Abstract
The gating component associated with the voltage-sensitive Na+ channel from electroplax membranes of Electrophorus electricus has been purified by using toxin gamma from the venom of the scorpion Tityus serrulatus serrulatus. The toxin-binding site was efficiently solubilized with Lubrol PX, resulting in an extract of high initial specific activity. Purification was achieved by adsorption of the toxin-binding component to DEAE-Sephadex A-25 followed by desorption at high ionic strength and chromatography on either wheat germ agglutinin-Ultrogel or Sepharose 6B. Maximal final specific activities were at least 42% of the specific activity expected for a pure toxin-binding component. The purified material exhibited a Stokes radius of 85 A, and sodium dodecyl sulfate/polyacrylamide gel electrophoresis demonstrated a single polypeptide component of Mr 270,000. Furthermore, tetrodotoxin binding activity and Tityus gamma toxin binding activity copurified, suggesting that the selectivity filter and the gating component of the Na+ channel are carried by the same polypeptide chain.
Full text
PDF
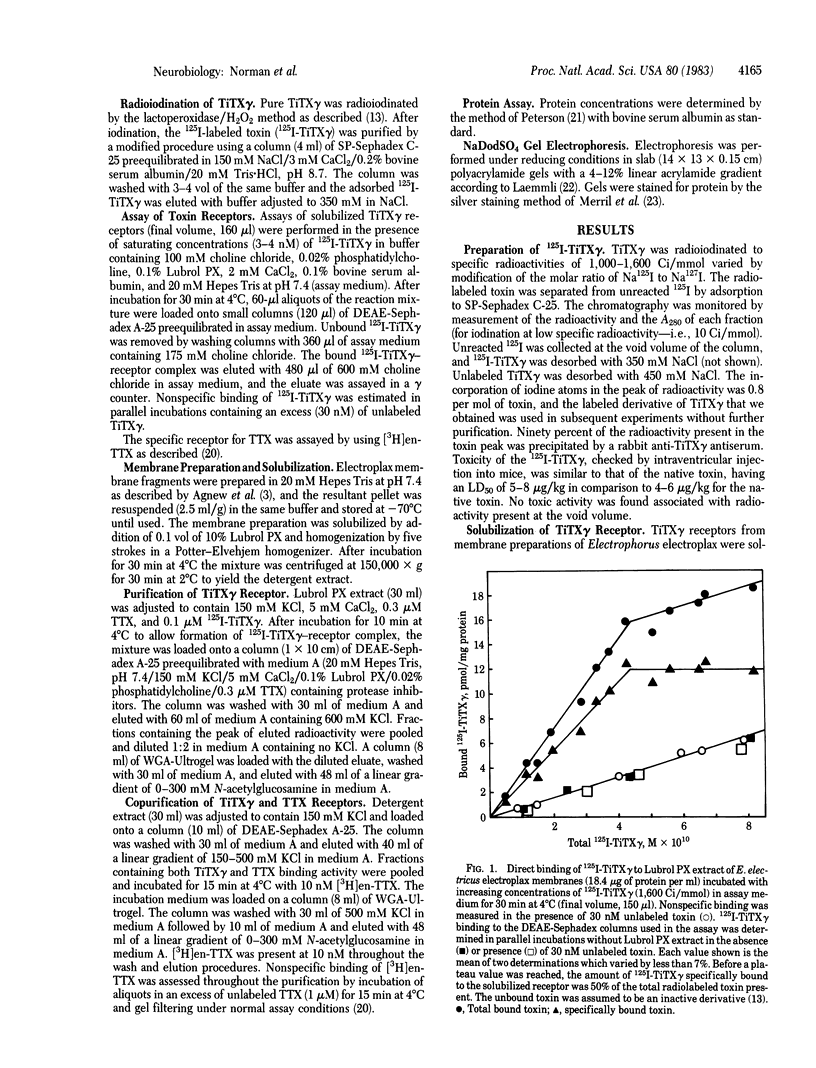
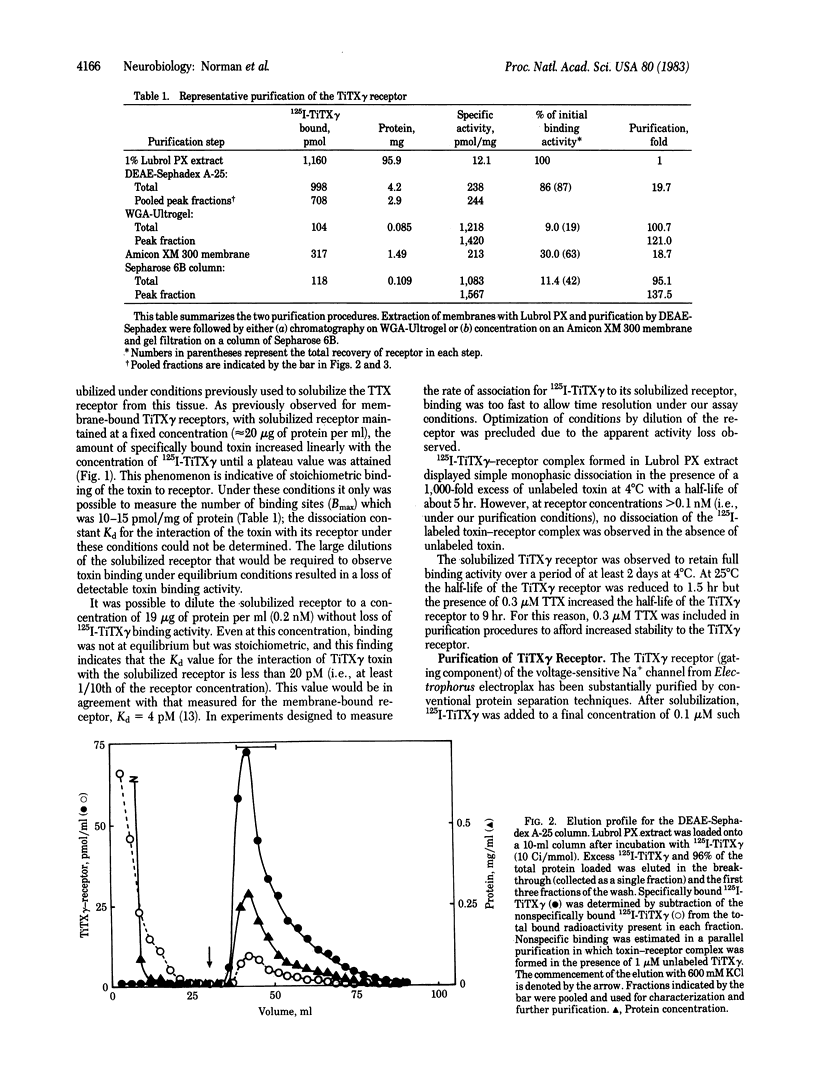
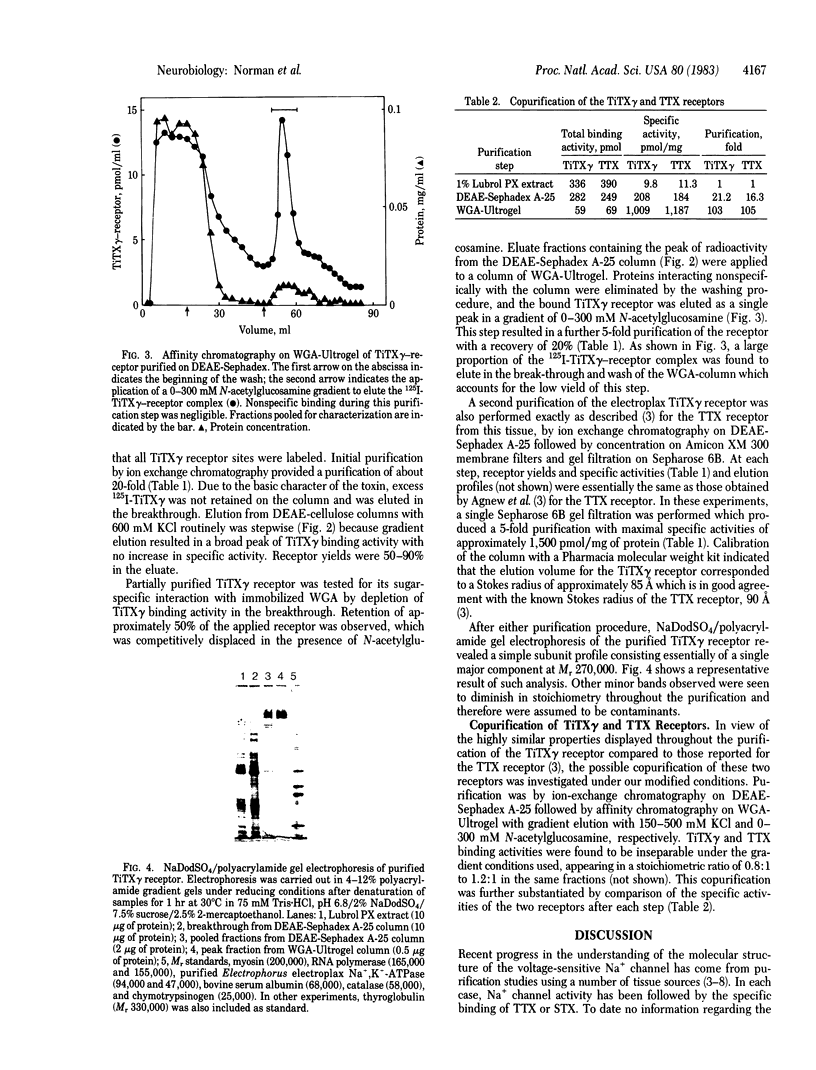

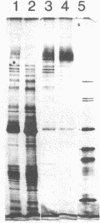
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnew W. S., Moore A. C., Levinson S. R., Raftery M. A. Identification of a large molecular weight peptide associated with a tetrodotoxin binding protein from the electroplax of Electrophorus electricus. Biochem Biophys Res Commun. 1980 Feb 12;92(3):860–866. doi: 10.1016/0006-291x(80)90782-2. [DOI] [PubMed] [Google Scholar]
- Barchi R. L., Cohen S. A., Murphy L. E. Purification from rat sarcolemma of the saxitoxin-binding component of the excitable membrane sodium channel. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1306–1310. doi: 10.1073/pnas.77.3.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barhanin J., Giglio J. R., Léopold P., Schmid A., Sampaio S. V., Lazdunski M. Tityus serrulatus venom contains two classes of toxins. Tityus gamma toxin is a new tool with a very high affinity for studying the Na+ channel. J Biol Chem. 1982 Nov 10;257(21):12553–12558. [PubMed] [Google Scholar]
- Barhanin J., Schmid A., Lombet A., Wheeler K. P., Lazdunski M., Ellory J. C. Molecular size of different neurotoxin receptors on the voltage-sensitive Na+ channel. J Biol Chem. 1983 Jan 25;258(2):700–702. [PubMed] [Google Scholar]
- Cahalan M. D. Modification of sodium channel gating in frog myelinated nerve fibres by Centruroides sculpturatus scorpion venom. J Physiol. 1975 Jan;244(2):511–534. doi: 10.1113/jphysiol.1975.sp010810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catterall W. A., Morrow C. S., Hartshorne R. P. Neurotoxin binding to receptor sites associated with voltage-sensitive sodium channels in intact, lysed, and detergent-solubilized brain membranes. J Biol Chem. 1979 Nov 25;254(22):11379–11387. [PubMed] [Google Scholar]
- Catterall W. A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Chicheportiche R., Balerna M., Lombet A., Romey G., Lazdunski M. Synthesis of new, highly radioactive tetrodotoxin derivatives and their binding properties to the sodium channel. Eur J Biochem. 1980 Mar;104(2):617–625. doi: 10.1111/j.1432-1033.1980.tb04466.x. [DOI] [PubMed] [Google Scholar]
- Couraud F., Jover E., Dubois J. M., Rochat H. Two types of scorpion receptor sites, one related to the activation, the other to the inactivation of the action potential sodium channel. Toxicon. 1982;20(1):9–16. doi: 10.1016/0041-0101(82)90138-6. [DOI] [PubMed] [Google Scholar]
- Hartshorne R. P., Catterall W. A. Purification of the saxitoxin receptor of the sodium channel from rat brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4620–4624. doi: 10.1073/pnas.78.7.4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard B. D., Gundersen C. B., Jr Effects and mechanisms of polypeptide neurotoxins that act presynaptically. Annu Rev Pharmacol Toxicol. 1980;20:307–336. doi: 10.1146/annurev.pa.20.040180.001515. [DOI] [PubMed] [Google Scholar]
- Jaimovich E., Ildefonse M., Barhanin J., Rougier O., Lazdunski M. Centruroides toxin, a selective blocker of surface Na+ channels in skeletal muscle: voltage-clamp analysis and biochemical characterization of the receptor. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3896–3900. doi: 10.1073/pnas.79.12.3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Renaud J. F. The action of cardiotoxins on cardiac plasma membranes. Annu Rev Physiol. 1982;44:463–473. doi: 10.1146/annurev.ph.44.030182.002335. [DOI] [PubMed] [Google Scholar]
- Levinson S. R., Ellory J. C. Molecular size of the tetrodotoxin binding site estimated by irradiation inactivation. Nat New Biol. 1973 Sep 26;245(143):122–123. doi: 10.1038/newbio245122a0. [DOI] [PubMed] [Google Scholar]
- Lombet A., Renaud J. F., Chicheportiche R., Lazdunski M. A cardiac tetrodotoxin binding component: biochemical identification, characterization, and properties. Biochemistry. 1981 Mar 3;20(5):1279–1285. doi: 10.1021/bi00508a036. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Meves H., Rubly N., Watt D. D. Effect of toxins isolated from the venom of the scorpion Centruroides sculpturatus on the Na currents of the node of Ranvier. Pflugers Arch. 1982 Mar;393(1):56–62. doi: 10.1007/BF00582392. [DOI] [PubMed] [Google Scholar]
- Moore H. P., Fritz L. C., Raftery M. A., Brockes J. P. Isolation and characterization of a monoclonal antibody against the saxitoxin-binding component from the electric organ of the eel Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1673–1677. doi: 10.1073/pnas.79.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Possani L. D., Alagón A. C., Fletcher P. L., Jr, Erickson B. W. Purification and properties of mammalian toxins from the venom of Brazilian Scorpion Tityus serrulatus Lutz and Mello. Arch Biochem Biophys. 1977 Apr 30;180(2):394–403. doi: 10.1016/0003-9861(77)90053-4. [DOI] [PubMed] [Google Scholar]
- Sampaio S. V., Laure C. J., Giglio J. R. Isolation and characterization of toxic proteins from the venom of the Brazilian scorpion Tityus serrulatus. Toxicon. 1983;21(2):265–277. doi: 10.1016/0041-0101(83)90011-9. [DOI] [PubMed] [Google Scholar]
- Weigele J. B., Barchi R. L. Functional reconstitution of the purified sodium channel protein from rat sarcolemma. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3651–3655. doi: 10.1073/pnas.79.11.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]