Abstract
Context:
The human adrenal zona fasciculata (ZF) and zona reticularis (ZR) are responsible for the production of cortisol and 19-carbon steroids (often called adrenal androgens), respectively. However, the gene profiles and exact molecular mechanisms leading to the functional phenotype of the ZF and ZR are still not clearly defined. In the present study, we identified the transcripts that are differentially expressed in the ZF and ZR.
Objective:
The objective of the study was to compare the transcriptome profiles of ZF and ZR.
Design and Methods:
ZF and ZR were microdissected from 10 human adrenals. Total RNA was extracted from 10 ZF/ZR pairs and hybridized to Illumina microarray chips. The 10 most differentially expressed transcripts were studied with quantitative RT-PCR (qPCR). Immunohistochemistry was also performed on four zone-specific genes.
Results:
Microarray results demonstrated that only 347 transcripts of the 47 231 were significantly different by 2-fold or greater in the ZF and ZR. ZF had 195 transcripts with 2-fold or greater increase compared with its paired ZR, whereas ZR was found to have 152 transcripts with 2-fold or greater higher expression than in ZF. Microarray and qPCR analysis of transcripts encoding steroidogenic enzymes (n = 10) demonstrated that only 3β-hydroxysteroid dehydrogenase, steroid sulfotransferase, type 5 17β-hydroxysteroid dehydrogenase, and cytochrome b5 were significantly different. Immunohistochemistry and qPCR studies confirmed that the ZF had an increased expression of lymphoid enhancer-binding factor 1 and nephroblastoma overexpressed, whereas ZR showed an increased expression of solute carrier family 27 (fatty acid transporter) (SLC27A2), member 2 and TSPAN12 (tetraspanin 12)
Conclusion:
Microarray revealed several novel candidate genes for elucidating the molecular mechanisms governing the ZF and ZR, thereby increasing our understanding of the functional zonation of these two adrenocortical zones.
The zonal classification of the mammalian adrenal cortex, as seen in light microscopy, was first provided by Arnold in 1866 (1). He also coined the terms zona glomerulosa (ZG), zona fasciculata (ZF), and zona reticularis (ZR) for the three concentric zones. Since then, many researchers have demonstrated the functional relevance of these zones by providing their distinct roles in steroid hormone biosynthesis: ZG synthesizes mineralocorticoids and ZF produces glucocorticoids (2, 3). The human ZR is the site of biosynthesis of the 19-carbon (C19) steroids dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS) in the prepubertal, pubertal, and adult human (4–6). The adrenal C19 steroid output that results from the expansion and differentiation of the adrenal zona reticularis in humans and some nonhuman primates is called adrenarche. The timing of adrenarche varies among primates, but in humans, serum levels of DHEAS are seen to increase at approximately 6 years of age (7, 8). Neither DHEA nor DHEAS are bioactive androgens, but they act as precursors for the production of more potent androgens, including T, in peripheral tissues that include prostate, adipose tissue, and skin (9).
Although some steroidogenic enzymes and cofactor proteins are common to all zones of the cortex, the zone-specific production of steroids results in part due to differential expression of key steroidogenic enzymes (10–13). The pathway leading to the synthesis of DHEAS is quite simple and requires only three steroidogenic enzymes, namely cytochrome P450 cholesterol side-chain cleavage (CYP11A1), CYP17 (a single enzyme catalyzing two biosynthetic activities: 17α -hydroxylase and 17,20-lyase), and steroid sulfotransferase (SULT2A1). It has been demonstrated that CYP11A1 is expressed in all zones of the adult human adrenal, whereas CYP17 is expressed in both ZF and ZR (14). Although 17α-hydroxylase activity is mandatory for production of the glucocorticoid, cortisol, in human adrenal ZF, both 17α-hydroxylase and 17,20-lyase activities are needed for C19 steroid production in the ZR (15–17). Cytochrome b5 (CYB5), an allosteric regulator of CYP17, enhances the 17,20-lyase activity of CYP17 and is found to be most evident in ZR (14). SULT2A1 is also predominantly expressed in the cytoplasm of ZR (14). The pattern of CYB5 and SULT2A1 expression is thus consistent with the ability of ZR to produce DHEA and DHEAS. The hallmark of ZR is the low expression of type 2 3β-hydroxysteroid dehydrogenase (HSD3B2) (12, 18, 19). The relative lack of HSD3B2 expression/activity facilitates increased DHEA and DHEAS synthesis because HSD3B2 competes with CYP17 and SULT2A1 for pregnenolone and 17α-hydroxypregnenolone (20, 21). It was also recently demonstrated that the adrenal ZR is also able to synthesize the potent androgen T owing to the higher expression of type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) in ZR as compared with ZF (22, 23).
Beyond steroidogenic enzymes and cofactor proteins, little is known about the differences in phenotypes of ZF and ZR. A handful of genes have been defined to have distinct expression patterns between these two zones using cDNA probe arrays for approximately 750 genes (6). However, the molecular mechanisms governing the distinct steroidogenic phenotype of the two zones have not been defined. In the present study, we have sought to identify the transcripts that are differentially expressed in the human adrenal ZF and ZR using visual microdissection, microarray, quantitative RT-PCR (qPCR), and immunohistochemistry. To better understand the biological aspect of the observed differences in gene expression, we also analyzed the same microarray data using gene ontology (GO) and pathway analyses. This approach has revealed several novel candidate genes for elucidating the molecular mechanisms governing the ZF and ZR, thereby increasing our understanding of the functional zonation of these two adrenocortical zones.
Materials and Methods
An expanded Materials and Methods section with statistical analyses is provided as a Supplemental File, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org.
Human tissue preparation
After informed consent from the family, human adult adrenal glands were obtained from cadaveric kidney donors that were transplanted at the Georgia Regents University (Augusta, Georgia). Use of these tissues was approved by the Institutional Review Board of Georgia Regents University.
Isolation of ZF and ZR tissue from human adult adrenals
Adrenal glands (n = 10) were trimmed free of fat and placed in culture medium DMEM/F14 medium (Gibco, Life Technologies), and ZF and ZR tissue was isolated by microdissection of the adrenal gland as previously described (6, 18).
RNA extraction and gene expression assays
RNA extraction and gene expression assays were performed as described previously (24).
Microarray analysis for human adrenal ZF and ZR cells
Total RNA extracted from ZF and ZR fragments was hybridized to an Illumina bead chip. The arrays were scanned at high resolution on the iScan system (Illumina). Results were analyzed by GeneSpring GX (version 12.1) software (Silicon Genetics) by customizing to the Illumina single-color analysis. To identify the differences among the two zones, a list of ZF and ZR markers was created by using transcripts satisfying the conditions including fold expression differences and statistical differences between the respective zones. Differences in the GO term were defined based on a list of genes having a 1.5-fold or greater and P < .05 difference in ZF and ZR expression using GeneSpring GX version 12.1 software. For the pathway analysis, a list of transcripts up-regulated in ZF and ZR was created (≥1.25-fold and P < .05).
Immunohistochemistry
Immunohistochemical analysis of lymphoid enhancer-binding factor 1 (LEF1), nephroblastoma overexpressed (NOV), solute carrier family 27 (fatty acid transporter), member 2 (SLC27A2) and tetraspanin 12 (TSPAN12) was performed using the streptavidin-biotin amplification method using a Histofine kit (Nichirei) or Envision+ kit (DAKO) as previously described (25).
Data analysis and statistical methods
Results are given as mean ± SEM where appropriate. Statistical analyses for microarray were done by a paired t test, followed by Benjamini and Hochberg false discovery rate correction. Significance was accepted at the level of probability of P = .0-.05. Statistical analyses for qPCR were done by paired t test followed by post hoc correction. Significance was accepted at the level of probability of P = .0-.05.
Results
Differentially expressed transcripts in ZF and ZR
Principal component analysis (PCA) showed that samples from the same group clustered together and thus indicated that the microarray data is highly reproducible (Figure 1A). Of the 47 231 probe sets on the Illumina array, the scatterplot showed that only 347 transcripts were significantly different by 2-fold or greater between the ZF and ZR (P < .05). On paired analysis, ZF was found to have 195 transcripts with a 2-fold or greater increase compared with ZR, whereas ZR had 152 transcripts with a 2-fold or greater higher expression than in ZF (P < .05) (Figure 1B). Some of these up-regulated transcripts could be involved in zone-dominant steroid hormone synthesis.
Figure 1.
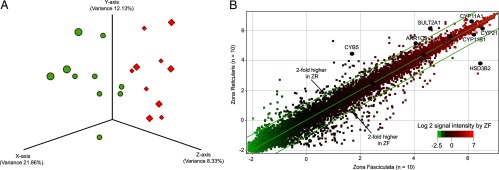
A, PCA of microarray data. PCA three-dimensional scatterplot uses the expression of the all of the 47 231 independent transcripts on the normalized microarray platform [diamonds in red and spheres in green represent individual ZF and ZR samples (n = 10 each)]. B, Microarray scatterplot showing pooled data for ZF and ZR from 10 adrenals. Each spot indicates a unique probe with a total of 47 231 independent transcripts based on normalized microarray data. Spots outside the parallel lines represent transcripts with greater than 2-fold differences in expression. The transcripts involved in steroidogenesis are indicated as black circles.
Transcripts encoding genes involved in steroidogenesis in ZF and ZR
As expected, signal intensities of transcripts that are involved in both cortisol and C19 steroid synthesis, namely steroidogenic acute regulatory protein, CYP11A1, 11β-hydroxylase, and CYP17 were highly and equally expressed in ZF and ZR (Figure 2A). P450 oxidoreductase was also expressed equally in the two zones, although the expression levels were relatively low.
Figure 2.
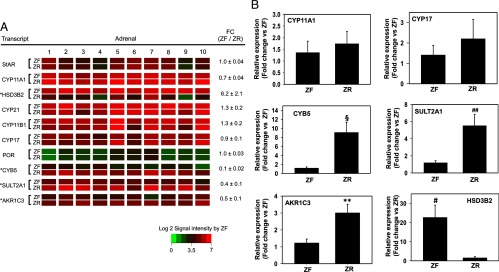
A, Heat map representation of microarray analysis for transcripts encoding genes involved steroidogenesis in ZF vs ZR (n = 10). Normalized log2 signal intensity acquired from microarray analysis is represented by color (see color bar). Fold change was calculated as the average of normalized ZF signal intensities divided by that of normalized ZR signal intensities. Data are represented as mean ± SEM. FC, fold change. *, P < .01, by paired t test. B, Validation of microarray analysis by real-time qPCR for selected transcripts encoding genes involved in steroidogenesis in ZF vs ZR. CYP11A1 and CYP17 remain unchanged, whereas HSD3B2, CYB5, AKR1C3, and SULT2A1 were found to be significantly different between ZF and ZR. #, P < .01, §, P < .005, **, P < .001, ##, P < .05, by paired t test. Ten ZF-ZR pairs were used for the qPCR analysis.
The transcripts encoding genes involved in steroidogenesis that were found to be significantly different in the two zones were HSD3B2 (higher in ZF), and CYB5, SULT2A1, and AKR1C3 (higher in ZR) as shown by microarray (Figure 2A) and confirmed by qPCR (Figure 2B). Previous studies have also shown the zonal specificity of these genes by immunohistochemistry (12, 20). We also confirmed the low level distribution of CYP11A1 and CYP17 between the two zones by qPCR (Figure 2B).
Transcripts up-regulated in ZF
Most of the transcripts for proteins with the highest differential expression in ZF vs ZR have not been previously studied with regard to adrenal physiology and function (Figure 3A). In our list, a transcription factor involved in the Wnt signaling pathway, LEF1, was the most up-regulated ZF transcript when compared with ZR. This was followed by plasma membrane calcium ATPase isoform 3 (ATP2B3), NOV, and Purkinje cell protein 4 (PCP4). HSD3B2 was also one of the transcripts with the highest differential expression in ZF as compared with ZR. The relative signal intensities between the 10 human ZF and ZR samples were consistently uniform through the 15 transcripts and the respective zones, suggesting consistent visual dissection of the adrenal tissue.
Figure 3.
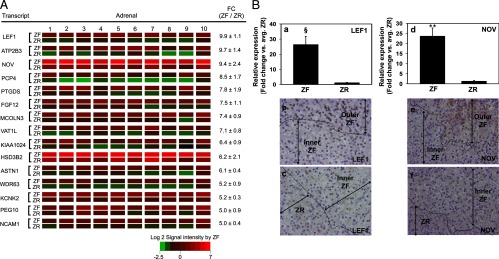
A, Heat map representation of microarray analysis for the 15 transcripts with the highest differential expression in ZF vs ZR (n = 10). Normalized log2 signal intensity acquired from microarray analysis is represented by color (see color bar). Fold change was calculated as the average of normalized ZF signal intensities divided by that of normalized ZR signal intensities. Data are represented as mean ± SEM. FC, fold change. All transcripts showed a significance of P < .05. B, Quantitative RT-PCR and immunohistochemical analysis for ZF-expressed LEF1 and NOV. Validation of microarray analysis was done by real-time qPCR (a and d) and immunohistochemistry (b, c, e, and f) for transcripts with high differential expression in ZF vs ZR, namely LEF1 and NOV. Both LEF1 and NOV showed a higher expression in the outer ZF as compared with the inner ZF and ZR. Ten ZF-ZR pairs were used for the qPCR analysis. §, P < .005, **, P < .001, by paired t test.
We also performed qPCR to confirm the difference in zonal expression for two transcripts with high differential expression in the ZF (Figure 3B). The protein expression was also examined by immunohistochemistry. HSD3B2 was used as the positive control for positive ZF staining. LEF1 and NOV were clearly defined in ZG (data not shown) as well as the outer ZF but not in the inner ZF and ZR (Figure 3B). The degree of differences in the expression of LEF1 and NOV was found to be higher with qPCR than immunohistochemistry. The most obvious explanation would be to conclude that mRNA and protein values do not always agree, but comparisons between mRNA and immunohistochemistry are likely more complex. Although immunohistochemistry detects the functional product of the biomarker gene, it may be influenced by tissue fixation as well as potential lack of specificity and sensitivity of the antibody. On the other hand, qPCR may be a good indicator of mRNA levels. ATPase isoform 3 and Purkinje cell protein 4 were found to be highly expressed in the ZG as opposed to ZF and ZR by immunohistochemistry (data not shown).
Transcripts up-regulated in ZR
Other than CYB5, none of the other 15 ZR-dominant transcripts have been associated with adrenal function (Figure 4A). Fibrinogen-γ (FGG) chain was the most up-regulated transcript in the ZR followed by transcripts encoding two transporters, solute carrier family 13 (sodium-dependent citrate transporter), member 5 (transporting sodium citrate) and SLC27A2 (transporting fatty acids). In addition, the steroidogenic enzymes SULT2A1 and AKR1C3 were also found to be more highly expressed in the ZR compared with the ZF (∼5.5- and 3-fold, respectively, as found by qPCR; data not shown). We confirmed the zonal difference in expression for SLC27A2 and TSPAN12 in the ZR by qPCR (Figure 4B). Additionally, immunohistochemistry was performed for three transcripts (CYB5, SLC27A2, and TSPAN12) based on the availability of antibodies. CYB5 was used as the positive control for positive ZR staining. SLC27A2 and TSPAN12 were expressed preferentially in ZR (Figure 4B). Thus, we obtained consistent results from microarray, qPCR, and immunohistochemistry.
Figure 4.
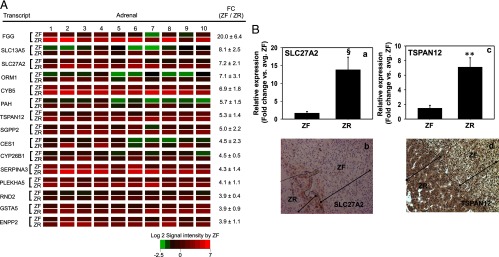
A, Heat map representation of microarray analysis for the 15 transcripts with the highest differential expression in ZR vs ZF (n = 10). Normalized log2 signal intensity acquired from the microarray analysis is represented by color (see color bar). Fold change was calculated as the average of normalized ZF signal intensities divided by that of normalized ZR signal intensities. Data are represented as mean ± SEM. FC, fold change. All transcripts showed a significance of P < .05. B, Quantitative RT-PCR and immunohistochemical analysis for ZR-expressed SLC27A2 and TSPAN12. Validation of microarray analysis was done by real-time qPCR (a and c) and immunohistochemistry (b and d) for transcripts with high differential expression in ZR vs ZF, namely SLC27A2 and TSPAN12. Ten ZF-ZR pairs were used for the qPCR analysis. §, P < .005, **, P < .001, by paired t test.
GO analysis of zonally expressed genes
GO analysis was done using 1187 probe sets that were significantly up- or down-regulated (≥1.5-fold, P < .05) in ZR (Table 1). A total of 11 GO classification terms were found to be significantly (P < .01) overrepresented among these 1187 transcripts: six terms were related to cellular components, one term to the molecular function category, and four terms to the biological process category. Interestingly, four of the 11 GO terms were associated with major histocompatibility complex (MHC) class II antigen processing. This trend suggests that the expression of MHC class II molecules likely differs in fundamental ways between the ZF and ZR.
Table 1.
GO Categories Significantly (P < .01) Overrepresented Among the 1187 Probe Sets Shown to Be Up- or Down-Regulated 1.5-Fold or Greater in ZR (P < .05)
| GO Accession | GO Term | Corrected P Value | Probes |
|
|---|---|---|---|---|
| Present | Total | |||
| Cellular Component | ||||
| GO:0042613 | MHC class II protein complex | 2.26E-07 | 11 | 16 |
| GO:0042611 | MHC protein complex | 6.74E-04 | 12 | 39 |
| GO:0005576 | Extracellular region | 3.06E-04 | 134 | 1788 |
| GO:0016020 | Membrane | 3.06E-04 | 381 | 6525 |
| GO:0044421 | Extracellular region part | 0.002 | 67 | 754 |
| GO:0044459 | Plasma membrane part | 0.005 | 106 | 1432 |
| Molecular function | ||||
| GO:0032395 | MHC class II receptor activity | 2.26E-07 | 10 | 13 |
| Biological process | ||||
| GO:0002504 | Antigen processing and presentation via MHC class II | 3.19E-06 | 11 | 20 |
| GO:0006629 | Lipid metabolic process | 3.06E-04 | 69 | 735 |
| GO:0046165 | Alcohol biosynthetic process | 0.002 | 13 | 51 |
| GO:0044699 | Single-organism process | 0.003 | 349 | 6043 |
Pathway analysis of ZF vs ZR
A list of genes defined by microarray to be up-regulated in ZF and ZR (≥1.25-fold and P < .05) was exported for pathway analysis. There were 92 pathways found to be significantly different in the two zones (P < .01) (Supplemental Table 1). To determine the pathways up-regulated in either zone, a list of genes in the selected pathways was made and their zonal expression was compared (P < .05) (Supplemental Table 2). Pathways involving cell proliferation (namely, Wnt signaling, canonical Wnt signaling, and regulation of telomerase) were up-regulated in ZF. Those involving cell death (namely, Fox O family signaling, proteasome degradation, senescence and autophagy, apoptosis modulation and signaling, oxidative stress, and glutathione metabolism) were up-regulated in ZR (Supplemental Table 2). Similarly, steroid metabolism genes (namely, cholesterol biosynthesis, sulfation biotransformation reaction, metabolism of lipids and lipoproteins, and glutathione metabolism) were up-regulated in ZR.
Discussion
Using microarray, GO and pathway analyses, we have identified several novel candidate genes that could help in elucidating the molecular mechanisms governing the differences between ZF and ZR.
Our microarray analysis identified several steroidogenic genes that are known to be differentially expressed between ZF and ZR, namely HSD3B2, CYB5, AKR1C3, and SULT2A1. Several other studies have shown results consistent with our data (6, 12, 14, 16, 17, 22). Wang et al (6) showed that an aldo-keto reductase, AKR1B1, was among the highly expressed genes in ZR. In our study, the only highly expressed aldo-keto reductase was AKR1C3 (2.1-fold), which is known to convert androstenedione to T. This observation agrees with the results obtained by Nakamura et al (22). Pathways analyzed in our studies revealed that the sulfation biotransformation reaction pathway was higher in the reticularis, with two genes that were significantly up-regulated (namely, SULT2A1 and PAPSS2). The sulfation of steroids by SULT2A1 requires a sulfate donor, 3′-phosphoadenosine 5′-phosphosulfate (PAPS) (26). In humans, PAPS synthesis requires two isoforms of the enzyme PAPS synthase, PAPSS1 and PAPSS2 (26). Our pathway analysis of steroidogenesis showed that cholesterol and lipid metabolism is elevated in ZR. Glutathione metabolism was also elevated in ZR. Previously the high expression of the glutathione-S-transferases in the adrenal was shown to be attributed to their participation in the metabolism of peroxides and the subsequent presence of lipofuscin in ZR, thereby giving it a reddish brown color (27–29). Recently it was demonstrated that these enzymes also play a role in the metabolism of Δ5 steroids (28, 30). Another interesting transcript that was up-regulated in the ZR was SLC27A2 (also called FATP2), a fatty acid transport protein, which confers increased ZR fatty acid uptake and also exhibits fatty acyl-CoA synthetase activity (31). SLC27A2 has been shown to mediate the channeling of exogenous long-chain fatty acids into phosphatidylinositol (32, 33). The phosphatidylinositol thereby produced could participate in cell membranes or signaling mechanisms within the ZR cell. However, further studies are needed to test the physiologic role of SLC27A2 in the ZR.
Adrenal development and zonation are generally thought to be driven by the centripetal migration theory. According to this theory, a common pool of progenitor cells located in the capsule gives rise to each zone by migrating inward, at first differentiating into ZG cells and then followed sequentially by the development of the ZF and ZR phenotypes; during this process, the cells senesce and die through an apoptotic mechanism (34, 35). As aging progresses, the size of ZR decreases, whereas that of the outer cortical zones increases (36, 37), thereby indicating that the adrenal tissue remodeling is attained by a balance between cell proliferation in the ZG and ZF, and apoptosis in the ZR (38, 39). Our pathway analyses also indicated that cell proliferation was up-regulated in the ZF, whereas pathways related to cell death were up-regulated in the ZR.
Wnt/β-catenin signaling pathways have been shown to play a very important role in adrenal development and homeostasis, in humans as well as mice (40–44). In humans, dysregulation of Wnt signaling has been found in a subset of sporadic adrenocortical adenomas and carcinomas (45, 46). In mice, the subcapsular region of the adrenal, which differentiates into ZG, shows higher β-catenin expression as well as more active Wnt/β-catenin signaling (43). In human adrenals, although β-catenin was detected throughout the entire cortex, activation of β-catenin, as shown by cytoplasmic and nuclear accumulation, was seen in ZG (45). It has also been proposed that Wnt4, a factor in the Wnt signaling pathway, may be needed for proper formation and function of ZG and that its deficiency decreases aldosterone production in mice (42). In humans, ZG was shown to exhibit higher expression of DKK3, a gene from the dickkopf family that acts as a morphogen in Wnt signaling. However, other Wnt signaling-related proteins like frizzled 2 and dishevelled showed no significant difference between ZG, ZF, and ZR (44).
Our pathway analysis showed that components of the canonical and noncanonical Wnt signaling mechanism, namely LEF1, Axil/conductin (AXIN)-2, cyclin D (CCND)-1, and CCND2 were up-regulated in ZF and ZG. LEF1 is a transcription factor that initiates the transcription of Wnt-responsive target genes involved in proliferation, differentiation, and survival, including c-myc and CCND1 (40, 47, 48). AXIN2 is a scaffold protein forming a part of the β-catenin destruction complex glycogen synthase kinase-3β/AXIN/adenomatous polyposis coli and is known to mediate the degradation of excess β-catenin and thereby maintain homeostasis (49). CCND1 and CCND2 are members of the highly conserved cyclin family involved in cell cycle regulation by positively coordinating cell cycle transition from the G1 phase to the S phase (50), thus promoting cell proliferation. Telomerase and the shelterin complex control adrenocortical homeostasis by the maintenance of telomeres in the proliferative adrenal subcapsular cells (51–53). Telomerase extends telomeres by addition of TTAGGG nucleotide repeats to the 3′-terminus of the chromosome, whereas shelterin protects the telomere from DNA damage (54). Interestingly, our pathway analysis also determined that some of the components of the shelterin complex, namely, TERF1 (telomeric repeat binding factor), TERF2, and TINF2 (TERF1-interacting nuclear factor), are significantly elevated in ZF. Thus, it can be speculated that along with ZG, cell proliferation may also occur in ZF to a limited extent. TSPAN12, a member of the phylogenetically ancient tetraspanin family, which is involved in norrin- but not Wnt-mediated frizzled 4/β-catenin signaling (55) is up-regulated in the ZR. TSPAN12 has been previously shown to play a role in promoting tumor growth (56), and it can be thus speculated that whatever little cell proliferation occurs in the ZR is norrin and not Wnt induced. Also, it is interesting that NOV, a growth-regulatory protein, was up-regulated in the ZG and ZF. It has been identified as a definitive zone marker of the human fetal adrenal and has been shown to inhibit growth while promoting differentiation (57). It has also been demonstrated that NOV is down-regulated in childhood adrenocortical tumors (58). It would therefore be interesting to identify a role for NOV in the ZG and ZF, as suggested by our immunohistochemistry studies.
Several studies have demonstrated that ZR is the zone of cell aging and senescence (36, 37, 59, 60). However, Wolkersdorfer et al (61) have shown that the highest apoptotic index was detected in the outermost zones of the human adrenal cortex, predominantly ZG. Sasano et al (39) studied cell proliferation by Ki67 immunostaining and apoptosis by a 3′-hydroxy nick end labeling technique and demonstrated that Ki67 immunoreactivity was predominantly observed in ZF, whereas cortical cells positive for nick end labeling were present in ZR and, in some cases, in ZG. The increased occurrence of cell aging in ZR has also been histologically shown by the increased presence of lipofuscin in ZR (62–64). The formation of lipofuscin was attributed to the high expression of the glutathione-S-transferases in ZR (28, 30), which was also confirmed by our pathway analysis.
One possible mechanism contributing to ZR apoptosis is the higher expression of MHC class II molecules in ZR cells, thereby priming them for eradication by the immune system (65). Our study demonstrated that several genes encoding components of the MHC are higher in ZR including HLA-DMA, HLA-DPB1, HLA-DRB3, HLA-DRB6, HLA-DRB4, and HLA-DRB6 (data not shown). Our GO analysis also showed that the expression of MHC class II molecules differed in fundamental ways between ZF and ZR. Earlier studies have confirmed that human leukocyte antigen (HLA) class II molecules are restricted to ZR cells (6, 66, 67). It has been shown that MHC class II expression and the maturation of the human adrenal cortex occur concomitantly (67). Thus, it has been proposed that MHC class II molecules facilitate interactions between cells in the ZR and immune cells, such as lymphocytes, that may regulate adrenal androgen production (68). Other studies have shown a related increase in the ZR-specific T lymphocytes during aging (69). The expression of the MHC class II antigens on ZR cells may also result from the effect of cytokines released by macrophages or other immune cells within the adrenal gland (70). However, Khoury et al (66) have shown the presence of expression of the MCH class II antigens on ZR cells in culture, although they were mixed with ZF cells, which remained MCH class II negative. These results suggested that the presence of MHC class II molecules on ZR cells was an inherent property of ZR rather than the effect of cytokines released by immune adrenal cells. Furthermore, a direct cell-to-cell contact has also been demonstrated between MHC class II molecules expressing ZR cells and T lymphocytes (71). The presence of the HLA antigens could play a role in differentiation or alternatively make the ZR more susceptible to apoptosis via immune attack.
Our pathway analyses shed more light on the cell death occurring in ZR. We have shown that ZR shows an increase in certain components of the cell death machinery including pathways involving Fox O family signaling (FoxO4), proteasome degradation (UCHL1 [ubiquitin carboxyl-terminal esterase L1] and PSMB8 [proteasome (prosome, macropain) subunit, beta type 8]), senescence and autophagy (ING2 [inhibitor of growth family, member 2] and lysosomal-associated membrane protein-2), and apoptosis signaling (TNF-related apoptosis-inducing ligand). FoxO4, a member of the forkhead transcription factor, has been found to promote apoptosis by negatively regulating the cell cycle (72) and inducing proteasome activity (73). UCHL1 is a ubiquitin-protein hydrolase involved in both the processing of ubiquitin precursors and ubiquitinated proteins and has been shown to prevent the degradation of free monoubiquitin in lysosomes (74), whereas PSMB8 is a component of the proteasome (75). Likewise, ING2 has been shown to be involved in cellular senescence (76). The role of lysosome-associated membrane protein-2 in the maintenance of the lysosome and that of TNF-related apoptosis-inducing ligand in inducing apoptosis has also been well characterized (77, 78).
In summary, our study helped to identify transcripts that are differentially expressed in the human adrenal ZF and ZR and provided a broad understanding of pathway differences in ZF and ZR. Detailed analysis of the newly defined gene differences should help to clarify the mechanisms leading to functional differences between the ZF and ZR.
Acknowledgments
We thank Dr Mary Bassett for her editorial assistance.
This work was supported by Grant DK069950 from the National Institutes of Health (to W.E.R.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- AKR1C3
- type 5 17β-hydroxysteroid dehydrogenase
- AXIN
- Axil/conductin
- C19
- 19-carbon
- CCND
- cyclin D
- CYB5
- cytochrome b5
- CYP11A1
- cytochrome P450 cholesterol side-chain cleavage
- DHEA
- dehydroepiandrosterone
- DHEAS
- DHEA sulfate
- GO
- gene ontology
- HLA
- human leukocyte antigen
- HSD3B2
- type 2 3β-hydroxysteroid dehydrogenase
- LEF1
- lymphoid enhancer-binding factor 1
- MHC
- major histocompatibility complex
- NOV
- nephroblastoma overexpressed
- PAPS
- 5′-phosphoadenosine 5′-phosphosulfate
- PCA
- principal component analysis
- qPCR
- quantitative RT-PCR
- SLC27A2
- solute carrier family 27 (fatty acid transporter)
- SULT2A1
- steroid sulfotransferase
- TERF
- telomeric repeat binding factor
- TSPAN12
- tetraspanin 12
- ZF
- zona fasciculata
- ZG
- zona glomerulosa
- ZR
- zona reticularis.
References
- 1. Arnold J. Ein beitrag zu der feiner struktur und dem chemismus der nebennieren. Virchows Arch. 1866;35:64–107 [Google Scholar]
- 2. Vinson GP. Adrenocortical zonation and ACTH. Microsc Res Tech. 2003;61:227–239 [DOI] [PubMed] [Google Scholar]
- 3. Vinson GP, Ho MM. Origins of zonation: the adrenocortical model of tissue development and differentiation. Clin Exp Pharmacol Physiol Suppl. 1998;25:S91–S96 [DOI] [PubMed] [Google Scholar]
- 4. Hornsby PJ. Biosynthesis of DHEA(S) by the human adrenal cortex and its age-related decline. Ann NY Acad Sci. 1995;774:29–46 [DOI] [PubMed] [Google Scholar]
- 5. Parker LN, Odell WD. Control of adrenal androgen secretion. Endocr Rev. 1980;1:392–410 [DOI] [PubMed] [Google Scholar]
- 6. Wang W, Yang L, Suwa T, Casson PR, Hornsby PJ. Differentially expressed genes in zona reticularis cells of the human adrenal cortex. Mol Cell Endocrinol. 2001;173:127–134 [DOI] [PubMed] [Google Scholar]
- 7. Cutler GB, Jr, Loriaux DL. Andrenarche and its relationship to the onset of puberty. Fed Proc. 1980;39:2384–2390 [PubMed] [Google Scholar]
- 8. Miller WL. Androgen synthesis in adrenarche. Rev Endocr Metab Disord. 2009;10:3–17 [DOI] [PubMed] [Google Scholar]
- 9. Labrie F. Intracrinology. Mol Cell Endocrinol. 1991;78:C113–C118 [DOI] [PubMed] [Google Scholar]
- 10. Aiba M, Fujibayashi M. Alteration of subcapsular adrenocortical zonation in humans with aging: the progenitor zone predominates over the previously well-developed zona glomerulosa after 40 years of age. J Histochem Cytochem. 2011;59:557–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Domalik LJ, Chaplin DD, Kirkman MS, et al. Different isozymes of mouse 11β-hydroxylase produce mineralocorticoids and glucocorticoids. Mol Endocrinol. 1991;5:1853–1861 [DOI] [PubMed] [Google Scholar]
- 12. Gell JS, Carr BR, Sasano H, et al. Adrenarche results from development of a 3β-hydroxysteroid dehydrogenase-deficient adrenal reticularis. J Clin Endocrinol Metab. 1998;83:3695–3701 [DOI] [PubMed] [Google Scholar]
- 13. Nishimoto K, Nakagawa K, Li D, et al. Adrenocortical zonation in humans under normal and pathological conditions. J Clin Endocrinol Metab. 2010;95:2296–2305 [DOI] [PubMed] [Google Scholar]
- 14. Suzuki T, Sasano H, Takeyama J, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf). 2000;53:739–747 [DOI] [PubMed] [Google Scholar]
- 15. Brock BJ, Waterman MR. Biochemical differences between rat and human cytochrome P450c17 support the different steroidogenic needs of these two species. Biochemistry. 1999;38:1598–1606 [DOI] [PubMed] [Google Scholar]
- 16. Miller WL. Early steps in androgen biosynthesis: from cholesterol to DHEA. Baillieres Clin Endocrinol Metab. 1998;12:67–81 [DOI] [PubMed] [Google Scholar]
- 17. Nakamura Y, Gang HX, Suzuki T, Sasano H, Rainey WE. Adrenal changes associated with adrenarche. Rev Endocr Metab Disord. 2009;10:19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Endoh A, Kristiansen SB, Casson PR, Buster JE, Hornsby PJ. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J Clin Endocrinol Metab. 1996;81:3558–3565 [DOI] [PubMed] [Google Scholar]
- 19. Sasano H, Mori T, Sasano N, Nagura H, Mason JI. Immunolocalization of 3β-hydroxysteroid dehydrogenase in human ovary. J Reprod Fertil. 1990;89:743–751 [DOI] [PubMed] [Google Scholar]
- 20. Conley AJ, Bird IM. The role of cytochrome P450 17α-hydroxylase and 3β-hydroxysteroid dehydrogenase in the integration of gonadal and adrenal steroidogenesis via the δ5 and δ4 pathways of steroidogenesis in mammals. Biol Reprod. 1997;56:789–799 [DOI] [PubMed] [Google Scholar]
- 21. Rainey WE, Carr BR, Sasano H, Suzuki T, Mason JI. Dissecting human adrenal androgen production. Trends Endocrinol Metab. 2002;13:234–239 [DOI] [PubMed] [Google Scholar]
- 22. Nakamura Y, Hornsby PJ, Casson P, et al. Type 5 17β-hydroxysteroid dehydrogenase (AKR1C3) contributes to testosterone production in the adrenal reticularis. J Clin Endocrinol Metab. 2009;94:2192–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rege J, Nakamura Y, Satoh F, et al. Liquid chromatography-tandem mass spectrometry analysis of human adrenal vein 19-carbon steroids before and after ACTH stimulation. J Clin Endocrinol Metab. 2013;98:1182–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang T, Satoh F, Morimoto R, et al. Gene expression profiles in aldosterone-producing adenomas and adjacent adrenal glands. Eur J Endocrinol. 2011;164:613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nakamura Y, Felizola SJ, Kurotaki Y, et al. Cyclin D1 (CCND1) expression is involved in estrogen receptor β (ERβ) in human prostate cancer. Prostate. 2013;73:590–595 [DOI] [PubMed] [Google Scholar]
- 26. Strott CA. Sulfonation and molecular action. Endocr Rev. 2002;23:703–732 [DOI] [PubMed] [Google Scholar]
- 27. Belloni AS, Mazzocchi G, Mantero F, Nussdorfer GG. The human adrenal cortex: ultrastructure and base-line morphometric data. J Submicrosc Cytol. 1987;19:657–668 [PubMed] [Google Scholar]
- 28. Raffalli-Mathieu F, Mannervik B. Human glutathione transferase A3–3 active as steroid double-bond isomerase. Methods Enzymol. 2005;401:265–278 [DOI] [PubMed] [Google Scholar]
- 29. Neville AM, O'Hare MJ. The Human Adrenal Cortex. Berlin: Springer Verlag; 1981 [Google Scholar]
- 30. Raffalli-Mathieu F, Orre C, Stridsberg M, Hansson Edalat M, Mannervik B. Targeting human glutathione transferase A3–3 attenuates progesterone production in human steroidogenic cells. Biochem J. 2008;414:103–109 [DOI] [PubMed] [Google Scholar]
- 31. Gimeno RE. Fatty acid transport proteins. Curr Opin Lipidol. 2007;18:271–276 [DOI] [PubMed] [Google Scholar]
- 32. Melton EM, Cerny RL, Watkins PA, DiRusso CC, Black PN. Human fatty acid transport protein 2a/very long chain acyl-CoA synthetase 1 (FATP2a/Acsvl1) has a preference in mediating the channeling of exogenous n-3 fatty acids into phosphatidylinositol. J Biol Chem. 2011;286:30670–30679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Melton EM, Cerny RL, Dirusso CC, Black PN. Overexpression of human fatty acid transport protein 2/very long chain acyl-CoA synthetase 1 (FATP2/Acsvl1) reveals distinct patterns of trafficking of exogenous fatty acids. Biochem Biophys Res Commun. 2013;440:743–748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pignatelli D, Ferreira J, Vendeira P, Magalhaes MC, Vinson GP. Proliferation of capsular stem cells induced by ACTH in the rat adrenal cortex. Endocr Res. 2002;28:683–691 [DOI] [PubMed] [Google Scholar]
- 35. Zajicek G, Ariel I, Arber N. The streaming adrenal cortex: direct evidence of centripetal migration of adrenocytes by estimation of cell turnover rate. J Endocrinol. 1986;111:477–482 [DOI] [PubMed] [Google Scholar]
- 36. Parker CR, Jr, Mixon RL, Brissie RM, Grizzle WE. Aging alters zonation in the adrenal cortex of men. J Clin Endocrinol Metab. 1997;82:3898–3901 [DOI] [PubMed] [Google Scholar]
- 37. Staton BA, Mixon RL, Dharia S, Brissie RM, Parker CR., Jr Is reduced cell size the mechanism for shrinkage of the adrenal zona reticularis in aging? Endocr Res. 2004;30:529–534 [DOI] [PubMed] [Google Scholar]
- 38. Hui XG, Akahira J, Suzuki T, et al. Development of the human adrenal zona reticularis: morphometric and immunohistochemical studies from birth to adolescence. J Endocrinol. 2009;203:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sasano H, Imatani A, Shizawa S, Suzuki T, Nagura H. Cell proliferation and apoptosis in normal and pathologic human adrenal. Mod Pathol. 1995;8:11–17 [PubMed] [Google Scholar]
- 40. Berthon A, Martinez A, Bertherat J, Val P. Wnt/β-catenin signalling in adrenal physiology and tumour development. Mol Cell Endocrinol. 2012;351:87–95 [DOI] [PubMed] [Google Scholar]
- 41. El Wakil A, Lalli E. The Wnt/β-catenin pathway in adrenocortical development and cancer. Mol Cell Endocrinol. 2011;332:32–37 [DOI] [PubMed] [Google Scholar]
- 42. Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365 [DOI] [PubMed] [Google Scholar]
- 43. Kim AC, Reuter AL, Zubair M, et al. Targeted disruption of β-catenin in Sf1-expressing cells impairs development and maintenance of the adrenal cortex. Development. 2008;135:2593–2602 [DOI] [PubMed] [Google Scholar]
- 44. Suwa T, Chen M, Hawks CL, Hornsby PJ. Zonal expression of dickkopf-3 and components of the Wnt signalling pathways in the human adrenal cortex. J Endocrinol. 2003;178:149–158 [DOI] [PubMed] [Google Scholar]
- 45. Boulkroun S, Samson-Couterie B, Golib-Dzib JF, et al. Aldosterone-producing adenoma formation in the adrenal cortex involves expression of stem/progenitor cell markers. Endocrinology. 2011;152:4753–4763 [DOI] [PubMed] [Google Scholar]
- 46. Giordano TJ, Kuick R, Else T, et al. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin Cancer Res. 2009;15:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. He TC, Sparks AB, Rago C, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512 [DOI] [PubMed] [Google Scholar]
- 48. Tetsu O, McCormick F. β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426 [DOI] [PubMed] [Google Scholar]
- 49. Yan Y, Tang D, Chen M, et al. Axin2 controls bone remodeling through the β-catenin-BMP signaling pathway in adult mice. J Cell Sci. 2009;122:3566–3578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 5th ed New York: Garland Science, Taylor, Francis Group; 2008 [Google Scholar]
- 51. Else T. Telomeres and telomerase in adrenocortical tissue maintenance, carcinogenesis, and aging. J Mol Endocrinol. 2009;43:131–141 [DOI] [PubMed] [Google Scholar]
- 52. Vlangos CN, O'Connor BC, Morley MJ, Krause AS, Osawa GA, Keegan CE. Caudal regression in adrenocortical dysplasia (acd) mice is caused by telomere dysfunction with subsequent p53-dependent apoptosis. Dev Biol. 2009;334:418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wood MA, Hammer GD. Adrenocortical stem and progenitor cells: unifying model of two proposed origins. Mol Cell Endocrinol. 2011;336:206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110 [DOI] [PubMed] [Google Scholar]
- 55. Junge HJ, Yang S, Burton JB, et al. TSPAN12 regulates retinal vascular development by promoting Norrin- but not Wnt-induced FZD4/β-catenin signaling. Cell. 2009;139:299–311 [DOI] [PubMed] [Google Scholar]
- 56. Knoblich K, Wang HX, Sharma C, Fletcher AL, Turley SJ, Hemler ME. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits β-catenin degradation [published online August 18, 2013]. Cell Mol Life Sci. doi:10.1007/s00018-013-1444-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ratcliffe J, Nakanishi M, Jaffe RB. Identification of definitive and fetal zone markers in the human fetal adrenal gland reveals putative developmental genes. J Clin Endocrinol Metab. 2003;88:3272–3277 [DOI] [PubMed] [Google Scholar]
- 58. Doghman M, Arhatte M, Thibout H, et al. Nephroblastoma overexpressed/cysteine-rich protein 61/connective tissue growth factor/nephroblastoma overexpressed gene-3 (NOV/CCN3), a selective adrenocortical cell proapoptotic factor, is down-regulated in childhood adrenocortical tumors. J Clin Endocrinol Metab. 2007;92:3253–3260 [DOI] [PubMed] [Google Scholar]
- 59. Hoerr N. The cells of the suprarenal cortex in the guinea pig. Am J Anat. 1931;48:139–197 [Google Scholar]
- 60. Nussdorfer GG. Cytophysiology of the adrenal cortex. Int Rev Cytol. 1986;98:1–405 [PubMed] [Google Scholar]
- 61. Wolkersdorfer GW, Ehrhart-Bornstein M, Brauer S, Marx C, Scherbaum WA, Bornstein SR. Differential regulation of apoptosis in the normal human adrenal gland. J Clin Endocrinol Metab. 1996;81:4129–4136 [DOI] [PubMed] [Google Scholar]
- 62. Kacsoh B. Endocrine Physiology. New York: McGraw-Hill; 2000 [Google Scholar]
- 63. Kawaoi A. Ultrastructural zonation of the human adrenal cortex. Acta Pathol Jpn. 1969;19:115–149 [DOI] [PubMed] [Google Scholar]
- 64. Sucheston ME, Cannon MS. Development of zonular patterns in the human adrenal gland. J Morphol. 1968;126:477–491 [DOI] [PubMed] [Google Scholar]
- 65. Hornsby PJ. Aging of the human adrenal cortex. Sci Aging Knowledge Environ. 2004;2004:re6. [DOI] [PubMed] [Google Scholar]
- 66. Khoury EL, Greenspan JS, Greenspan FS. Adrenocortical cells of the zona reticularis normally express HLA-DR antigenic determinants. Am J Pathol. 1987;127:580–591 [PMC free article] [PubMed] [Google Scholar]
- 67. Marx C, Bornstein SR, Wolkersdorfer GW, Peter M, Sippell WG, Scherbaum WA. Relevance of major histocompatibility complex class II expression as a hallmark for the cellular differentiation in the human adrenal cortex. J Clin Endocrinol Metab. 1997;82:3136–3140 [DOI] [PubMed] [Google Scholar]
- 68. Dharia S, Parker CR., Jr Adrenal androgens and aging. Semin Reprod Med. 2004;22:361–368 [DOI] [PubMed] [Google Scholar]
- 69. Lebrethon MC, Jaillard C, Naville D, Begeot M, Saez JM. Effects of transforming growth factor-β1 on human adrenocortical fasciculata-reticularis cell differentiated functions. J Clin Endocrinol Metab. 1994;79:1033–1039 [DOI] [PubMed] [Google Scholar]
- 70. Ehrhart-Bornstein M, Bornstein SR, Scherbaum WA. Sympathoadrenal system and immune system in the regulation of adrenocortical function. Eur J Endocrinol. 1996;135:19–26 [DOI] [PubMed] [Google Scholar]
- 71. Hayashi Y, Hiyoshi T, Takemura T, Kurashima C, Hirokawa K. Focal lymphocytic infiltration in the adrenal cortex of the elderly: immunohistological analysis of infiltrating lymphocytes. Clin Exp Immunol. 1989;77:101–105 [PMC free article] [PubMed] [Google Scholar]
- 72. Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787 [DOI] [PubMed] [Google Scholar]
- 73. Vilchez D, Boyer L, Morantte I, et al. Increased proteasome activity in human embryonic stem cells is regulated by PSMD11. Nature. 2012;489:304–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Setsuie R, Wada K. The functions of UCH-L1 and its relation to neurodegenerative diseases. Neurochem Int. 2007;51:105–111 [DOI] [PubMed] [Google Scholar]
- 75. Lichter DI, Danaee H, Pickard MD, et al. Sequence analysis of β-subunit genes of the 20S proteasome in patients with relapsed multiple myeloma treated with bortezomib or dexamethasone. Blood. 2012;120:4513–4516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ludwig S, Klitzsch A, Baniahmad A. The ING tumor suppressors in cellular senescence and chromatin. Cell Biosci. 2011;1:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Eskelinen EL, Illert AL, Tanaka Y, et al. Role of LAMP-2 in lysosome biogenesis and autophagy. Mol Biol Cell. 2002;13:3355–3368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682 [DOI] [PubMed] [Google Scholar]


