Abstract
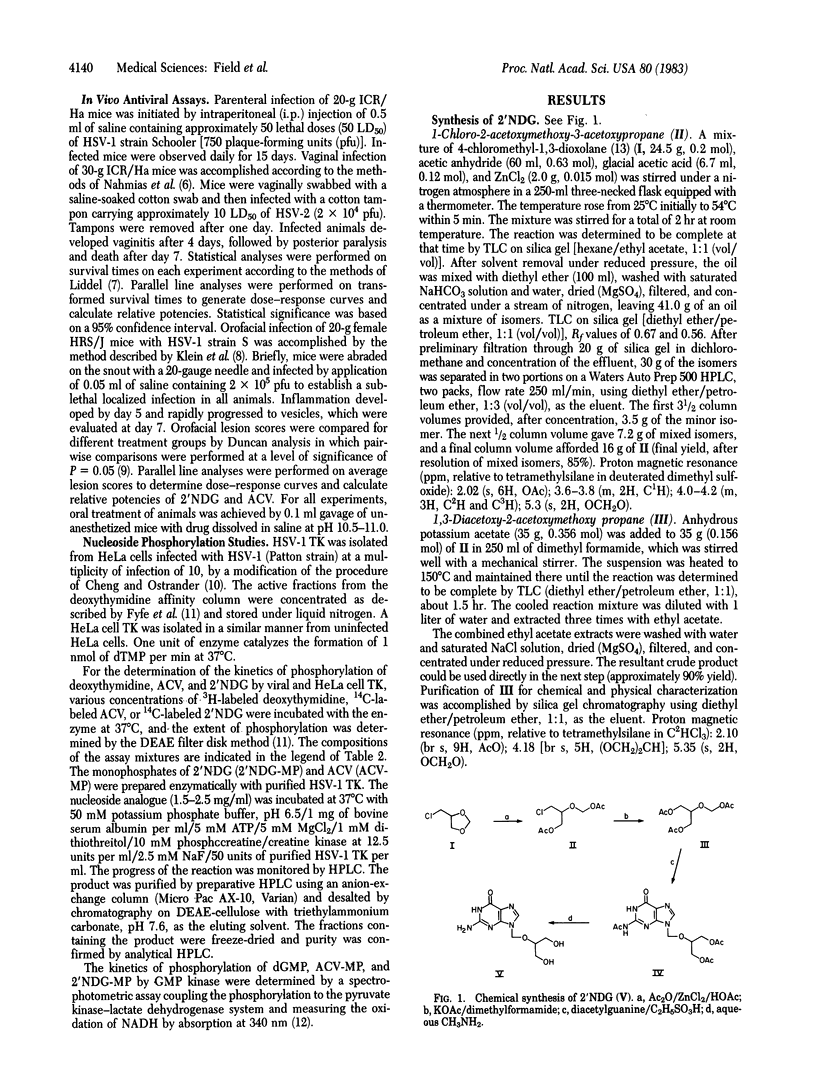
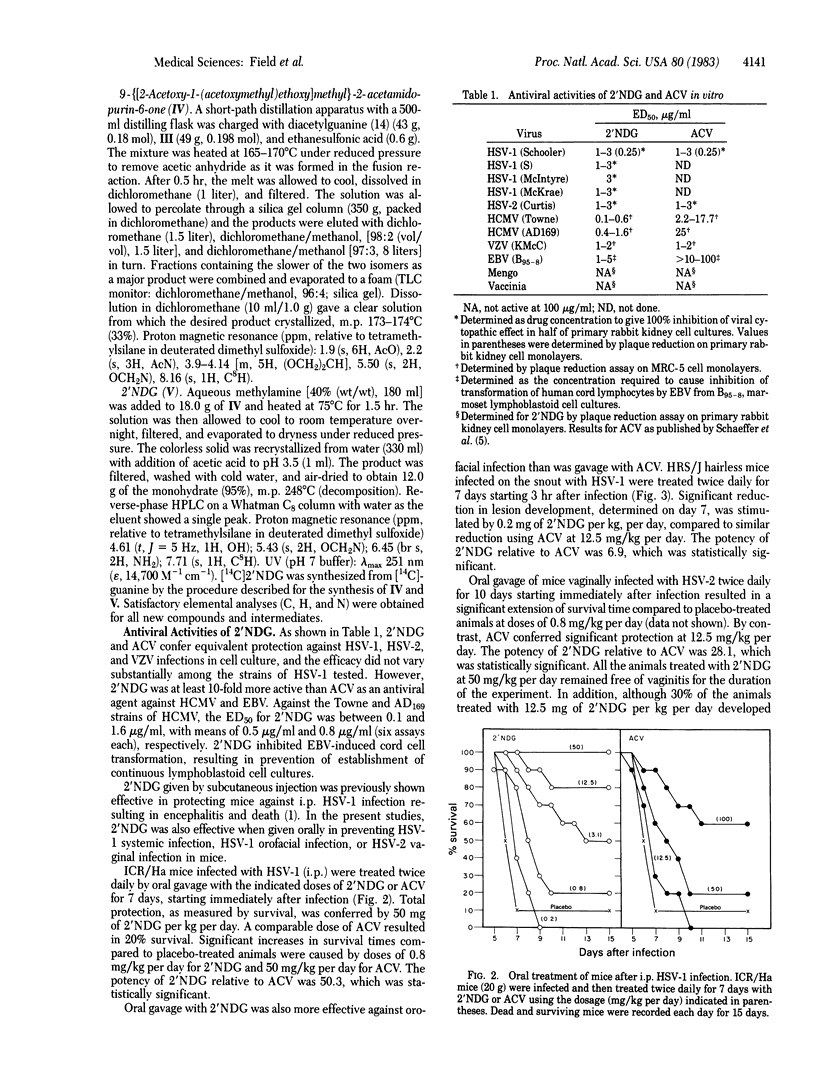
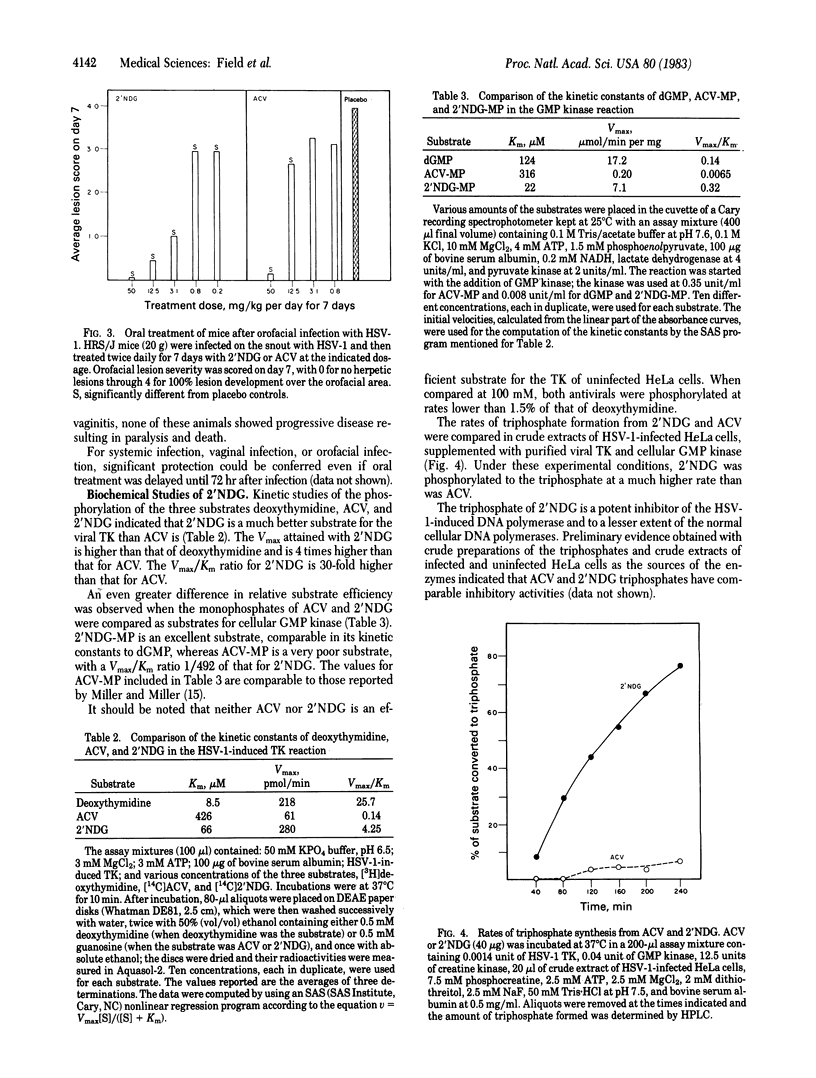
9-([2-Hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine (2'-nor-2'-deoxyguanosine; 2'NDG) selectively inhibits the replication of herpes group viruses. In cell culture studies 2'NDG was at least 10-fold more potent than acyclovir (ACV) in inhibition of human cytomegalovirus replication and Epstein-Barr virus-induced lymphocyte transformation and was about as effective as ACV in inhibition of herpes simplex viruses 1 and 2 and varicella zoster virus. Orally administered 2'NDG was 6- to 50-fold more efficacious than ACV in treating systemic or local HSV-1 infection or HSV-2 intravaginal infection in mice. The mode of action of 2'NDG appears to involve phosphorylation by herpes simplex virus thymidine kinase and subsequent phosphorylations by cellular kinases to produce 2'NDG triphosphate, which is a potent inhibitor of herpes virus DNA polymerase. Compared to ACV, 2'NDG was a more efficient substrate for HSV-1 thymidine kinase (Vmax/Km for 2'NDG 30-fold higher than that of ACV), whereas 2'NDG monophosphate is a more efficient substrate for GMP kinase (Vmax/Km for 2'NDG monophosphate 492-fold higher than that for ACV monophosphate). The combined effect is more rapid production of the inhibitory triphosphate from 2'NDG than from ACV.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashton W. T., Karkas J. D., Field A. K., Tolman R. L. Activation by thymidine kinase and potent antiherpetic activity of 2'-nor-2'-deoxyguanosine (2'NDG). Biochem Biophys Res Commun. 1982 Oct 29;108(4):1716–1721. doi: 10.1016/s0006-291x(82)80109-5. [DOI] [PubMed] [Google Scholar]
- Balfour H. H., Jr, Bean B., Mitchell C. D., Sachs G. W., Boen J. R., Edelman C. K. Acyclovir in immunocompromised patients with cytomegalovirus disease. A controlled trial at one institution. Am J Med. 1982 Jul 20;73(1A):241–248. doi: 10.1016/0002-9343(82)90099-7. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Ostrander M. Deoxythymidine kinase induced in HeLa TK- cells by herpes simplex virus type I and type II. II. Purification and characterization. J Biol Chem. 1976 May 10;251(9):2605–2610. [PubMed] [Google Scholar]
- Colby B. M., Furman P. A., Shaw J. E., Elion G. B., Pagano J. S. Phosphorylation of acyclovir [9-(2-hydroxyethoxymethyl)guanine] in Epstein-Barr virus-infected lymphoblastoid cell lines. J Virol. 1981 May;38(2):606–611. doi: 10.1128/jvi.38.2.606-611.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes J. E., Huang E. S. Stimulation of cellular thymidine kinases by human cytomegalovirus. J Virol. 1977 Oct;24(1):13–21. doi: 10.1128/jvi.24.1.13-21.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyfe J. A., Keller P. M., Furman P. A., Miller R. L., Elion G. B. Thymidine kinase from herpes simplex virus phosphorylates the new antiviral compound, 9-(2-hydroxyethoxymethyl)guanine. J Biol Chem. 1978 Dec 25;253(24):8721–8727. [PubMed] [Google Scholar]
- Klein R. J., Friedman-Kien A. E., Brady E. Herpes simplex virus skin infection in hairless mice: treatment with antiviral compounds. Antimicrob Agents Chemother. 1974 Mar;5(3):318–322. doi: 10.1128/aac.5.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddell F. D. Evaluation of survival in challenge experiments. Microbiol Rev. 1978 Mar;42(1):237–249. doi: 10.1128/mr.42.1.237-249.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. H., Miller R. L. Phosphorylation of acyclovir (acycloguanosine) monophosphate by GMP kinase. J Biol Chem. 1980 Aug 10;255(15):7204–7207. [PubMed] [Google Scholar]
- Schaeffer H. J., Beauchamp L., de Miranda P., Elion G. B., Bauer D. J., Collins P. 9-(2-hydroxyethoxymethyl) guanine activity against viruses of the herpes group. Nature. 1978 Apr 13;272(5654):583–585. doi: 10.1038/272583a0. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Galloway K. S., Kennell W. L., Ogilvie K. K., Radatus B. K. A new nucleoside analog, 9-[[2-hydroxy-1-(hydroxymethyl)ethoxyl]methyl]guanine, highly active in vitro against herpes simplex virus types 1 and 2. Antimicrob Agents Chemother. 1982 Jul;22(1):55–61. doi: 10.1128/aac.22.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade J. C., Hintz M., McGuffin R., Springmeyer S. C., Connor J. D., Meyers J. D. Treatment of cytomegalovirus pneumonia with high-dose acyclovir. Am J Med. 1982 Jul 20;73(1A):249–256. doi: 10.1016/0002-9343(82)90100-0. [DOI] [PubMed] [Google Scholar]
- Závada V., Erban V., Rezácová D., Vonka V. Thymidine-kinase in cytomegalovirus infected cells. Arch Virol. 1976;52(4):333–339. doi: 10.1007/BF01315622. [DOI] [PubMed] [Google Scholar]
- de Miranda P., Krasny H. C., Page D. A., Elion G. B. The disposition of acyclovir in different species. J Pharmacol Exp Ther. 1981 Nov;219(2):309–315. [PubMed] [Google Scholar]


