Abstract
Context:
Research examining the source of excess soluble fms-like tyrosine kinase 1 (sFLT1) in preeclampsia has focused on the placenta. The potential contribution of the releasable store of sFLT1 in the systemic vasculature is unknown.
Objective:
We asked whether the nonplacental releasable store of sFLT1 is larger in women with previous preeclampsia than in women with a previous uncomplicated pregnancy.
Design:
We administered heparin to nulligravid women and to women with previous preeclampsia or a previous uncomplicated pregnancy. We compared post-heparin sFLT1 concentrations with those observed in uncomplicated pregnancy and preeclampsia.
Setting:
The study was performed at Magee-Womens Hospital.
Patients:
Participants included nulligravidas (n = 8), women 6–24 months postpartum (previous uncomplicated pregnancy, n = 16; previous preeclampsia, n = 15), and pregnant women (uncomplicated pregnancy, n = 30; preeclampsia, n = 25).
Intervention:
Nonpregnant women received an unfractionated heparin bolus.
Main Outcome Measures:
Pre- and post-heparin plasma sFLT1, placental growth factor, and vascular endothelial growth factor were measured.
Results:
In nonpregnant women, heparin increased plasma sFLT1 by 250-fold (P < .01), increased placental growth factor by 7-fold (P < .01), and decreased free vascular endothelial growth factor (P < .01). These changes did not differ between nulligravidas, women with previous preeclampsia, and women with a previous uncomplicated pregnancy. Post-heparin sFLT1 in nonpregnant women was higher than sFLT1 in uncomplicated pregnancy, but lower than sFLT1 in preeclampsia. Baseline and post-heparin sFLT1 were positively correlated (r2 = 0.19; P < .01). Heparin increased the concentration of the 100-kDa sFLT1 isoform. Adding heparin to whole blood or plasma did not increase sFLT1.
Conclusions:
Nonpregnant women have a significant vascular store of releasable sFLT1. The size of this store does not differ between women with previous preeclampsia vs women with previous uncomplicated pregnancy.
Preeclampsia affects 2–7% of pregnancies (1, 2), and is a leading cause of maternal (3) and fetal (4) morbidity and mortality. An excess of the antiangiogenic protein soluble fms-like tyrosine kinase 1 (sFLT1) is thought to contribute to this syndrome (5). sFLT1 increases significantly during uncomplicated pregnancy, and concentrations are even higher in early onset preeclampsia (5, 6). sFLT1 reduces the bioavailability of placental growth factor (PGF) and vascular endothelial growth factor (VEGF) by binding these proangiogenic proteins as a nonsignaling decoy (5, 7).
Research examining the source of excess sFLT1 in preeclampsia has focused on the placenta (5, 8), although there are reports of slightly, but significantly, increased sFLT1 in nonpregnant women years after a preeclamptic pregnancy (9, 10). This could reflect a subtle vasculopathic process in women with prior preeclampsia. The systemic vasculature contains a substantial releasable store of sFLT1 (11–13). The potential contribution of this store to elevated sFLT1 in disease is unknown. This vascular store of sFLT1 is bound to heparin sulfate proteoglycans (HSPGs), likely in the extracellular matrix and on the glycocalyx (11). The glycocalyx is a hydrated matrix of proteoglycans and glycoproteins that coats the external (apical) surface of epithelial cells (14). HSPGs are the most abundant glycocalyx proteoglycans (14). They consist of core proteins bound to the plasma membrane and covalently bound HSPG side chains (14). HSPGs bind sFLT1 electrostatically, regulating sFLT1 bioavailability by buffering systemic release (15). Transgenic heparanase overexpression in mice significantly increases circulating sFLT1 (15). This suggests that most sFLT1 is bound to HSPGs and only a small amount normally enters the circulation (15).
Intravenous heparin administration reveals this releasable vascular store of sFLT1 by transiently displacing proteins from HSPGs into the circulation (11, 12). sFLT1 (100 kDa) and sFLT1–14 (145 kDa), the two predominant isoforms present in pregnancy and preeclampsia, have heparin binding domains (16) and could be released by this method. Heparin administration dramatically increases sFLT1 (100 kDa) in older, nonpregnant individuals undergoing coronary angiography with (11, 12) or without (12) percutaneous coronary intervention for suspected or confirmed coronary artery disease. The absence of any sFLT1–14 in these patients after heparin (12) suggests that sFLT1 may be the predominant circulating isoform in nonpregnant individuals. The effect of heparin on sFLT1 in healthy young men and women has not been examined.
We quantified the releasable store of sFLT1 in three groups of women of childbearing age, namely, nulligravid women and women with previous preeclampsia or a previous uncomplicated pregnancy (6–24 months postpartum). We hypothesized that administration of unfractionated heparin in nonpregnant women would increase plasma sFLT1 to concentrations reported in the third trimester of uncomplicated pregnancy. We further posited that the amount of this releasable store would be greater in women with a history of preeclampsia and that the 100-kDa sFLT1 isoform would be the predominant circulating form in nonpregnant women before and after heparin. We also added heparin to blood samples from pregnant and nonpregnant women to test our hypothesis that blood components do not significantly contribute to this releasable store of sFLT1.
Subjects and Methods
Subjects for heparin administration study
We recruited nulligravid women (n = 8) and nonpregnant women with prior preeclampsia (n = 15) or a prior uncomplicated pregnancy (n = 16). The University of Pittsburgh Institutional Review Board (IRB) approved the study (0404185). All subjects provided written informed consent before participating. The nonpregnant women with prior preeclampsia or uncomplicated pregnancy were part of a previous study comparing circulating myeloperoxidase before and after heparin administration (17). Ten of the 15 women with prior preeclampsia and 11 of the 16 women with a prior uncomplicated pregnancy were part of a larger cohort in whom baseline plasma sFLT1 and VEGF concentrations were reported previously (9).
Subjects were nonsmokers, did not use illicit drugs, and had no history of renal or vascular disease. Women with a prior pregnancy delivered at Magee-Womens Hospital between 1999 and 2005, were studied 6–24 months postpartum, and were not lactating. Gestational hypertension was defined as persistent, new onset hypertension (systolic blood pressure ≥ 140 mm Hg and/or diastolic blood pressure ≥ 90 mm Hg) appearing after 20 weeks gestation, in the absence of proteinuria or hyperuricemia. Preeclampsia was defined as gestational hypertension with proteinuria in accordance with the National High Blood Pressure Education Program Working Group Report on High Blood Pressure in Pregnancy (18). To ensure that preeclamptic women had the specific disease of interest, our research definition also required gestational hyperuricemia (>1 SD above normal values for gestational age) (19). This definition is thought to minimize the number of women with hypertension in pregnancy who are misclassified as preeclamptics (19). Proteinuria was the excretion of ≥ 300 mg of protein per 24 hours, a dipstick of 2+, a catheterized sample of 1+, or protein:creatinine ≥ 0.3. Hyperuricemia was defined as a plasma uric acid ≥ 1 SD above the mean value for gestational age (20). Women with a previous uncomplicated pregnancy were normotensive and without proteinuria throughout gestation, and they delivered healthy babies at term.
Samples from pregnant women
Concentrations of angiogenic factors in nonpregnant women after heparin administration were compared with banked samples from pregnant women with an uncomplicated pregnancy (n = 30, third trimester), gestational hypertension (n = 12), or preeclampsia (n = 25). Diagnostic criteria were identical to those for women who received heparin. Samples were obtained from 2003 to 2006 as part of our ongoing Pregnancy Exposures and Preeclampsia Prevention Study (University of Pittsburgh IRB PRO08050339). All subjects provided written informed consent.
Blood was collected into EDTA-coated Vacutainers. Samples were centrifuged within 1 (n = 27), 2 (n = 26), or 3 (n = 7) hours of collection for 20 minutes at 2000 × g. Collected plasma was stored at −80°C.
Heparin administration
Participants were admitted to the Magee-Womens Hospital Clinical and Translational Research Center after an overnight fast. Weight, height, age, race, date of the last menstrual period, oral contraceptive use, blood pressure, and pulse were recorded. A urine pregnancy test confirmed that women were not pregnant. Blood was collected to verify that hematocrit and platelets were normal (hematocrit >35%; platelets >100 000/μL). Unfractionated heparin (70 IU/kg body weight) was administered iv over 1 minute. Blood was drawn into heparinized Vacutainers before and 15 minutes after the heparin bolus. Samples were kept on ice for less than 1 hour, then centrifuged for 10 minutes at 2000 × g. Collected plasma was stored at −80°C. Patients were discharged after 2 hours of observation.
Measurement of proangiogenic and antiangiogenic factors
Plasma sFLT1, unbound (free) VEGF, and free PGF concentrations were measured by ELISA (R&D Systems), as previously validated by our laboratory (9, 21). Intra-assay and interassay coefficients of variation were 5 and 7% for VEGF, 5 and 11% for PGF, and 4 and 14% for sFLT1. Changes in sFLT1 after heparin administration were confirmed by Western blot of enriched samples. Heparin-agarose enrichment of sFLT1 was performed in 100-μL plasma samples as described previously (16). sFLT1 was detected using a mouse monoclonal antibody (V4262, Sigma Chemical) that recognizes the extracellular domain present in sFLT1 and FLT1.
Blood sample incubation with heparin
See Supplemental Data (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Statistical analysis
Comparisons of subject characteristics between nulligravid women, women with a previous uncomplicated pregnancy, and women with previous preeclampsia were conducted using a one-way ANOVA, Mann-Whitney U test, or χ2 test, as appropriate. Pregnancy outcome data in pregnant and postpartum patient groups were compared by the Kruskal-Wallis test followed by Dunn's post hoc test. The effects of unfractionated heparin on angiogenic factors were assessed by the Wilcoxon signed rank test. The effects of incubation with and without heparin on sFLT1 concentrations in predelivery and postpartum blood samples were assessed by repeated measures ANOVA, followed by the Student-Newman-Keuls post hoc test. Correlations between sFLT1 and PGF were analyzed by Pearson correlation coefficients. Statistical analyses were performed using SPSS 18 (IBM), and JMP 9 (SAS Institute Inc).
Results
Heparin study subject characteristics
Age, race, and the proportion of women in each menstrual cycle phase were not significantly different between nonpregnant subject groups (Table 1). Compared to nulligravid women, body mass index and resting heart rate were significantly higher in women with a previous pregnancy. Women with a history of preeclampsia had significantly higher systolic and diastolic blood pressures than nulligravid women and women with a history of uncomplicated pregnancy. Compared to women with an uncomplicated pregnancy, women with preeclampsia had higher blood pressures in labor, delivered smaller infants at earlier gestational ages, and had a lower percentage of female babies (P < .05 for all; Table 2). Testing was completed approximately 1 year postpartum in women with prior preeclampsia or a prior uncomplicated pregnancy (P > .05). All women with previous uncomplicated pregnancies were primiparous. Two of 15 women with previous preeclampsia were multiparous. One of these women had preeclampsia in both of her previous pregnancies. The second had preeclampsia in her most recent pregnancy; the outcome of her first pregnancy was not known.
Table 1.
Subject Characteristics
| Variable | Nulligravid | Prior Uncomplicated Pregnancy | Prior Preeclampsia |
|---|---|---|---|
| n | 8 | 16 | 15 |
| Age, y | 25 ± 4 | 26 ± 6 | 29 ± 4 |
| Race, n (%) | |||
| White | 7 (88) | 11 (69) | 10 (67) |
| Black | 0 (0) | 4 (25) | 5 (33) |
| Asian | 1 (12) | 1 (6) | 0 (0) |
| Body mass index, kg/m2 | 21.5 (20.8–22.5) | 27.3 (23.1–31.0)b | 30.0 (24.0–35.2)b |
| Resting heart rate, beats/min | 64 (60–67) | 78 (68–84)b | 72 (68–84)b |
| Hormonal contraceptive use, n (%)a | 2 (29) | 10 (66) | 7 (50) |
| Menstrual cycle phase, n (%) | |||
| Follicular | 0 (0) | 3 (19) | 5 (33) |
| Luteal | 6 (75) | 8 (50) | 7 (47) |
| Cycle >35 d | 1 (13) | 4 (25) | 1 (7) |
| Not known | 1 (12) | 1 (6) | 2 (13) |
| Blood pressure, mm Hg | |||
| Systolic | 109 ± 8 | 109 ± 8 | 119 ± 10b,c |
| Diastolic | 68 ± 9 | 73 ± 9 | 81 ± 10b,c |
| Parity, n (%) | |||
| Nulliparous | 8 (100%) | 0 | 0 |
| Primiparous | 0 | 12 (100%) | 13 (87%) |
| Multiparous | 0 | 0 | 2 (13%) |
| Significance | b | b | |
| Days after delivery | NA | 388 ± 151 | 389 ± 114 |
Abbreviation: NA, not applicable. Values are expressed as mean ± SD or median (interquartile range) unless otherwise indicated.
Data were missing for one woman in each group.
Significant difference (P < .05) from: b nulligravid women; c prior uncomplicated pregnancy.
Table 2.
Pregnancy Outcome
| Variable | Previous Uncomplicated Pregnancy | Previous Preeclampsia | Uncomplicated Pregnancy | Gestational Hypertension | Preeclampsia |
|---|---|---|---|---|---|
| n | 15 | 15 | 30 | 12 | 25 |
| Blood pressure in labor, mm Hg | |||||
| Systolic | 123 (116–129) | 158 (145–165)b,c | 126 (118–129) | 144 (139–152)b,c | 153 (143–163)b,c |
| Diastolic | 72 (67–75) | 97 (93–102)b,c | 74 (69–81) | 84 (79–93)b,c | 96 (89–99)b,c |
| Gestational age of sample, wk | Postpartum | Postpartum | 36.0 (33.8–39.0) | 40.0 (37.0–41.0)c | 33.9 (32.0–36.7)d |
| Gestational age at delivery, wk | 39.7 (38.7, 40.6) | 36.0 (32.7, 37.7)b,c,d | 39.4 (38.6, 40.6) | 39.9 (39.0, 40.9) | 34.3 (32.4, 38.2)b,c,d |
| Baby weight, ga | 3660 (3120, 3935) | 2555 (1725, 2869)b,c,d | 3509 (3195, 3810) | 3554 (3231, 3867) | 2016 (1510, 2995)b,c,d |
Values are expressed as median (interquartile range). All data were missing for one woman with a previous uncomplicated pregnancy.
Baby weight data were missing for one woman with an uncomplicated pregnancy and two women with preeclampsia.
Significant difference (P < .05) from: b previous uncomplicated pregnancy; c uncomplicated pregnancy; and d gestational hypertension.
Pregnancy outcome of postpartum heparin patients and pregnancy comparison groups
The postpartum heparin patients and pregnancy comparison groups were well matched for blood pressure in labor, gestational age at delivery, and baby weight within each pregnancy outcome (uncomplicated pregnancy; preeclampsia) (Table 2). Women in the preeclampsia groups had higher blood pressures in labor and delivered smaller babies at an earlier gestational age than women in the uncomplicated pregnancy groups. Among women with gestational hypertension, gestational age at delivery and baby weight were similar to women in the uncomplicated pregnancy groups, whereas blood pressure in labor was similar to women in the preeclampsia groups.
Effect of pregnancy history and heparin administration on angiogenic factors
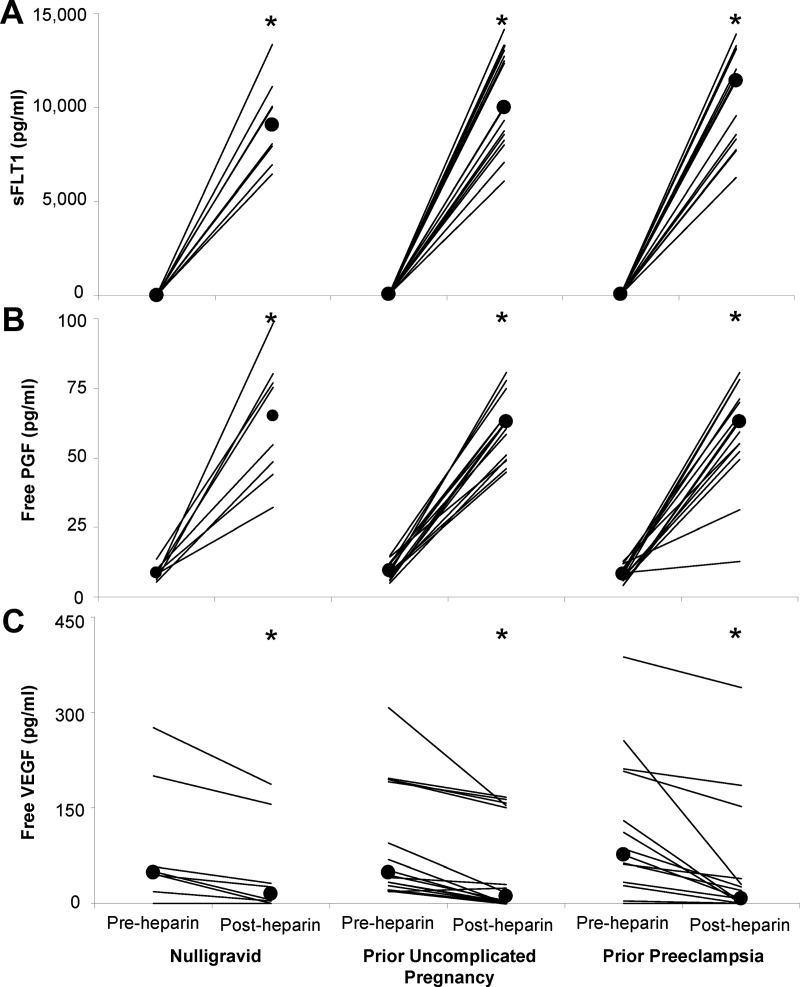
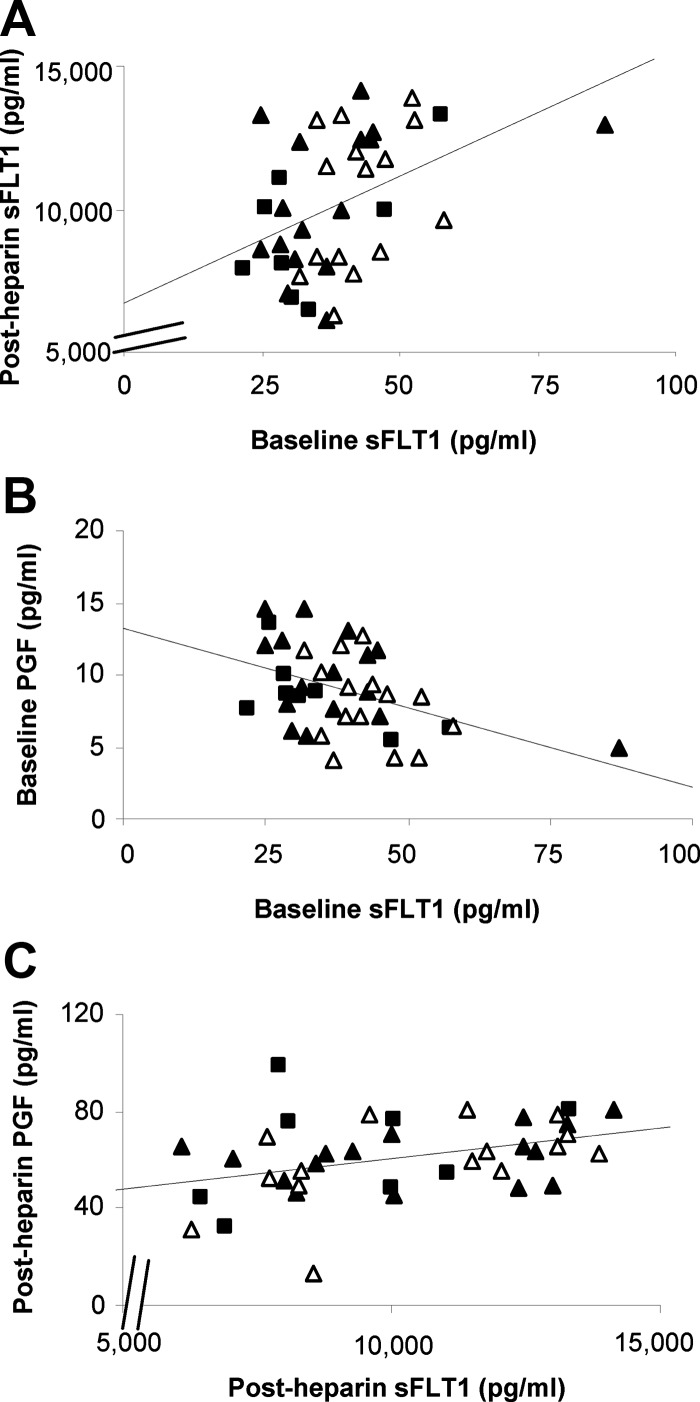
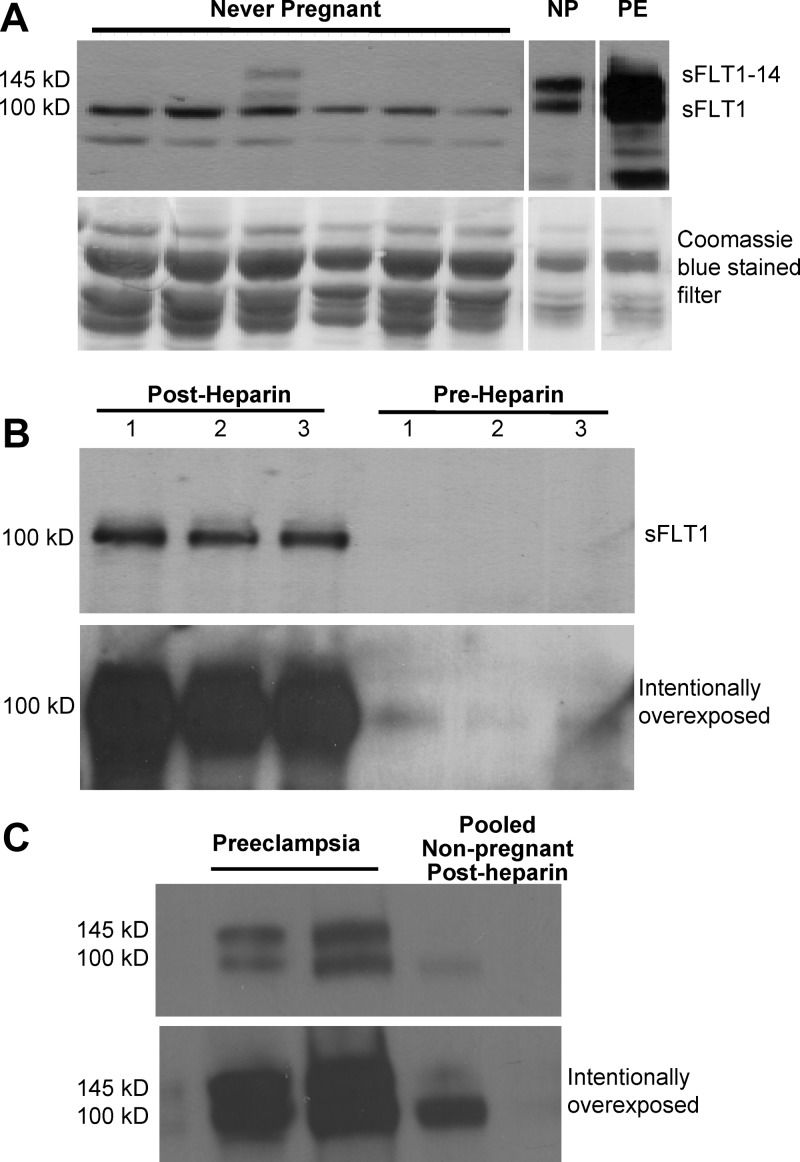
Women with previous preeclampsia had slightly, but significantly, higher baseline plasma sFLT1 than nulligravid women (Supplemental Figure 1; P = .03) and women with a previous uncomplicated pregnancy (P = .04). Similar results were obtained in our previous study, which included 10 of the 15 women with prior preeclampsia and 11 of the 16 women with a prior uncomplicated pregnancy as part of a larger cohort (9). Heparin increased sFLT1 by approximately 250-fold (P < .01; Figure 1A). Baseline and post-heparin sFLT1 were positively correlated (r2 = 0.19; P < .01; Figure 2A) even after removing one prior uncomplicated pregnancy subject with a high baseline sFLT1 (r2 = 0.18; P < .01). sFLT1 was present as a single 100-kDa isoform before and after heparin administration (Figure 3, A and B). Western blot confirmed the dramatic increase in sFLT1 after heparin administration (Figure 3B). Comparison with villous placental homogenates from women with preeclampsia confirmed that the 145 kD sFLT1–14 was not present in plasma from nonpregnant women after heparin administration (Figure 3C).
Figure 1.
Heparin administration increases plasma sFLT1 and unbound PGF and decreases unbound VEGF in nonpregnant women. Plasma sFLT1 (A), unbound PGF (B), and unbound VEGF (C) concentrations before and after heparin in nulligravid women and women with a prior uncomplicated pregnancy or prior preeclampsia. Lines show pre- and post-heparin concentrations for individual subjects. Large circles show the group median. Median sFLT1 concentrations at baseline were 30 pg/mL in nulligravid women, 34 pg/mL in women with a previous uncomplicated pregnancy, and 42 pg/mL in women with prior preeclampsia (Supplemental Figure 1). After the heparin bolus, median sFLT1 concentrations increased to 9032 pg/mL in nulligravid women, 10 003 pg/mL in women with a previous uncomplicated pregnancy, and 11 424 pg/mL in women with prior preeclampsia. *, Significant increases in sFLT1 and PGF and significant decreases in VEGF after heparin administration in all subject groups (P < .001 for all).
Figure 2.
Correlations between sFLT1 and PGF before and after heparin. Each graph shows data for nulligravid women (black squares) and women with previous preeclampsia (open triangles) or a previous uncomplicated pregnancy (black triangles). All groups were pooled for this analysis. Baseline sFLT1 was correlated with post-heparin sFLT1 (A, r2 = 0.19; P < .01). Unbound PGF and total sFLT1 were negatively correlated at baseline (B, r2 = 0.20; P < .01) but positively correlated after heparin (C, r2 = 0.15; P = .02).
Figure 3.
Heparin administration in nonpregnant women dramatically increases the 100-kDa sFLT1 isoform. A, Western blotting demonstrated that sFLT1 was the predominant isoform in plasma from never-pregnant women, whereas sFLT1 and sFLT1–14 are both present in plasma from normal pregnant (NP) and preeclamptic (PE) women. Coomassie blue staining on the lower blot demonstrates that three times more sample was loaded into lanes from never-pregnant women because sFLT1 concentrations in nonpregnant women are lower than sFLT1 concentrations in pregnant and preeclamptic women. B, The dramatic increase in sFLT1 after heparin administration was confirmed by Western blotting. A single sFLT1 band was seen at 100 kDa after heparin administration to three different nonpregnant women. The upper blot shows a normal exposure. The lower blot was intentionally overexposed to demonstrate very low levels of sFLT1 before heparin administration. C, Lanes 1 and 2 show both 100- and 145-kDa sFLT1 bands from separately run villous placental homogenates from two women with preeclampsia. In contrast, a single 100-kDa band was obtained from post-heparin plasma pooled from four nonpregnant women. The upper blot shows a normal exposure, whereas the lower blot was intentionally overexposed.
Baseline concentrations of free PGF (P = .32) and free VEGF (P = .83) did not differ between subject groups. PGF increased by approximately 7-fold after heparin (P < .01; Figure 1B), whereas free VEGF significantly decreased (P < .01; Figure 1C). Post-heparin concentrations and changes in sFLT1, PGF, and VEGF were not significantly different between subject groups. Free PGF and total sFLT1 were negatively correlated at baseline (r2 = 0.20; P < .01; Figure 2B) but positively correlated after heparin administration (r2 = 0.15; P = .02; Figure 2C). The baseline correlation remained significant after removing one woman with high sFLT1 (r2 = 0.17; P = .01). sFLT1 was not correlated with free VEGF at baseline (r2 = 0.00) or after heparin (r2 = 0.00).
sFLT1 and PGF in nonpregnant women after heparin vs pregnant women
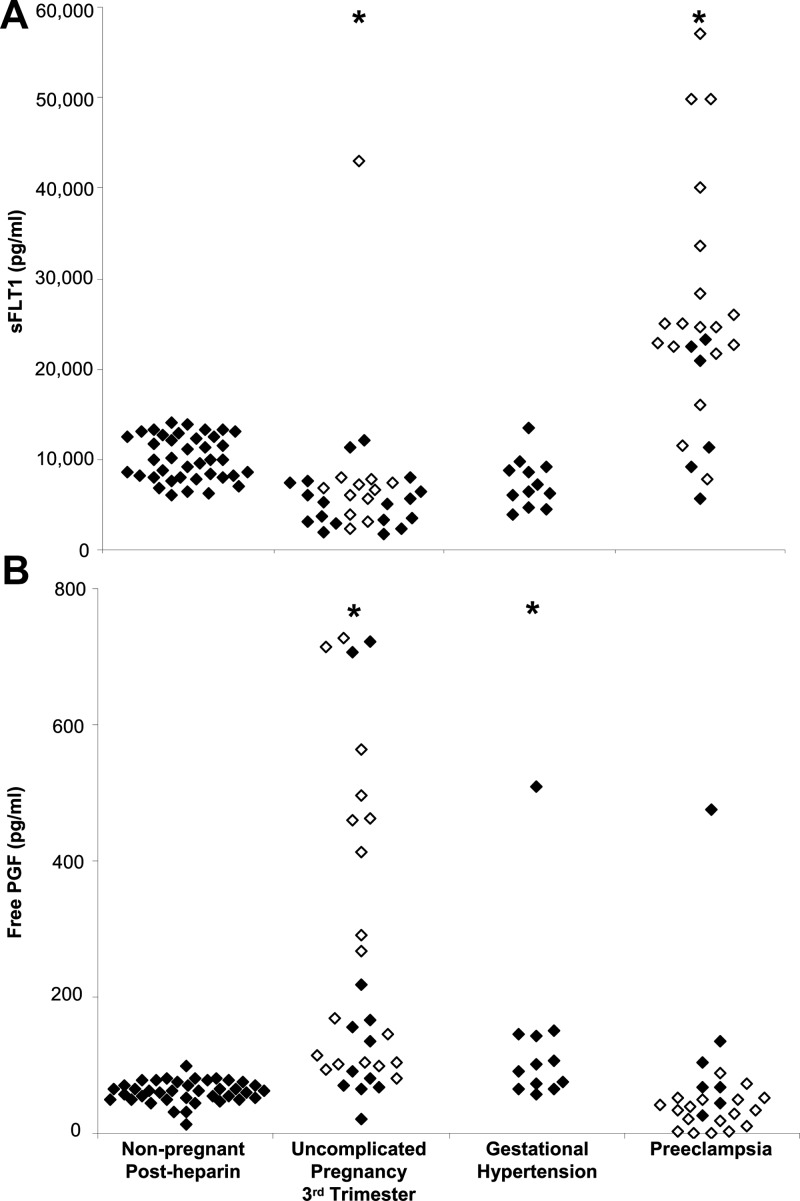
Nulligravid women, women with previous preeclampsia, and women with a previous uncomplicated pregnancy were pooled for this analysis because post-heparin sFLT1 and PGF did not differ significantly between these groups. Post-heparin plasma sFLT1 concentrations in nonpregnant women were on average 1.4-fold higher than sFLT1 concentrations in the third trimester of uncomplicated pregnancy (median, 9980 vs 5857 pg/mL; P < .001; Figure 4A), not different from concentrations in women with gestational hypertension (P = .10), and 1.3-fold lower than concentrations in women with preeclampsia (median, 9980 vs 23 032 pg/mL; P < .01). PGF concentrations in nonpregnant women after heparin were not different from PGF concentrations in women with preeclampsia (P = .39; Figure 4B), but lower than PGF concentrations in uncomplicated pregnancy (P < .001) and gestational hypertension (P = .01).
Figure 4.
sFLT1 after heparin in nonpregnant women exceeds concentrations observed in late pregnancy. Plasma sFLT1 (A) and PGF (B) concentrations in nonpregnant women after heparin administration are compared with sFLT1 concentrations in uncomplicated pregnancy, gestational hypertension, and preeclampsia. Open symbols indicate samples collected before 37 weeks gestation. Samples collected before 37 weeks gestation in the uncomplicated pregnancy group were obtained from women who later delivered at term, whereas samples obtained before 37 weeks gestation in the preeclampsia group were predelivery samples obtained from women who delivered before 37 weeks. All pregnant groups were compared to the nonpregnant group using the Kruskal-Wallis test followed by Dunn's post hoc test for comparison to a control group. *, Significant difference from nonpregnant group, P < .01.
In vitro heparin administration
Predelivery samples were obtained from four women between 36 and 40 weeks gestation (one uncomplicated pregnancy, one woman with hypertension and hyperuricemia, one woman with proteinuria but no hypertension, and one woman with a positive toxicology screen). Plasma sFLT1 was significantly lower in samples processed within 2 hours than in samples in which whole blood was incubated for 24 hours, or in which whole blood was incubated for 24 hours and low molecular weight heparin was then added to plasma (Supplemental Table 1; P < .05). Incubating whole blood or plasma with low molecular weight or unfractionated heparin did not further increase sFLT1 in predelivery samples; in fact, sFLT1 was significantly lower in all conditions in which low molecular weight or unfractionated heparin was added to plasma compared to samples incubated for 24 hours without heparin (P < .05).
Samples were obtained from seven women postpartum (13 ± 5 months postpartum; four uncomplicated pregnancies, one woman with gestational hypertension, one woman with hypertension in whom proteinuria was not assessed, and one woman with hypertension and hyperuricemia). Plasma sFLT1 was slightly but significantly higher in all conditions in which whole blood was incubated for 24 hours compared to samples processed immediately (Supplemental Table 1; P < .05 for all). Adding unfractionated heparin to whole blood or plasma had no additional effect on plasma sFLT1 in postpartum samples.
Discussion
This study reveals several important findings. First, nonpregnant women of reproductive age have an extremely large store of releasable sFLT1 and a smaller store of releasable PGF, which are not derived from the placenta. sFLT1 increased approximately 250-fold 15 minutes after heparin administration, reaching concentrations that exceed those observed in the third trimester of uncomplicated pregnancy. This perturbation resulted in a significant decline in unbound (free) circulating VEGF. However, post-heparin sFLT1 concentrations in nonpregnant women were still significantly less than those observed in preeclampsia. Second, the amount of the releasable store of sFLT1 and PGF did not differ significantly between nulligravid women and nonpregnant women with a previous uncomplicated or preeclamptic pregnancy. However, the correlation between baseline and post-heparin sFLT1 suggests that baseline sFLT1 may be proportionate to the amount of this releasable store. Third, blood components were not a major source of releasable sFLT1. sFLT1 was not elevated when heparin was added to whole blood or to plasma from pregnant or postpartum women. The in vivo effect of heparin on sFLT1 is therefore consistent with our hypothesis that sFLT1 is bound to HSPGs in the vascular glycocalyx. A previous study demonstrating that sFLT1 returns to normal levels by 6 to 10 hours after heparin administration (12) also supports this hypothesis. Fourth, we find that circulating sFLT1 in nonpregnant women at baseline and after heparin is the 100-kDa sFLT1 isoform. In contrast, sFLT1 and sFLT1–14 both contribute to elevated plasma sFLT1 in pregnancy and preeclampsia (16). This supports the hypothesis that sFLT1–14 originates from the placenta. Our combined observations suggest that the large nonplacental releasable store of sFLT1 is insufficient to achieve the high sFLT1 concentrations seen in preeclampsia, in part due to the absence of sFLT1–14.
Our results are consistent with two previous studies that demonstrated that therapeutic doses of iv heparin significantly increase plasma sFLT1 and also increase PGF in older men and women with known or suspected coronary artery disease (11, 12). Patients received unfractionated heparin before coronary angiography with (11, 12) or without (12) percutaneous coronary intervention. In contrast, a third study observed no change in PGF after unfractionated heparin administration in healthy participants or patients undergoing coronary angiography (22). sFLT1 was not measured (22), and the reason for this contrasting result is unclear. The anticoagulant bivalirudin does not affect plasma sFLT1 (11), supporting our hypothesis that the releasable store of sFLT1 is bound to HSPGs in the vascular glycocalyx.
In nonpregnant individuals, elevations in plasma sFLT1 that are well below pregnancy levels are associated with pathological antiangiogenic effects in several syndromes, including chronic kidney disease (23). Furthermore, anti-VEGF antibodies that sequester VEGF in a manner similar to sFLT1 have been associated with preeclampsia/eclampsia-like changes (hypertension, proteinuria, reversible posterior leukoencephalopathy) in nonpregnant individuals undergoing treatment for cancer-related angiogenesis (24). We postulate that VEGF deprivation caused by excess placental sFLT1 contributes to glomerular and peripheral vascular glycocalyx disruption (25), causing further inappropriate release of sFLT1 from the vascular endothelial glycocalyx. This could lead to reduced glomerular permselectivity (25), vasospasm, and the spectrum of maternal manifestations of preeclampsia.
Although therapeutic doses of iv heparin reveal a substantial releasable store of sFLT1 in nonpregnant individuals, the amount of this store has not been examined in pregnancy. Prophylactic doses of sc heparin were associated with higher sFLT1 in the third trimester, but not the first or second trimesters (26). The lower dose (prophylactic instead of therapeutic) and slower absorption (sc instead of iv administration) could potentially explain lesser effects of heparin in the pregnancy study, compared to our study and the coronary angiography studies. Therapeutic doses of iv heparin likely cause a near-complete disassociation of sFLT1 from endothelial surface HSPGs, resulting in a large spike in plasma sFLT1. In contrast, sFLT1 shedding may be more gradual with prolonged exposure to low heparin concentrations.
However, differences in dose and route of administration cannot explain why heparin increased sFLT1 in the third trimester, but not earlier in gestation (26). The reservoir of sFLT1 on the syncytiotrophoblast surface and/or syncytial knots (8) may be larger or more sensitive to heparin in the third trimester when the placenta is larger. Alternatively, unfractionated heparin may dissociate sFLT1 from HSPGs more effectively than low molecular weight heparin. Women switched from low molecular weight to unfractionated heparin at 35–36 weeks gestation, and the effect of heparin on sFLT1 was most pronounced after 37 weeks (26). Unfractionated heparin causes greater increases in circulating sFLT1 than low molecular weight heparin in mice (12). However, a second pregnancy study reported that women receiving low molecular weight heparin prophylaxis had higher sFLT1 at 35.8 weeks gestation than pregnant controls (27).
In healthy nonpregnant women, the 100-kDa sFLT1 is the predominant circulating isoform. Heparin only caused release of this isoform. This is consistent with previous reports in patients undergoing percutaneous coronary intervention (12). The production of sFLT1 vs sFLT1–14 appears to be highly cell-type specific (28). Endothelial cells from the saphenous vein express sFLT1, whereas vascular smooth muscle cells express sFLT1–14 (28). Peripheral blood mononuclear cells mainly produce sFLT1 (16).
sFLT1 released from shed syncytial microparticles clearly contributes to elevated plasma sFLT1 in preeclampsia (8). However, we propose that altered interaction of vascular endothelial HSPGs and sFLT1 might also contribute to elevated circulating sFLT1 in preeclampsia or other angiogenesis-dependent diseases. Heparin is an iatrogenic stimulus for sFLT1 release that is not present in uncomplicated or preeclamptic pregnancy. However, different physiological stimuli can also promote shedding of glycocalyx-bound proteins, including sFLT1. For example, ischemia-reperfusion injury promotes glycocalyx damage and shedding (14). Failed adaptation of the maternal spiral arteries that supply the placenta contributes to ischemia-reperfusion injury in women who later develop preeclampsia (30). Diabetes also causes glycocalyx damage and shedding (14). Gestational diabetes increases preeclampsia risk (31), and 15–18% of women with type 1 diabetes develop preeclampsia (32, 33). Even uncomplicated pregnancy is an insulin-resistant state (34). Alternatively, elevated sFLT1 levels in trauma patients correlate with markers of inflammation (IL-6), glycocalyx degradation (syndecan-1), endothelial activation (angiopoietin-2), and cell damage (soluble thrombomodulin) (13). Angiopoietin-2 (35) is elevated in severe preeclampsia, compared to uncomplicated pregnancy. IL-6 (36) and thrombomodulin are also elevated in preeclampsia (37). Finally, heparanase regulates sFLT1 release from placental villi (15). Additional experiments should determine whether heparanase or glycocalyx dysfunction contributes to the pathophysiology of preeclampsia.
The increase in total PGF after heparin was likely larger than the observed 7-fold increase in free PGF. PGF-2 and PGF-4 have heparin binding domains and should be released after heparin administration (38). However, any PGF that bound to sFLT1 would have been undetectable by ELISA. Increased binding with sFLT1 over time may explain previous observations that free PGF decreases from 30 to 60 minutes after heparin administration (11).
Limitations
This study has two limitations that should be considered. First, blood samples were drawn 15 minutes after heparin administration because samples were collected primarily to measure myeloperoxidase and lipoprotein lipase. The effect of heparin on sFLT1 is transient because sFLT1 returns to baseline levels by 6 to 10 hours after heparin administration (12). We lacked serial samples to demonstrate the time course of increases in sFLT1 and PGF and decreases in unbound VEGF after heparin administration. Kinetic studies of other biomolecules that similarly bind sulfated glycosaminoglycans on the endothelial cell surface, such as xanthine oxidase, show a spike in circulating concentrations within minutes of an iv heparin bolus (29). Values return to baseline after several hours (29). Second, the amount of this releasable store may differ in pregnancy. We measured responses in nonpregnant women to investigate constitutional differences in sFLT1 stores and to demonstrate that this releasable store of sFLT1 and PGF was not derived from the placenta. Notably, heparanase-overexpressing nonpregnant and pregnant mice exhibit significantly higher steady-state circulating sFLT1 levels (1.65-fold and >3-fold on gestational days 11.5 and 16.5, respectively) compared to control nonpregnant and pregnant mice, indicating that vascular and placental surface HSPGs are a major reservoir of releasable sFLT1 (15).
Conclusions
Nonpregnant women of reproductive age have a substantial releasable store of sFLT1 and a smaller store of PGF that are not derived from the placenta. Heparin increased plasma sFLT1 by approximately 250-fold, increased PGF by 7-fold, and decreased free VEGF. The magnitude of these changes did not differ between nulligravid women, women with previous preeclampsia, and women with a previous uncomplicated pregnancy. Post-heparin sFLT1 in nonpregnant women was higher than sFLT1 in uncomplicated pregnancy but lower than sFLT1 in preeclampsia. The positive correlation between baseline and post-heparin sFLT1 suggests that baseline sFLT1 may be proportionate to the amount of this releasable store. Future studies should investigate the potential contribution of glycocalyx shedding to elevated sFLT1 during and after preeclampsia.
Acknowledgments
The authors thank the Preeclampsia Program Project core staff for pregnancy participant recruitment and blood sample acquisition, and laboratory staff Marcia Gallaher and David Lykins for assistance in data collection and sample assays.
This project was supported by National Institutes of Health Grants P01HD030367 (to C.A.H.) and UL1RR024153 and UL1TR000005 (to the University of Pittsburgh Clinical and Translational Science Institute), and by the State of Pennsylvania Health Research Formula Fund (to C.A.H.). T.L.W. was supported by a Canadian Institute of Health Research Fellowship, the Amy Roberts Health Promotion Research Award, and the Office of Women's Health Research (Building Interdisciplinary Careers in Women's Health award K12HD065987).
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- HSPG
- heparin sulfate proteoglycan
- PGF
- placental growth factor
- sFLT1
- soluble fms-like tyrosine kinase 1
- VEGF
- vascular endothelial growth factor.
References
- 1. Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Intensification of fetal and maternal surveillance in pregnant women with hypertensive disorders. Int J Gynaecol Obstet. 1998;61:127–133 [DOI] [PubMed] [Google Scholar]
- 2. Hauth JC, Ewell MG, Levine RJ, et al. Pregnancy outcomes in healthy nulliparas who developed hypertension. Calcium for Preeclampsia Prevention Study Group. Obstet Gynecol. 2000;95:24–28 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization The World Health Report 2005–Make Every Mother and Child Count. Geneva, Switzerland: World Health Organization; 2005 [Google Scholar]
- 4. Altman D, Carroli G, Duley L, et al. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359:1877–1890 [DOI] [PubMed] [Google Scholar]
- 5. Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111:649–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noori M, Donald AE, Angelakopoulou A, Hingorani AD, Williams DJ. Prospective study of placental angiogenic factors and maternal vascular function before and after preeclampsia and gestational hypertension. Circulation. 2010;122:478–487 [DOI] [PubMed] [Google Scholar]
- 7. Mutter WP, Karumanchi SA. Molecular mechanisms of preeclampsia. Microvasc Res. 2008;75:1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rajakumar A, Cerdeira AS, Rana S, et al. Transcriptionally active syncytial aggregates in the maternal circulation may contribute to circulating soluble fms-like tyrosine kinase 1 in preeclampsia. Hypertension. 2012;59:256–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wolf M, Hubel CA, Lam C, et al. Preeclampsia and future cardiovascular disease: potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab. 2004;89:6239–6243 [DOI] [PubMed] [Google Scholar]
- 10. Sandvik MK, Leirgul E, Nygård O, et al. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am J Obstet Gynecol. 2013;209:569.e1–569.e10 [DOI] [PubMed] [Google Scholar]
- 11. Kapur NK, Shenoy C, Yunis AA, et al. Distinct effects of unfractionated heparin versus bivalirudin on circulating angiogenic peptides. PLoS One. 2012;7:e34344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Searle J, Mockel M, Gwosc S, et al. Heparin strongly induces soluble fms-like tyrosine kinase 1 release in vivo and in vitro–brief report. Arterioscler Thromb Vasc Biol. 2011;31:2972–2974 [DOI] [PubMed] [Google Scholar]
- 13. Ostrowski SR, Sørensen AM, Windeløv NA, et al. High levels of soluble VEGF receptor 1 early after trauma are associated with shock, sympathoadrenal activation, glycocalyx degradation and inflammation in severely injured patients: a prospective study. Scand J Trauma Resusc Emerg Med. 2012;20:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reitsma S, Slaaf DW, Vink H, van Zandvoort MA, oude Egbrink MG. The endothelial glycocalyx: composition, functions, and visualization. Pflugers Arch. 2007;454:345–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sela S, Natanson-Yaron S, Zcharia E, Vlodavsky I, Yagel S, Keshet E. Local retention versus systemic release of soluble VEGF receptor-1 are mediated by heparin-binding and regulated by heparanase. Circ Res. 2011;108:1063–1070 [DOI] [PubMed] [Google Scholar]
- 16. Rajakumar A, Powers RW, Hubel CA, et al. Novel soluble Flt-1 isoforms in plasma and cultured placental explants from normotensive pregnant and preeclamptic women. Placenta. 2009;30:25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gandley RE, Rohland J, Zhou Y, et al. Increased myeloperoxidase in the placenta and circulation of women with preeclampsia. Hypertension. 2008;52:387–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22 [PubMed] [Google Scholar]
- 19. Chesley LC. Hypertension in pregnancy: definitions, familial factor, and remote prognosis. Kidney Int. 1980;18:234–240 [DOI] [PubMed] [Google Scholar]
- 20. Lind T, Godfrey KA, Otun H, Philips PR. Changes in serum uric acid concentrations during normal pregnancy. Br J Obstet Gynaecol. 1984;91:128–132 [DOI] [PubMed] [Google Scholar]
- 21. Shibata E, Rajakumar A, Powers RW, et al. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. J Clin Endocrinol Metab. 2005;90:4895–4903 [DOI] [PubMed] [Google Scholar]
- 22. Steppich BA, Kaufmann J, Sepp D, et al. Increased circulating placental growth factor during percutaneous coronary intervention is associated with applied radiocontrast agent. Coron Artery Dis. 2009;20:130–137 [DOI] [PubMed] [Google Scholar]
- 23. Di Marco GS, Reuter S, Hillebrand U, et al. The soluble VEGF receptor sFlt1 contributes to endothelial dysfunction in CKD. J Am Soc Nephrol. 2009;20:2235–2245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Obeidat M, Obeidat M, Ballermann BJ. Glomerular endothelium: a porous sieve and formidable barrier. Exp Cell Res. 2012;318:964–972 [DOI] [PubMed] [Google Scholar]
- 26. Rosenberg VA, Buhimschi IA, Lockwood CJ, et al. Heparin elevates circulating soluble fms-like tyrosine kinase-1 immunoreactivity in pregnant women receiving anticoagulation therapy. Circulation. 2011;124:2543–2553 [DOI] [PubMed] [Google Scholar]
- 27. Sobel ML, Kingdom J, Drewlo S. Angiogenic response of placental villi to heparin. Obstet Gynecol. 2011;117:1375–1383 [DOI] [PubMed] [Google Scholar]
- 28. Sela S, Itin A, Natanson-Yaron S, et al. A novel human-specific soluble vascular endothelial growth factor receptor 1: cell-type-specific splicing and implications to vascular endothelial growth factor homeostasis and preeclampsia. Circ Res. 2008;102:1566–1574 [DOI] [PubMed] [Google Scholar]
- 29. Adachi T, Fukushima T, Usami Y, Hirano K. Binding of human xanthine oxidase to sulphated glycosaminoglycans on the endothelial-cell surface. Biochem J. 1993;289:523–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wendland EM, Torloni MR, Falavigna M, et al. Gestational diabetes and pregnancy outcomes - a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jensen DM, Damm P, Moelsted-Pedersen L, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care. 2004;27:2819–2823 [DOI] [PubMed] [Google Scholar]
- 33. Persson M, Norman M, Hanson U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: a large, population-based study. Diabetes Care. 2009;32:2005–2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Landon MB, Gabbe SG. Gestational diabetes mellitus. Obstet Gynecol. 2011;118:1379–1393 [DOI] [PubMed] [Google Scholar]
- 35. Han SY, Jun JK, Lee CH, Park JS, Syn HC. Angiopoietin-2: a promising indicator for the occurrence of severe preeclampsia. Hypertens Pregnancy. 2012;31:189–199 [DOI] [PubMed] [Google Scholar]
- 36. Xiao JP, Yin YX, Gao YF, et al. The increased maternal serum levels of IL-6 are associated with the severity and onset of preeclampsia. Cytokine. 2012;60:856–860 [DOI] [PubMed] [Google Scholar]
- 37. Hsu CD, Copel JA, Hong SF, Chan DW. Thrombomodulin levels in preeclampsia, gestational hypertension, and chronic hypertension. Obstet Gynecol. 1995;86:897–899 [DOI] [PubMed] [Google Scholar]
- 38. Yang W, Ahn H, Hinrichs M, Torry RJ, Torry DS. Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J Reprod Immunol. 2003;60:53–60 [DOI] [PubMed] [Google Scholar]