Abstract
Background
Researchers have generally focused on tissue reactions occurring within the periodontal ligament and bone to find possible explanation for various clinical phenomena, with less attention being paid to the inherent bone density. Recently, regional differences in jaw anatomy and bone structure including bone density have become important issue to explain some of the variation in clinical practice with respect to tooth movement, implant success rate, anchorage loss etc.
Materials and methods
The intent of this review is to discuss various methods and classification proposed to determine bone density in particular area and its importance in field of orthodontia. Various clinical studies and research done in relation to bone density were searched using PubMed.
Results and conclusion
This review endeavours to compile the research of bone density in maxilla and mandible. Many clinical studies have demonstrated relation between bone density and various clinical phenomena in dentistry. Knowledge of bone density in particular area of oral cavity may help the clinician to plan proper site for implant placement and various anchorage augmentation techniques in order to increase success rate of the treatment.
Keywords: Bone density, Maxilla, Mandible, Mini-implant, Anchorage
1. Introduction
The term density has been used in a variety of different meaning by various skeletal tissue investigators. To some, density is the quality of radiopaqueness of roentgenograms. A weight-per-volume concept is based on the fact that the x-ray absorption is proportional to the mass of calcium in that unit of bone volume. Others have used density to be weight of bone per unit volume as reflected by the external envelope of the organ bone. Density has been used as an expression of specific gravity of bone tissue. Lastly, density has been used to describe the relative amount of marrow spaces present in a unit of bone tissue.1
Knowledge of bone density in the maxillofacial region has numerous advantages for dental research and clinical practice. Muscle loading forces influence bone formation as well as bone density. The knowledge of three dimensional distribution of bone density would permit a more comprehensive assessment of the intricate relationship between adaptive deformation of the skeleton and its biomechanical environment. An increase in the bone density on the skeletal surface indicates active addition of mineral. Changes in its distribution during growth could reveal the growth sites. Measurement of these properties would be useful for planning sites for implant placement and determination of bone healing in dental implantalogy, as well as evaluation of orthodontic tooth movement.2
2. Various methods of assessing bone density
A wide range of methods have been developed for the measurement of bone mineral density.
-
i.
Radiogrammetry (RG) measures the thickness of the cortex of metacarpal or other tubular bones on standard anteroposterior roentgenograms of the hand, from which various derived indices of cortical bone volume are calculated. This technique is widely available, simple and requires only the ability to take reproducible bone roentgenograms and make fine calibre measurements. Radiogrammetric measurements are usually precise and reproducible and can be compared with large normal population. However, it does not reflect absolute bone mineral content reliably. It only provides information on relative change in bone volume and has been mainly applied to appendicular skeleton.3
-
ii.
Compton scattering technique4 takes advantage of the scattering of a beam of gamma rays into a detector, whereby the level of activity is a function of the density of bone target. It reflects both organic and inorganic component of the volume of bone studies. The scattering volume can be located entirely within weight bearing trabecular bone with high precision.
-
iii.
Radiographic Photodensitometry (RP) uses the bone mineral image on standard radiographic film as an indicator of photon absorption by bone, thereby indirectly measuring the bone mineral content. The degree of film whitening is measured by a photodensitometer. Calibration of each film is accomplished by simultaneous exposure of a reference aluminium alloy wedge that has a similar rate of X-ray absorption as bone.3 As routinely obtained radiographs vary widely in density, a strict standardization of kilovoltage, exposure time and film processing is essential for these measurements. This method is very sensitive to changes in overlying tissue and is therefore restricted to appendicular bones.5
-
iv.
Single energy photon absorptiometry (SPA) was first introduced in 1963 by Cameron and Sorenson. It measures mineral content in the appendicular skeleton and usually is done on the radius. A monoenergetic photon source, such as iodine-125, is coupled with a collimated sodium iodide scintillation counter detector. The difference in photon absorption between bone and soft tissue allows calculation of the total bone mineral content found in the scan path. Bone mineral content is expressed as bone mineral per square centimetre scanned.3 However; the technique requires a uniform soft tissue thickness surrounding the bone, thus limiting its use to extremities. Since it does not distinguish cortical from trabecular bone, there is poor correlation between the measurements.5
-
v.
Dual-energy photon absorptiometry (DPA) is a modification of the single-energy technique using a radioisotope that emits photons at two different energy levels. Dual photon absorption measurement eliminates the need for a constant soft tissue thickness across a scan path and measures the total integrated mineral in the path of the beam.4 Von Wowern was the first author to describe the application of DPA to analyze the mandibular bone mineral content.6
-
vi.
Neutron activation analysis uses a source of high energy neutrons to activate body calcium-48 to calcium-49. The subsequent decay back to calcium-48 can be measured with a gamma radiation counter which eventually provides a measurement of the total body calcium. Since more than 98% of total body calcium is skeletal, this technique assesses total bone calcium content.3
-
vii.
Quantitative computed tomography (QCT) – The data that is displayed as a CT image is a representation of the X-ray attenuation coefficients of a series of voxels, which are defined by their size and position within the reconstructed image. These calculated attenuated coefficients are expressed as “CT numbers” with the use of an absolute linear scale (Hounsfield scale) defined only by the two points of −1000 for the attenuation of dry air and 0 for that of pure water at 25 °C at the effective scanning energy used. Therefore, the Hounsfield unit varies from scanner to scanner and with different energies on the same scanner. In a perfect CT scanner, the CT number of each voxel would be an accurate reflection of the true tissue attenuation coefficient in that element. Since the CT number is affected by changes in the effective energy of the X-ray source, beam hardening effect and object size and/or positioning, to achieve accurate measurement of bone density, reference calibration standards are needed. A number of reference materials have been introduced such as dipotassium hydrogen phosphate (K2HPO4), calcium carbonate (CaCO3) and calcium hydroxyapatite (Ca10 (PO4)6OH2). Calibrating the CT number with a reference phantom quantifies its calcium content in the subject scanned.2 This technique is capable of measuring trabecular and cortical bone density separately. Variable fat content may produce inaccurate results with the single-energy technique which can be solved by using the dual-energy configuration. However this technique is more expensive, less precise, and results in higher radiation exposure.5,7
-
viii.
Dual energy X-ray absorptiometry (DXA) allows fast, non-invasive and highly precise measurement of BMD. This technique is based on the principle that bone and soft tissue exhibit differing attenuation properties as a function of photon energy. Thus, an X-ray source is used in DXA to produce a beam of two discrete energies, which is attenuated as it travels through the patient. Computerized analysis of the emergent beam produces separate attenuation profiles for bone and soft tissue structures and from this the BMD is automatically calculated.8
-
ix.
Panormic X-ray – Epistatu D et al9 evaluated bone density as precisely as possible using Panormic X-ray. It can be used for preliminary evaluation of bone density based on the opacity given by the bony structures and on identifying the inter-trabecular spaces. Appreciation of bone density depends on use of uniform X-ray technique with same parameters, mineralization of compact bone and subjectivism and experience of the observer.
3. Classifications of bone density
Various classifications of bone density have been proposed in literature in order to simplify understanding.
-
i.
Linkow LI, Chercheve R (1970)10 classified bone density into three categories namely Class I, Class II and Class III bone structure. Class I bone structure is the ideal bone type consisting of evenly spaced trabeculas with small cancellated spaces. Class II bone structure is the bone that has slightly larger cancellated spaces with less uniformity of the osseous pattern. Class III bone structure has large marrow filled spaces existing between trabeculas.
-
ii.
Lekholm U, Zarb GA (1985)11 subjectively classified bone density using radiographs into four bone types based on the amount of cortical versus trabecular bone. Type 1 bone composed of homogenous compact bone. Type 2 is a thick layer of compact bone surrounding a core of dense trabecular bone. Type 3 is a thin layer of cortical bone surrounding dense trabecular bone of favourable strength. Type 4 is a thin layer of cortical bone surrounding a core of low density trabecular bone. (Fig. 1)
-
iii.
Roberts WE, Turley PK, Brezniak N, Fiedler PJ (1987)12 macroscopically classified the bone densities into four categories. These categories can be arranged from the most dense to the least dense as follows – dense cortical followed by porous cortical, coarse trabecular and fine trabecular. Dense and porous cortical bone is found on the outer surfaces of the bone and includes the crest of edentulous ridge. Coarse and fine trabecular bone is found within the outer shell of cortical bone.
-
iv.
Misch CE (1988)13 described four bone densities found in the edentulous regions of the maxilla and the mandible based on macroscopic cortical and trabecular bone characteristics. D1 bone is primarily dense cortical bone, D2 bone has dense to thick porous cortical bone on the crest and coarse trabecular bone underneath, D3 bone has thinner porous cortical crest and fine trabecular bone within and D4 has almost no crestal cortical bone and fine trabecular bone composes almost all of the total volume of bone.
-
v.
Based on clinical hardness of bone as perceived during drilling prior to implant placement Misch CE (1993)14 categorized the bone density into four groups. Drilling and placing implants in D1 has the tactile analogue of oak or maple wood. D2 bone is similar to the tactile sensation of drilling into spruce or white pine wood. Drilling into D3 bone has the tactile analogue of balsa wood. D4 bone is similar to drilling into styrofoam.
-
vi.
Misch CE, Kircos LT (1999)15 classified the bone density into five groups based on number of Hounsfield units (HU). D1 corresponds to values greater than 1250 HU, D2 has 850–1250 HU, D3 refers to density within 350–850 HU, D4 has 150–350 HU and D5 less than 150 HU. D1 is primarily found in the anterior mandible, buccal shelf and midpalatal region. D2 is found primarily in the anterior maxilla, the midpalatal region and the posterior mandible. D3 is found primarily in the posterior maxilla and mandible. D4 is found primarily in the tuberosity region.16 (Fig. 2)
Fig. 1.
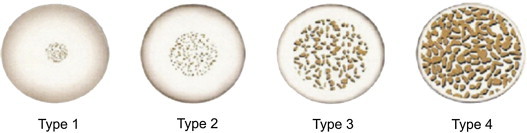
Bone types classified according to Lekholm and Zarb based on the amount of cortical versus trabecular bone. Type 1 bone composed of homogenous compact bone, type 2 is a thick layer of compact bone surrounding a core of dense trabecular bone, type 3 is a thin layer of cortical bone surrounding dense trabecular bone of favourable strength, and type 4 is a thin layer of cortical bone surrounding a core of low density trabecular bone.
Fig. 2.
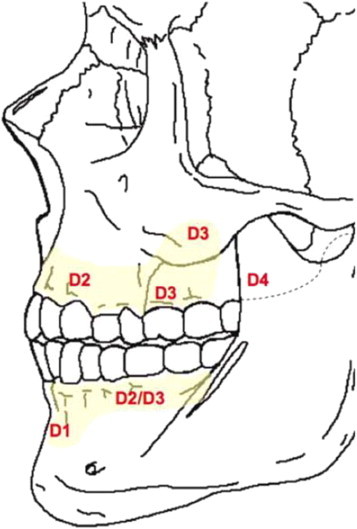
Bone density in maxilla and mandible according to Misch. D1 is primarily found in the anterior mandible, buccal shelf and midpalatal region, D2 primarily in the anterior maxilla, the midpalatal region and the posterior mandible, D3 in the posterior maxilla and mandible, and D4 is found primarily in the tuberosity region.
4. Bone density as a parameter in treatment planning of mini-implants
4.1. Influence of bone density on the load transfer
During early stages, bone density appears to be a key determinant for stationary anchorage of mini-implants in the sites with inadequate cortical bone thickness because primary retention of mini-implants is achieved by mechanical means rather than through osseointegration.17 The mechanical distribution of the stress occurs primarily where bone is in contact with the implant. The smaller the area of bone contacting the implant body, the greater the overall stress, when all other factors are equal. The bone density influences the amount of bone in contact with the implant surface. Jaffin and Berman18 analyzed the Branemark fixture loss over a period of five years in various types of bone. They found that type I, II and III bone offer good strength. Type IV bone, which has a thin cortex and poor medullary strength with low trabeculae density, was associated with excessive loss of these fixtures. Since less dense bone is found in the posterior maxilla, it will offer less area of contact with the body of the implant. Consequently, greater implant surface area is required to obtain a similar amount of bone-implant contact in soft bone compared with denser bone quality.
As the bone density decreases, the strength of the bone also decreases. Bone density is related directly to the strength of bone before microfracture. Misch et al19 observed a tenfold difference in bone strength from D1 to D4 bone. D2 bone exhibited a 47%–68% greater ultimate compressive strength compared with D3 bone. Statistically significant correlation have been found between implant placement resistance and the bone density values of the recipient site,20,21 and hence insertion torque measurements for the evaluation of bone quality seemed to be reliable. Whenever forces are applied Ti implant or when the implant is driven into bone, microfractures occur in adjacent bone. To decrease the amount of microfracture of bone, the strain on the bone should be reduced. Strain is directly related to the stress. Consequently, the stress to the implant system should be reduced as the bone density decreases. Stress is equal to the force divided by the functional area over which it is applied. One of the ways to reduce stress is to reduce the biomechanical forces on the implant. The amount of force applied to mini-implants is usually kept between 50–80 g for intrusion depending on the number of teeth and 150 g for retraction. This amount of force is much less when compared to masticatory forces which are applied to teeth.22 The stress can also be reduced by increasing the functional area over which the force is applied. It can be done by increasing the length or width of the implant. Tada S et al23 performed a 3- dimensional finite element analysis to evaluate the influence of implant length as well as that of bone quality, on the stress/strain in bone and implant. The results of this study suggest that bone of higher rather than lower density might ensure a better biomechanical environment for implants. Moreover, longer screw-type implants could be a better choice in a jaw with bone of low density.
In the comparatively weak cortical bone area, the stress is known to be distributed to the cancellous bone and the cortical bone, whereas the stress is centred on the cortical bone where it is thick and dense.24 When considering this with Hedia's study25 showing that stress can be concentrated at the cortical bone with weak or no cancellous bone, the cancellous bone in the maxilla might have a greater influence for success than that of the mandible. For these reasons, when selecting screw implants, a clinician should choose longer screw implants in the maxilla. But in the mandible, the most support for screw implants originates from the cortical bone because it is thick and dense. Therefore, longer screw implants in the mandible might not enhance stability as in the maxilla, but the diameter might affect stability. According to Cheng et al,26 the most favourable site for miniscrew placement for canine retraction is between the roots of the maxillary second permanent premolars and the first molars.
4.2. Bone density and method of insertion
While selecting the method of insertion of implants, bone density of the area should also be considered. Whenever the mini-implants are placed in the thick, dense cortical bone, insertion torque increases20,21 and thereby chances of fracture or breakage of implant increases and more amount of bone is damaged. Therefore, while placing the mini-implants in the thick and dense cortical bone area, it is advisable to use pre-drilling method.
4.3. Bone density and implant failure
Regions of D1–D3 bone have been found to be adequate for temporary anchorage device (TAD) insertion. TADs placed in D1 and D2 bone exhibit lower stress at the screw-bone interface and may provide greater stationary anchorage during loading. Placement in D4 bone is not recommended owing to the high failure rate associated with it (35–50 percent).16 Previous investigations dealing with the success of screw implants showed high failure rates in the posterior mandible. Cheng et al26 speculated that movable soft oral mucosa was the cause, since it is more prone to inflammation. However, Park27 speculated that failures might be caused by movable oral mucosa, irritation from food or excessive heat generated during placement because presence of thick and dense cortical bone in posterior mandible. Heat generated at 47 °C is known to cause bone necrosis and can adversely affect the success of dental implants. Bone necrosis becomes extensive with increase in temperature and exposure time to heat. Tehemar28 stated that heat generation increases during drilling in dense bone. Therefore, when placing the mini-implants into high density areas such as retromolar and posterior areas in the mandible, clinicians must be careful not to generate heat. Heat generation can be prevented by irrigating abundantly with saline solution, not applying too much pressure on the bone and not using a worn drill. Also, large-diameter drill can be used instead of a small diameter drill.
Santiago et al29 conducted a study to correlate the clinical and radiographic stability of titanium miniscrews when used for maxillary canine retraction and to assess bone quality. They concluded that the regions between the maxillary second premolars and first molars, and mesial to the maxillary second premolars are ideal, as far as bone quality is concerned, for miniscrew placement during the first 90 days of canine distalization.
5. Bone density and rate of tooth movement
As the bone density reduces, the rate of tooth movement increases. Mandibular molars have been found to have higher anchorage value than maxillary molars. The alveolar process supporting the mandibular molars has been found to be denser than maxillary molars, thereby offering more resistance to tooth movement. The enhanced anchorage value of mandibular molars is related to the high density bone formed as the leading roots are moved mesially. After few months of mesial translation, the trailing roots engage the high density bone formed by leading root and the rate of tooth movement declines. In general, the rate of tooth movement is inversely related to the bone density. An observation supporting this concept is the fact that the faster tooth movement occurs in children when compared to adults.30 Thus in the areas of low bone density, it is necessary to augment the anchorage as per requirement.
6. Conclusion
-
1.
Knowledge of low density sites prior to implant placement allows clinician to use longer implant in these areas to improve retention.
-
2.
In areas of high bone density, use of pre-drilling method avoids the breakage of implant. Sufficient irrigation should be done to prevent overheating of bone in that area.
-
3.
Immediate loading of mini-implants is possible because of higher bone density in all the areas of cortical bone.
-
4.
In areas of low bone density, it is necessary to augment the anchorage as per requirement.
Conflicts of interest
All authors have none to declare.
References
- 1.Buck D.L., Wheeler P.W. A density comparison of human alveolar and retromolar bone. Angle Orthod. 1969;39:133–136. doi: 10.1043/0003-3219(1969)039<0133:ADCOHA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Maki K., Okano T., Morohashi T., Yamada S., Shibaski Y. The application of three dimensional quantitative computed tomography to the maxillofacial skeleton. Dentomaxillofac Radiol. 1997;26:39–44. doi: 10.1038/sj.dmfr.4600220. [DOI] [PubMed] [Google Scholar]
- 3.Kimmel P.L. Health and public policy committee: radiologic methods to evaluate bone mineral content (position paper) Ann Intern Med. 1984;100:908–911. [PubMed] [Google Scholar]
- 4.Andresen J., Nielsen H.E. Assessment of bone mineral content and bone mass by non invasive radiologic methods. Acta Radiol. 1986;27:609–617. doi: 10.1177/028418518602700601. [DOI] [PubMed] [Google Scholar]
- 5.Punn K.K., Wong F.H.W. Importance of measurement of bone density in the management of osteoporosis. Singapore Med J. 1990;31:390–396. [PubMed] [Google Scholar]
- 6.Solar P., Ulm C.W., Thorton B., Matejka M. Sex related differences in the bone mineral density of atropic mandibles. J Prosthet Dent. 1994;71:345–349. doi: 10.1016/0022-3913(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 7.Cann C.E. Quantitative CT for determination of bone mineral density: a review. Radiology. 1988;166:509–522. doi: 10.1148/radiology.166.2.3275985. [DOI] [PubMed] [Google Scholar]
- 8.Devlin H., Horner K., Ledgerton D. A comparison of maxillary and mandibular bone mineral densities. J Prosthet Dent. 1998;79:323–327. doi: 10.1016/s0022-3913(98)70245-8. [DOI] [PubMed] [Google Scholar]
- 9.Epistatu D., Dumitru I., Pascutoi I., Oana G. A study of interpreting bone density on panoramic X-rays. Proc Rom Acad Series B. 2008;1–2:43–48. [Google Scholar]
- 10.Linkow L.I., Chercheve R. vol. 1. Mosby; St Louis: 1970. (Theories and Techniques of Oral Implantalogy). [Google Scholar]
- 11.Lekholm U., Zarb G.A. Patient selection and preparation. In: Brånemark P.-I., Zarb G.A., Albrektsson T., editors. Tissue-integrated Prostheses: Osseointegration in Clinical Dentistry. Quintessence; Chicago: 1985. pp. 199–209. [Google Scholar]
- 12.Roberts W.E., Turley P.K., Brezniak N., Fiedler P.J. Bone physiology and metabolism. J Calif Dent Assoc. 1987;15:54–61. [PubMed] [Google Scholar]
- 13.Misch C.E. Bone character: second vital implant criterion. Dent Today. 1988;7(5):39. [Google Scholar]
- 14.Misch C.E. Density of bone: effect on treatment planning, surgical approach, and healing. In: Misch C.E., editor. Contemporary Implant Dentistry. Mosby; St. Louis: 1993. pp. 469–485. [Google Scholar]
- 15.Misch C.E., Kircos L.T. Diagnostic imaging and techniques. In: Misch C.E., editor. Contemporary Implant Dentistry. 2nd ed. Mosby; St. Louis: 1999. pp. 73–87. [Google Scholar]
- 16.Kravitz N.D., Kusnoto B., Tsay T.P., Hohit W.F. The use of temporary anchorage devices for molar intrusion. J Am Dent Assoc. 2007;138(1):56–64. doi: 10.14219/jada.archive.2007.0021. [DOI] [PubMed] [Google Scholar]
- 17.Chun Y.S., Lim W.H. Bone density at interradicular sites: implications for orthodontic mini-implant placement. Orthod Craniofac Res. 2009;12:25–32. doi: 10.1111/j.1601-6343.2008.01434.x. [DOI] [PubMed] [Google Scholar]
- 18.Jaffin R.A., Berman C.L. The excessive loss of Branemark fixtures in type IV bone: a 5-year analysis. J Periodontol. 1991;62:2–4. doi: 10.1902/jop.1991.62.1.2. [DOI] [PubMed] [Google Scholar]
- 19.Misch C.E. Density of bone: effect on treatment plans, surgical approach, and healing, and progressive loading. Int J Oral Implantol. 1990;6:23–31. [PubMed] [Google Scholar]
- 20.Friberg B., Sennerby L., Roos J., Lekholm U. Identification of bone quality in conjunction with insertion of titanium implants. A pilot study in jaw autopsy specimens. Clin Oral Implants Res. 1995;6:213–219. doi: 10.1034/j.1600-0501.1995.060403.x. [DOI] [PubMed] [Google Scholar]
- 21.Turkyilmaz I., McGlumphy E.A. Influence of bone density on implant stability parameters and implant success: a retrospective clinical study. BMC Oral Health. 2008;8:32. doi: 10.1186/1472-6831-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Proffit W.R., Fields H.W., Sarver D.M. 4th ed. Mosby Inc.; St. Louis, MO: 2007. Contemporary Orthodontics. [Google Scholar]
- 23.Tada S., Stegaroiu R., Kitamura E., Miyakawa O., Kusakari H. Influence of implant design and bone quality on stress/strain distribution in bone around implants: a 3-dimensional finite element analysis. Int J Oral Maxillofac Implants. 2003;18:357–368. [PubMed] [Google Scholar]
- 24.Park H.S., Lee Y.J., Jeong S.H., Kwon T.G. Density of the alveolar and basal bones of the maxilla and the mandible. Am J Orthod Dentofacial Orthop. 2008;133:30–37. doi: 10.1016/j.ajodo.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Hedia H.S. Stress and strain distribution behavior in the bone due to the effect of cancellous bone, dental implant material and the bone height. Biomed Mater Eng. 2002;12:111–119. [PubMed] [Google Scholar]
- 26.Cheng S.J., Tseng I.Y., Lee J.J., Kok S.H. A prospective study of risk factors associated with failure of mini-implants used for orthodontic anchorage. Int J Oral Maxillofac Implants. 2004;19:100–106. [PubMed] [Google Scholar]
- 27.Park H.S. Clinical study on success rate of microscrew implants for orthodontic anchorage. Korean J Orthod. 2003;33:151–156. [Google Scholar]
- 28.Tehemar S.H. Factors affecting heat generation during implant site preparation: a review of biologic observations and future considerations. Int J Oral Maxillofac Implants. 1999;14:127–136. [PubMed] [Google Scholar]
- 29.Santiago R.C., de Paula F.O., Fraga M.R., Assis N.M.S.P., Vitral R.W.F. Correlation between miniscrew stability and bone mineral density in orthodontic patients. Am J Orthod Dentofacial Orthop. 2009;136:243–250. doi: 10.1016/j.ajodo.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 30.Roberts W.E. Bone physiology, metabolism, and biomechanics in orthodontic practice. In: Graber T.M., Vanarsdall R.L., Vig K.W.L., editors. Orthodontics: Current Principles and Techniques. 4th ed. Mosby; St Louis: 2005. pp. 221–292. [Google Scholar]


