and climate change effects on soil CO2 efflux
Warming significantly increased soil CO2 efflux at both study sites, albeit the effects of this treatment were affected by both RE and biocrust cover in Aranjuez. Our findings agree with results from experiments conducted in a wide variety of environments, which have reported significant increases in soil CO2 efflux with warming during the first years (typically between 20% and 40%), which are later reduced due to acclimatization processes (Rustad et al., 2001; Luo et al., 2001; Niinistö et al., 2004; but see Lellei-Kovács et al., 2008; de Dato et al., 2010). Differences between sites in the magnitude of warming effects with biocrust development may have caused by variations in overall fertility, as soil CO2 efflux has been found to be influenced not only by moisture and temperature, but also by the amount of available soil organic carbon (Sponseller, 2007; Moyano et al., 2012). At the beginning of the experiment, soil organic C contents were higher in Sorbas than in Aranjuez (Fig. S10). Relative differences in this variable between high and low biocrust cover areas were, however, larger in Aranjuez than in Sorbas (77% vs. 55% increase, Fig. S10), and this could explain the lack of stimulatory effects of warming on soil CO2 efflux in low cover areas found in Aranjuez. At this site, the lack of significant warming effects in biocrust-dominated microsites when rainfall was also excluded may have been caused by the overall reduction in soil moisture promoted by this treatment (Fig. S6), which likely limited microbial activity and soil CO2 efflux (Castillo-Monroy et al., 2011).
The absence of significant effects of RE per se on soil CO2 efflux was initially unexpected. This result contrasts with previous observations from Mediterranean drylands, which have found significant reductions in soil CO2 efflux with RE (Emmett et al., 2004; de Dato et al., 2010; Miranda et al., 2011). It is important to note that these studies have been conducted in shrublands, where reduced rainfall effects on soil CO2 efflux are mostly driven by the responses they induce on plants (Emmett et al., 2004; de Dato et al., 2010), and thus their results may not be translated to biocrust-dominated ecosystems such as those studied here. Previous studies conducted in Aranjuez (Castillo-Monroy et al., 2011) have shown that soil CO2 efflux is driven by temperature during the wettest part of the year, when soil water contents are higher than 25% and 11% for low and high biocrust cover microsites, respectively, and by soil moisture during the dry season, when soil temperatures exceed 25°C and 18°C for low and high biocrust cover microsites, respectively. The main reductions in soil moisture achieved with the RE treatment were observed during the wettest part of the year at both Aranjuez and Sorbas (Fig. S6), when soil moisture was highest and soil CO2 efflux is largely driven by temperature. This may explain the lack of strong responses observed in this variable in response to reduced rainfall inputs.
Increases in soil CO2 efflux in biocrust-dominated microsites compared to bare ground areas have been found in S. tenacissima steppes from calcareous soils (Maestre & Cortina, 2003). Therefore, while our study sites were located in areas with gypsum soils, we would expect to find similar responses to the climate change treatments evaluated in areas with lichen-dominated biocrusts growing on other soil types.
Biocrusts and climate change effects on soil biogeochemistry and microbial communities
Warming caused profound changes in the different soil C variables evaluated. The temporal increase in soil organic C with warming was initially unexpected, given the observed effects of this treatment on soil CO2 efflux and net CO2 uptake. While our experimental design and measurements cannot provide a mechanistic explanation for these results, the relationships found between the changes in biocrust cover and the different soil C variables evaluated (Fig. 5) suggest that they are due to the mortality and subsequent decomposition of biocrust-forming lichens. These organisms are rich in recalcitrant C compounds (e.g., phenols; Kranner et al., 2008; Stark et al., 2007), and thus their decomposition could explain the observed increases in organic C, and those of recalcitrant sources of C in particular. The decomposition dynamics of biocrust-forming lichens are largely unknown, as to our knowledge no previous studies have been conducted with these organisms in drylands. Decomposition of lichen tissues provides an important source of C in arctic and boreal ecosystems (Wetmore, 1982; Esseen & Renhorn, 1998), and is a process that can occur over short temporal scales. For instance, Lang et al. (2009) compared the decomposition of 17 arctic lichens, and reported average mass loss ~ 60% after two years (range between 10% and 90% of initial mass loss). Albeit our results will need to be confirmed by future experiments, they suggest that decomposition processes could effectively incorporate C from biocrust-forming lichens into the soil in a few years in drylands. The activity of β-glucosidase, which acts upon bonds of labile C molecules, was not affected by warming, suggesting that the observed increase in soil CO2 efflux rate was caused by the decomposition of recalcitrant C (Biasi et al., 2005). Well-developed biocrusts can also enhance the utilization rates of aromatic acids, carbohydrates and carboxylic acids, increasing soil CO2 efflux (Yu et al., 2012). Another important result is the observed increase in the ratio phenols: hexoses through time with warming in plots with high biocrust cover (Fig. 4e). These results indicate that warming is promoting a shift toward greater recalcitrance in the soil C pool and a reduction in the quality of soil organic matter (Rovira & Vallejo, 2002). This fact, together with the observed decrease in biocrust cover with warming, may decrease the use of C by soil microorganisms and the rate of nutrient cycling (Rovira & Vallejo, 2002), favoring the immobilization of nutrients and increasing the abundance of fungi (Thorn & Lynch, 2007).
The greater relative dominance of fungi over bacteria found with warming agrees with results reported in other studies (e.g., Zhang et al., 2005; Castro et al., 2010). It is interesting to note that this ratio was associated with recalcitrant C sources 46 months after the beginning of the experiment in Aranjuez (phenols, ρ = 0.526, P = 0.002; aromatic compounds, ρ = 0.567, P = 0.001, n = 33). Overall, our findings suggest that differences in microbial communities induced by warming were associated with modifications in C cycling promoted by this treatment, which were also linked to changes in biocrust cover (Fig. 5). While warming increased the amount of organic C over the course of the experiment, we expect that this effect will disappear as lichens die and are subsequently decomposed. Reductions in soil C at the mid to long term should occur for three main reasons: i) the warming-induced losses in biocrust cover and photosynthetic capacity will progressively reduce C inputs to the soil, ii) increased CO2 efflux rates with warming will intensify C losses, and iii) fungi are able to decompose virtually all classes of litter compounds, while bacteria mainly decompose labile substrates (De Boer et al., 2005). Therefore, the increased dominance of fungi with warming may further accelerate the decomposition of recalcitrant C sources, augmenting soil CO2 efflux and reducing the amount of C stored in soils (van der Heijden et al., 2008).
Concluding remarks
Our results indicate that climate change, and a 2–3°C warming in particular, will reduce the abundance of well-developed and lichen-dominated biocrusts, which are prevalent communities in drylands worldwide and need decades to centuries to fully develop (Fig. S11; Belnap & Lange, 2003). Such warming effects will hamper the successional trajectories of these communities (Lázaro et al., 2008), affecting the organisms and ecosystem processes that depend on them (Belnap & Lange, 2003; Elbert et al., 2012; Bowker et al., 2011). Here we show how changes in biocrusts drive responses of microbial communities (increase of fungal abundance) and C cycling (reduced net CO2 uptake by soils, increased soil CO2 efflux and variations in the content of different soil C fractions) to climate change in drylands. Our results can have major implications for the C cycle in these ecosystems, and indicate that the capacity of drylands to fix atmospheric CO2 and store it into the soil will be substantially reduced in a warmer world.
Supplementary Material
Acknowledgements
We thank M. D. Puche and E. Valencia for their help with field and laboratory work, I. Martínez and M. Prieto for their help with the identification of lichens, and M. A. Bowker, F. de Vries, P. García-Palacios, J. I. Querejeta, A. Rey, S. Soliveres and two anonymous reviewers for their comments and suggestions on earlier versions of this article. This research was funded by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement 242658 (BIOCOM), by the Spanish Ministry of Economy and competitiveness (projects CGL2007-63258/BOS and CGL2010-21381/BOS), and by the Junta de Andalucía (COSTRAS project, RNM-3614). C. E. and M.L.G. were supported by graduate fellowships from the British Ecological Society (Studentship 231/1975) and the Spanish National Research Council (CSIC, JAE-Pre 029 Grant), respectively. We would like to thank IMIDRA and Lindy Walsh for allowing us working in their properties, as well as to the Junta de Andalucia for allowing us to work in the Paraje Natural Karst en Yesos de Sorbas.
References
- M Anderson, JSI Ingramm. Tropical Soil Biology and Fertility: A Handbook of Methods. 2nd ed. CAB International; Wallington: 1993. [Google Scholar]
- Belnap J. Nitrogen fixation in biological soil crusts from southeast Utah, USA. Biology and Fertility of Soils. 2002;35:128–135. [Google Scholar]
- Belnap J, Lange OL. Biological Soil Crusts: Structure, Function and Management. Springer-Verleg; Berlin: 2003. [Google Scholar]
- Belnap J, Phillips SL, Miller ME. Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia. 2004;141:306–316. doi: 10.1007/s00442-003-1438-6. [DOI] [PubMed] [Google Scholar]
- Belnap J, Phillips SL, Troxler T. Soil lichen and moss cover and species richness can be highly dynamic: The effects of invasion by the annual exotic grass Bromus tectorum, precipitation, and temperature on biological soil crusts in SE Utah. Applied Soil Ecology. 2006;32:63–76. [Google Scholar]
- Biasi C, Rusalimova O, Meyer H, Kaiser C, Wanek W, Barsukov P, Junger H, Richter A. Temperature-dependent shift from labile to recalcitrant carbon sources of arctic heterotrophs. Rapid Communications in Mass Spectrometry. 2005;19:1401–1408. doi: 10.1002/rcm.1911. [DOI] [PubMed] [Google Scholar]
- Bond-Lamberty B, Thomson A. A global database of soil respiration data. Biogeosciences. 2010;7:1915–1926. [Google Scholar]
- Bowker MA, Mau RL, Maestre FT, Escolar C, Castillo-Monroy AP. Functional profiles reveal unique ecological roles of various biological soil crust organisms. Functional Ecology. 2011;25:787–795. [Google Scholar]
- Cardinale BJ, Duffy E, Gonzalez A, et al. Biodiversity loss and its impact on humanity. Nature. 2012;486:59–67. doi: 10.1038/nature11148. [DOI] [PubMed] [Google Scholar]
- Castillo-Monroy AP, Maestre FT, Delgado-baquerizo M, Gallardo A. Biological soil crusts modulate nitrogen availability in semi-arid ecosystems: insights from a Mediterranean grassland. Plant and Soil. 2010;333:21–34. [Google Scholar]
- Castillo-Monroy AP, Maestre FT, Rey A, Soliveres S, García-Palacios P. Biological soil crust microsites are the main contributor to soil respiration in a semiarid ecosystem. Ecosystems. 2011;14:835–847. [Google Scholar]
- Castro HF, Classen AT, Austin EE, Norby RJ, Schadt CW. Soil microbial community response to multiple experimental climate change drivers. Applied and Environmental Microbiology. 2012;76:999–1007. doi: 10.1128/AEM.02874-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamizo S, Cantón Y, Lázaro R, Solé-Benet A, Domingo F. Crust composition and disturbance drive infiltration through biological soil crusts in semiarid ecosystems. Ecosystems. 2012;15:148–161. [Google Scholar]
- Chantigny MH, Angers DA, Kaiser K, Kalbitz K. In: Soil Sampling and Methods of analysis. Carter MR, Gregorich EG, editors. Canadian Society of Soil Science; 2006. pp. 617–635. [Google Scholar]
- Ciais P, Bombelli A, Williams M, et al. The carbon balance of Africa: synthesis of recent research studies. Philosophical Transactions of the Royal Society of London, Series A. 2011;369:1–20. doi: 10.1098/rsta.2010.0328. [DOI] [PubMed] [Google Scholar]
- Coxson DS, Curteanu M. Decomposition of hair lichens (Alectoria sarmentosa and Bryoria spp.) under snowpack in montane forest, Cariboo Mountains, British Columbia. The Lichenologist. 2002;34:395–402. [Google Scholar]
- De Boer W, Folman LB, Summerbell RC, Boddy L. Living in a fungal world: impact of fungi on soil bacterial niche development. FEMS Microbiology Reviews. 2005;29:795–811. doi: 10.1016/j.femsre.2004.11.005. [DOI] [PubMed] [Google Scholar]
- De Castro M, Martín-Vide J, Alonso S. El clima de España: pasado, presente y escenarios de clima para el siglo XXI. In: Moreno JM, editor. Evaluación Preliminar de los Impactos en España por Efecto del Cambio Climático. Ministerio Medio Ambiente; Madrid: 2005. pp. 1–64. [Google Scholar]
- de Dato GD, Angelis P, De Sirca C, Beier C. Impact of drought and increasing temperatures on soil CO2 emissions in a Mediterranean shrubland (gariga) Plant and Soil. 2010;327:153–166. [Google Scholar]
- Delgado-Baquerizo M, Castillo-Monroy AP, Maestre FT, Gallardo A. Plants and biological soil crusts modulate the dominance of N forms in a semi-arid grassland. Soil Biology and Biochemistry. 2010;42:376–378. [Google Scholar]
- del Prado R, Sancho LG. Dew as a key factor for the distribution pattern of the lichen species Teloschistes lacunosus in the Tabernas Desert (Spain) Flora. 2007;202:417–428. [Google Scholar]
- Eivazi F, Tabatabai MA. Glucosidases and galactosidases in soils. Soil Biology & Biochemistry. 1988;20:601–606. [Google Scholar]
- Elbert W, Weber B, Burrows S, Steinkamp J, Büdel B, Andreae MO, Pöschl U. Contribution of cryptogamic covers to the global cycles of carbon and nitrogen. Nature Geoscience. 2012;5:459–462. [Google Scholar]
- Emmett BA, Beier C, Estiarte M, Tietema A, Kristensen HL, Williams D, Peñuelas J, Schmidt I, Sowerby A. The response of soil processes to climate change: results from manipulation studies across an environmental gradient. Ecosystems. 2004;7:625–637. [Google Scholar]
- Escolar C, Martínez I, Bowker MA, Maestre FT. Warming reduces the growth and diversity of biological soil crusts in a semi-arid environment: implications for ecosystem structure and functioning. Philosophical Transactions of the Royal Society of London, Series B. 2012;367:3087–3099. doi: 10.1098/rstb.2011.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esseen PA, Renhorn KE. Mass loss of epiphytic lichen litter in a boreal forest. Annales Botanici Fennici. 1998;35:211–217. [Google Scholar]
- Evans SE, Wallenstein MD. Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry. 2011;109:101–116. [Google Scholar]
- Green TGA, Sancho LG, Pintado A. Ecophysiology of Desiccation/Rehydration Cycles in Mosses and Lichens. In: Lüttge U, Beck E, Bartels D, editors. Plant Desiccation Tolerance. Springer; Berlin: 2011. pp. 89–120. [Google Scholar]
- Grote EE, Belnap J, Housman DC, Sparks JP. Carbon exchange in biological soil crust communities under differential temperatures and soil water contents: implications for global change. Global Change Biology. 2010;16:2763–2774. [Google Scholar]
- Hartley IP, Garnett MH, Sommerkorn M, et al. A potential loss of carbon associated with greater plant growth in the European Arctic. Nature Climate Change. 2012;2:875–879. [Google Scholar]
- Hollister RD, Weber PJ. Biotic validation of small open-top chambers in a tundra ecosystem. Global Change Biology. 2000;6:835–842. [Google Scholar]
- Housman DC, Powers HH, Collins AD, Belnap J. Carbon and nitrogen fixation differ between successional stages of biological soil crusts in the Colorado Plateau and Chihuahuan Desert. Journal of Arid Environments. 2006;66:620–634. [Google Scholar]
- IUSS Working Group WRB . World Reference base for soil resources 2006. FAO; Rome: 2006. World Soil Resources Reports No. 103. [Google Scholar]
- Johnson SL, Kuske CR, Carney TD, Housman DC, Gallegos-Garves LV, Belnap J. Increased temperature and altered summer precipitation have differential effects on biological soil crusts in a dryland ecosystem. Global Change Biology. 2012;18:2583–2593. [Google Scholar]
- Kranner I, Beckett R, Hochman A, Nash TH, III, Beckett R. Desiccation-tolerance in lichens: a review. The Bryologist. 2008;111:576–593. [Google Scholar]
- Lang SI, Cornelissen JHC, Klahn T, Van RSP, Broekman R, Schweikert W, Aerts R. An experimental comparison of chemical traits and litter decomposition rates in a diverse range of subarctic bryophyte, lichen and vascular plant species. Journal of Ecology. 2009;97:886–900. [Google Scholar]
- Lange OL, Green ATG, Melze B, Meyer A, Zellner H. Water relations and CO2 exchange of the terrestrial lichen Teloschistes capensis in the Namib fog desert: measurements during two seasons in the field and under controlled conditions. Flora. 2006;201:268–280. [Google Scholar]
- Lázaro R, Cantón Y, Solé-Benet A, Bevan J, Alexander R, Sancho LG, Puigdefábregas J. The influence of competition between lichen colonization and erosion on the evolution of soil surfaces in the badlands (SE Spain) and its landscape effects. Geomorphology. 2008;102:252–266. [Google Scholar]
- Lellei-Kovács E, Kovács-Láng E, LKalapos T, Botta-Dukát Z, Barabás S, Beier C. Experimental warming does not enhance soil respiration in a semiarid temperate forest-steppe ecosystem. Community Ecology. 2008;9:29–37. [Google Scholar]
- Luo Y, Shiqiang W, Dafeng H, Wallace LL. Acclimatization of soil respiration to warming in a tall grass prairie. Nature. 2001;413:622–625. doi: 10.1038/35098065. [DOI] [PubMed] [Google Scholar]
- Maestre FT, Cortina J. Small-scale spatial variation in soil CO2 efflux in a Mediterranean semiarid steppe. Applied Soil Ecology. 2003;23:199–209. [Google Scholar]
- Maestre FT, Bowker MA, Escolar C, et al. Do biotic interactions modulate ecosystem functioning along abiotic stress gradients? Insights from semi-arid Mediterranean plant and biological soil crust communities. Philosophical Transactions of the Royal Society of London, Series B. 2010;365:2057–2070. doi: 10.1098/rstb.2010.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Quero JL, Gotelli NJ, Escudero A, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012b;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestre FT, Salguero-Gómez R, Quero JL. It’s getting hotter in here: determining and projecting the impacts of global change on drylands. Philosophical Transactions of the Royal Society of London, Series B. 2012a;367:3062–3075. doi: 10.1098/rstb.2011.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maphangwa KW, Musil CF, Raitt L, Zedda L. Experimental climate warming decreases photosynthetic efficiency of lichens in an arid South African ecosystem. Oecologia. 2012;169:257–268. doi: 10.1007/s00442-011-2184-9. [DOI] [PubMed] [Google Scholar]
- Miralles I, Trasar-Cepeda C, Leirós MC, Gil-Sotres F. Labile carbon in biological soil crusts in the Tabernas desert, SE Spain. Soil Biology & Biochemistry. 2013;58:1–8. [Google Scholar]
- Miranda JD, Armas C, Padilla FM, Pugnaire FI. Climatic change and rainfall patterns: Effects on semi-arid plant communities of the Iberian Southeast. Journal of Arid Environments. 2011;75:1302–1309. [Google Scholar]
- Moyano FE, Vasilyeva N, Bouckaert L, et al. The moisture response of soil heterotrophic respiration: interaction with soil properties. Biogeosciences. 2012;9:1173–1182. [Google Scholar]
- Niinistö SM, Silvola J, Kellomäki S. Soil CO2 efflux in a boreal pine forest under atmospherc CO2 enrichment and air warming. Global Change Biology. 2004;10:1363–1376. [Google Scholar]
- Pintado LG, Sancho JM, Blanquer TG, Green A, Lázaro R. Microclimatic factors and photosynthetic activity of crustose lichens from the semiarid southeast of Spain: long-term measurements for Diploschistes diacapsis. Bibliotheca Lichenologica. 2010;105:211–224. [Google Scholar]
- Quinn GP, Keough MJ. Experimental design and data analysis for biologists. Cambridge University Press; Cambridge: 2002. [Google Scholar]
- Reed SC, Koe K, Sparks JP, Housman D, Zelikova TJ, Belnap J. Changes to dryland rainfall result in rapid moss mortality and altered soil fertility. Nature Climate Change. 2012;2:752–755. [Google Scholar]
- Rey A, Pegoraro E, Oyonarte C, Were A, Escribano P, Raimundo J. Impact of land degradation on soil respiration in a steppe (Stipa tenacissima L.) semi-arid ecosystem in the SE of Spain. Soil Biology & Biochemistry. 2011;43:393–403. [Google Scholar]
- Rovira P, Vallejo VR. Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil : an acid hydrolysis approach. Geoderma. 2002;107:109–141. [Google Scholar]
- Rustad LE, Campbell JL, Mariom GM, et al. A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia. 2001;126:543–562. doi: 10.1007/s004420000544. [DOI] [PubMed] [Google Scholar]
- Rustad LE, Huntington TG, Boone D. Controls on soil respiration: Implications for climate change. Biogeochemistry. 2000;48:1–6. [Google Scholar]
- Safriel U, Adeel Z. Dryland systems. In: Hassan R, Scholes R, Neville A, editors. Ecosystems and Human well-being: Current State and Trends. Vol. 1. Island Press; 2005. pp. 623–662. [Google Scholar]
- Solomon S, Qin D, Manning RB, et al. Technical Summary. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, Averyt KB, Tignor M, Miller HL, editors. Climate Change 2007 The Physical Science Basis. Contribution of Working Group I to the Fourth Assesment Report of the Intergovernmental Panel in Climate Change. Cambridge University Press; Cambridge and New York: 2007. [Google Scholar]
- Sponseller RA. Precipitation pulses and soil CO2 flux in a Sonoran Desert ecosystem. Global Change Biology. 2007;13:426–436. [Google Scholar]
- Stark S, Kytöviita M, Neumann AB. The phenolic compounds in Cladonia lichens are not antimicrobial in soils. Oecologia. 2007;152:299–306. doi: 10.1007/s00442-006-0644-4. [DOI] [PubMed] [Google Scholar]
- Strassburg BBN, Kelly A, Balmford A, et al. Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conservation Letters. 2012;3:98–105. [Google Scholar]
- Sugiura N. Further analysis of the data by Akaike’s information criterion and the finite corrections. Communications in Statistics, Theory and Methods. 1978;A7:13–26. [Google Scholar]
- Thomas AD. Impact of grazing intensity on seasonal variations of soil organic carbon and soil CO2 efflux in two semi-arid grasslands in southern Botswana. Philosophical Transactions of the Royal Society of London, Series B. 2012;367:3076–3086. doi: 10.1098/rstb.2012.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorn RG, Lynch MDJ. Fungi and eukaryotic algae. In: Paul EA, editor. Soil Microbiology and Biochemistry. 3rd ed Elsevier Academic; Amsterdam: 2007. pp. 145–162. [Google Scholar]
- van der Heijden MGA, Bardgett RD, van Straalen N. The unseen majority: soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecology Letters. 2008;11:296–310. doi: 10.1111/j.1461-0248.2007.01139.x. [DOI] [PubMed] [Google Scholar]
- Veste M, Littmann T, Friedrich H, Breckle S-W. Microclimatic boundary conditions for activity of soil lichen crusts in sand dunes of the north-western Negev desert, Israel. Flora. 2001;196:465–476. [Google Scholar]
- Wetmore CM. Lichen decomposition in a black spruce bog. The Lichenologist. 1982;14:267–271. [Google Scholar]
- Wilske B, Burgheimer J, Karnieli A, Zaady E, Andreae MO, Yakir D, Kesselmeier J. The CO2 exchange of biological soil crusts in a semiarid grass-shrubland at the northern transition zone of the Negev desert, Israel. Biogeosciences. 2008;5:1411–1423. [Google Scholar]
- Wilske B, Burgheimer J, Karnieli A, Zaady E. Modeling the variability in annual carbon fluxes related to biological soil crusts in a Mediterranean shrubland. Biogeosciences Discussions. 2009;6:7295–7324. [Google Scholar]
- Yeager CM, Kornosky JL, Housman DC, Grote EE, Belnap J, Kuske CR. Diazotrophic community structure and function in two successional stages of biological soil crusts from the Colorado Plateau and Chihuahuan Desert. Applied and Environmental Microbiology. 2004;70:973–983. doi: 10.1128/AEM.70.2.973-983.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Kidron GJ, Pen-Mouratov S, Wasserstrom H, Barness G, Steinberger Y. Do development stages of biological soil crusts determine activity and functional diversity in a sand-dune ecosystem? Soil Biology & Biochemistry. 2012;51:66–72. [Google Scholar]
- Zaady E, Arbel S, Barkai D, Sarig S. Long-term impact of agricultural practices on biological soil crusts and their hydrological processes in a semiarid landscape. Journal of Arid Environments. 2013;90:5–11. [Google Scholar]
- Zelikova TJ, Housman DC, Grote EE, Neher DA, Belnap J. Warming and increased precipitation frequency on the Colorado Plateau: implications for biological soil crusts and soil processes. Plant and Soil. 2012;355:265–282. [Google Scholar]
- Zhang W, Parker KM, Luo Y, Wan S, Wallace LL, Hu S. Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Global Change Biology. 2005;11:266–277. [Google Scholar]
- Zhou J, Xue K, Xie J, et al. Microbial mediation of carbon-cycle feedbacks to climate warming. Nature Climate Change. 2012;2:106–110. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.