Abstract
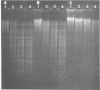
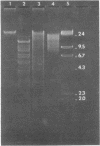
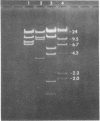
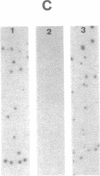
A library of cloned Mycoplasma hyorhinis genomic sequences was constructed by incorporation of EcoRI digestion fragments of mycoplasma DNA into the lambda Charon 4A bacteriophage vector. Immunological screening of recombinant phage plaques identified clones containing genes encoding mycoplasma antigenic structures expressed in an Escherichia coli host. Two such recombinant phage isolates, lambda Ch4A-MhrG1 and lambda Ch4A-MhrG28, were defined and found to contain distinct genomic sequences by analysis of restriction endonuclease fragments. Inoculation of mice with recombinant gene products from lambda Ch4A-MhrG1 yielded antiserum selectively recognizing a Mr 29,500 trypsin-sensitive mycoplasma constituent. This established a means for producing selected immunogenic mycoplasma component in a bacterial host. The cloned genomic sequences of M. hyorhinis encoding expressed mycoplasma antigens represent molecular probes that can be characterized both by specific DNA sequences and by the antigenic structure of corresponding gene products. These genomic fragments define initial physical markers of the M. hyorhinis genome and may be useful in assessing antigenic and molecular genetic relationships within the genus Mycoplasma and among other members of the class Mollicutes.
Full text
PDF

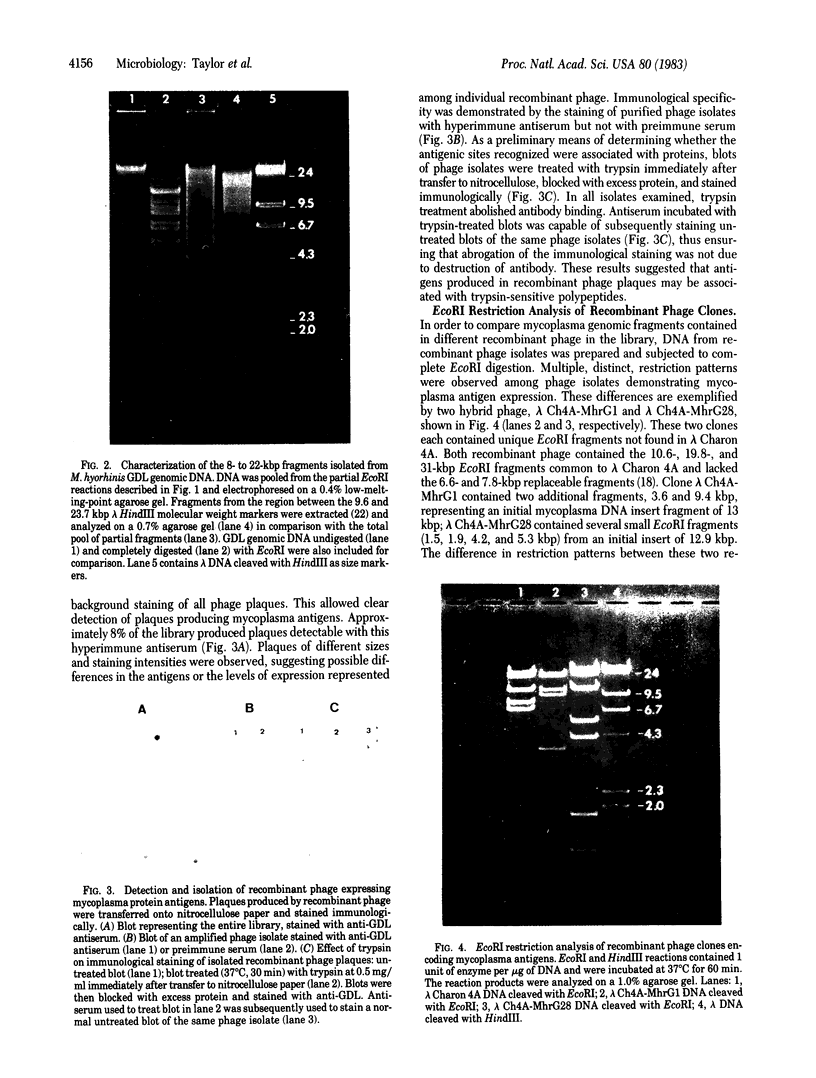


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amikam D., Razin S., Glaser G. Ribosomal RNA genes in Mycoplasma. Nucleic Acids Res. 1982 Jul 24;10(14):4215–4222. doi: 10.1093/nar/10.14.4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baseman J. B., Cole R. M., Krause D. C., Leith D. K. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982 Sep;151(3):1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- Darai G., Zöller L., Matz B., Delius H., Speck P. T., Flügel R. M. Analysis of Mycoplasma hyorhinis genome by use of restriction endonucleases and by electron microscopy. J Bacteriol. 1982 May;150(2):788–794. doi: 10.1128/jb.150.2.788-794.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinter Z., Taylor-Robinson D. Susceptibility and resistance of various strains of Mycoplasma hyorhinis to antisera, polymyxins and low pH values. J Gen Microbiol. 1969 Aug;57(2):263–272. doi: 10.1099/00221287-57-2-263. [DOI] [PubMed] [Google Scholar]
- Feldner J., Göbel U., Bredt W. Mycoplasma pneumoniae adhesin localized to tip structure by monoclonal antibody. Nature. 1982 Aug 19;298(5876):765–767. doi: 10.1038/298765a0. [DOI] [PubMed] [Google Scholar]
- Gois M., Kuksa F., Franz J., Taylor-Robinson D. The antigenic differentiation of seven strains of Mycoplasma hyorhinis by growth-inhibition, metabolism-inhibition, latex-agglutination, and polyacrylamide-gel-electrophoresis tests. J Med Microbiol. 1974 Feb;7(1):105–115. doi: 10.1099/00222615-7-1-105. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Cole R. M., Huang Y. S., Graham J. A., Gardner D. E., Collier A. M., Clyde W. A., Jr Mycoplasma pneumoniae infection: role of a surface protein in the attachment organelle. Science. 1982 Apr 16;216(4543):313–315. doi: 10.1126/science.6801766. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Neimark H., London J. Origins of the mycoplasmas: sterol-nonrequiring mycoplasmas evolved from streptococci. J Bacteriol. 1982 Jun;150(3):1259–1265. doi: 10.1128/jb.150.3.1259-1265.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolau C., Rottem S. Expression of a beta-lactamase activity in Mycoplasma capricolum transfected with the liposome-encapsulated E.coli pBR 322 plasmid. Biochem Biophys Res Commun. 1982 Oct 15;108(3):982–986. doi: 10.1016/0006-291x(82)92096-4. [DOI] [PubMed] [Google Scholar]
- Robbins J., Rosteck P., Jr, Haynes J. R., Freyer G., Cleary M. L., Kalter H. D., Smith K., Lingrel J. B. The isolation and partial characterization of recombinant DNA containing genomic globin sequences from the goat. J Biol Chem. 1979 Jul 10;254(13):6187–6195. [PubMed] [Google Scholar]
- Sternberg N., Tiemeier D., Enquist L. In vitro packaging of a lambda Dam vector containing EcoRI DNA fragments of Escherichia coli and phage P1. Gene. 1977 May;1(3-4):255–280. doi: 10.1016/0378-1119(77)90049-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise K. S., Cassell G. H., Action R. T. Selective association of murine T lymphoblastoid cell surface alloantigens with Mycoplasma hyorhinis. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4479–4483. doi: 10.1073/pnas.75.9.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M., Nomura M. Organization of genes for transcription and translation in the rif region of the Escherichia coli chromosome. J Bacteriol. 1979 Jan;137(1):584–594. doi: 10.1128/jb.137.1.584-594.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]