Abstract
Context:
A subset of patients diagnosed with idiopathic hypogonadotropic hypogonadism (IHH) later achieves activation of their hypothalamic-pituitary-gonadal axis with normalization of steroidogenesis and/or gametogenesis, a phenomenon termed reversal.
Objective:
The objective of this study was to determine the natural history of reversal and to identify associated phenotypes and genotypes.
Design, Setting, and Subjects:
This was a retrospective review of clinical, biochemical, and genetic features of patients with IHH evaluated at an academic medical center.
Main Outcome Measures:
History of spontaneous fertility, regular menses, testicular growth, or normalization of serum sex steroids, LH secretory profiles, brain imaging findings, and sequences of 14 genes associated with IHH were reviewed.
Results:
Of 308 patients with IHH, 44 underwent reversal. Time-to-event analysis estimated a lifetime incidence of reversal of 22%. There were no differences in the rates of cryptorchidism, micropenis, or partial pubertal development in patients with reversal vs IHH patients without reversal. Fifteen patients with reversal (30%) had Kallmann syndrome (IHH and anosmia); one had undetectable olfactory bulbs on a brain magnetic resonance imaging scan. Subjects with reversal were enriched for mutations affecting neurokinin B signaling compared with a cohort of IHH patients without reversal (10% vs 3%, P = .044), had comparable frequencies of mutations in FGFR1, PROKR2, and GNRHR, and had no mutations in KAL1. Five men did not sustain their reversal and again developed hypogonadotropism.
Conclusions:
Reversal of IHH may be more widespread than previously appreciated and occurs across a broad range of genotypes and phenotypes. Enrichment for mutations that disrupt neurokinin B signaling in patients who reversed indicates that, despite the importance of this signaling pathway for normal pubertal timing, its function is dispensable later in life. The occurrence of reversal in a patient with no olfactory bulbs demonstrates that these structures are not essential for normal reproductive function. Patients with IHH require lifelong monitoring for reversal and, if reversal occurs, subsequent relapse also may occur.
Puberty begins with a dramatic increase in GnRH secretion from the hypothalamus, stimulating a cascade of hormonal events that culminates in the secretion of sex steroids and the appearance of secondary sexual characteristics (1). Inability to synthesize, secrete, or respond to GnRH results in a condition called idiopathic hypogonadotropic hypogonadism (IHH), which is characterized by low sex steroids and low/normal gonadotropins in adulthood (2). IHH that occurs in combination with anosmia is known as Kallmann syndrome (KS), an association that stems from the fact that, during embryonic development, GnRH neurons migrate along the olfactory axons. In the absence of olfactory bulbs, this migration is disrupted, leading to hypogonadotropic hypogonadism (3, 4).
IHH has traditionally been regarded as a permanent condition, and patients are typically counseled that they will require lifelong treatment (2). However, in the last two decades there have been multiple reports of individuals with IHH who spontaneously recovered reproductive endocrine function (5–19). In a prospective series, reversal was found to occur in 10% of patients with IHH (13). Although the criteria for reversal have varied between studies, patients have uniformly demonstrated dramatic improvement in their reproductive endocrine function, including normalization of circulating sex steroids, spontaneous increases in testicular volume, and paternity/maternity without the use of medications.
In this report, records of 308 patients with IHH were reviewed to assemble the largest cohort of individuals with reversal of IHH reported to date. Clinical, laboratory, neuroendocrine, and neuroradiologic characteristics of these patients were reviewed to determine whether any phenotypic features predicted reversal and to examine the natural history of reversal, and genetic findings of these patients were analyzed to identify genes positively or negatively associated with reversal.
Materials and Methods
Patient identification and clinical/neuroendocrine evaluation
All protocols were approved by the Massachusetts General Hospital Human Research Committee, which determined that informed consent was not required for review of medical records alone. Written informed consent was obtained from subjects who underwent genetic and/or neuroendocrine studies. IHH was defined as hypogonadal sex-steroid levels (testosterone <100 ng/dL in men; estradiol <20 pg/mL in women) in the setting of low or normal gonadotropin levels at age ≥18 years and the absence of any identifiable medical condition that could cause hypogonadotropic hypogonadism (2). Anosmia was demonstrated by either formal smell testing using the University of Pennsylvania Smell Identification Test (20) or a self-reported inability to smell (which has been demonstrated to correlate well with formal smell testing) (21).
A subset of patients underwent detailed neuroendocrine phenotyping with blood sampling every 10 minutes for 8 to 24 hours to evaluate endogenous GnRH-induced LH secretion. Before all neuroendocrine evaluations, men discontinued pulsatile GnRH administration for ≥2 weeks, transdermal testosterone therapy for ≥4 weeks, and im testosterone therapy or human chorionic gonadotropin injections for ≥8 weeks (13); women discontinued hormonal therapy for ≥4 weeks (22). Measurements of LH for each sample and testosterone/estradiol on 6-hour pools were performed by the Massachusetts General Hospital Clinical Laboratory Research Core as previously described (23). LH pulses were identified using modified Santen and Bardin methodology (24, 25).
Criteria for reversal
Reversal of IHH in men was defined as meeting one or more of the following criteria: 1) fertility without use of exogenous GnRH or gonadotropin therapy; 2) serum testosterone ≥250 ng/dL (after an appropriate washout of pre-existing medical therapy as described above); 3) testicular volumes ≥4 mL and at least 2 mL increase in testicular volume in the absence of GnRH or gonadotropin therapy (for patients who had cryptorchidism, which was unilateral in all cases, only the volume of the descended testis was used); and 4) LH pulse frequency and amplitude within the normal range for men (frequency, 5.1 ± 3.4 pulses/12 h; amplitude, 2.5 ± 2.1 IU/L [mean ± 2 SD]) (26).
In women, reversal of IHH was defined as one or more of the following: 1) fertility without use of GnRH or gonadotropin therapy; 2) spontaneous menstrual cycling for at least 3 months in the absence of any treatment; and 3) LH pulse frequency and amplitude within the normal range for women (frequency, 7.0 ± 1.8 pulses/12 h; amplitude, 2.3 ± 1.0 IU/L [mean ± 2 SD]) (22).
Relapse after reversal was defined as again having hypogonadal sex-steroid levels (serum testosterone level <100 ng/dL in men, serum estradiol <20 pg/mL in women).
Genetic evaluation
Exonic and proximal intronic (≥15 bp from exon borders) DNA were amplified from genomic DNA by PCR and sequenced bidirectionally for 14 IHH genes: CHD7, FGF8, FGFR1, GNRH1, GNRHR, HS6ST1, KAL1, KISS1, KISS1R, NELF, PROK2, PROKR2, TAC3, and TACR3. Rare sequence variants (RSVs) were defined as having a minor allele frequency of <1% in the 1000 Genomes Project and the National Heart, Lung, and Blood Institute GO Exome Sequencing Project (27). RSVs were considered deleterious if they resulted in a premature termination codon, if they were demonstrated to be loss-of-function by previous in vitro studies (15, 16, 28–30), or if they were predicted to be damaging by five in silico prediction programs: Condel (31), PolyPhen (32), SIFT (33), pMUT (34), Mutation Taster (35).
Statistical analysis
Data are presented as mean ± SD unless noted otherwise. Fisher's exact test was used to compare the prevalence of cryptorchidism and micropenis in reversal patients vs a previously reported cohort of IHH patients unselected for reversal status (36) and the frequency of gene mutations between reversal probands and 1156 IHH probands that had no known evidence for reversal. P values of <.05 were considered statistically significant.
Results
Of 308 IHH patients for whom adequately detailed medical information was available to evaluate for reversal, 39 men and 5 women had evidence for reversal, with spontaneous improvement in their reproductive endocrine function. Their clinical and biochemical features are summarized in Tables 1 and 2. Of these patients, 12 had previously been reported in Ref. 13 and four had previously been reported in Ref. 37. The criteria used for reversal were designed to capture any physiologic evidence of recovery of reproductive endocrine function. A stricter set of criteria based on outcomes of direct clinical impact (recovery of serum testosterone, menstrual cyclicity, and/or fertility) was met by 29 men and 4 women.
Table 1.
Clinical Characteristics of Patients who Achieved Reversal of IHH
| Men |
Women |
|||
|---|---|---|---|---|
| % or Mean (range) | N | % or Mean (range) | n | |
| Kallmann syndrome | 31% | 12/39 | 20% | 1/5 |
| Normosmic IHH | 69% | 27/39 | 80% | 4/5 |
| Age at last evidence for hypogonadotropism, y | 23.8 (18–41.5) | 39 | 24.0 (18–35) | 5 |
| Age at first evidence for reversal, y | 28.3 (19.1–53.6) | 39 | 26.5 (19.5–35.5) | 5 |
| Duration of therapy before reversal, y | 4.4 (0.5–21) | 32 | 6.7 (0.4–11) | 4 |
| Micropenis | 27% | 9/33 | — | |
| Cryptorchidism | 15% | 6/39 | — | |
—, not applicable.
Table 2.
Features of Reversal of IHH
| Men |
Women |
|||
|---|---|---|---|---|
| % or Mean (range) | n | % or Mean (range) | n | |
| Spontaneous fertility, number of subjects | 7 | a | 2 | a |
| Normal LH pulse frequency and amplitude | 93% | 25/27 | 25% | 1/4 |
| Mean frequency, pulses/12 h | 6.7 (3–13) | 25 | 10 | |
| Mean amplitude, IU/L | 1.8 (0.5–4.6) | 25 | 1.5 | |
| Normalization of testosterone | 66% | 25/38 | — | |
| Testosterone at baseline, ng/dL | 48 (13–132) | 23 | — | |
| Testosterone at first detection of reversal, ng/dL | 379 (254–764) | 25 | — | |
| Highest testosterone achieved, ng/dL | 451 (257–918) | 25 | — | |
| Testicular growth | 97% | 28/29 | — | |
| Mean TV at baseline, mL | 7.4 (2–17.5) | 28 | — | |
| Mean TV at first detection of reversal, mL | 14.0 (6–25) | 28 | — | |
| Highest mean TV achieved, mL | 15.3 (8–25) | 28 | — | |
| Menstrual cyclicity | — | 60% | 3/5 | |
Abbreviation: TV, testicular volume. —, not applicable.
Unknown how many subjects sought fertility.
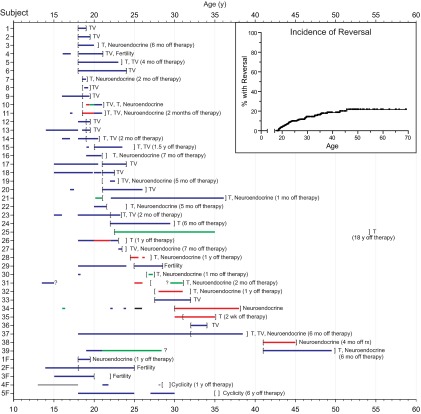
Reversal occurred across a broad range of ages. Pinpointing precisely when a patient achieved reversal was limited by the frequency of evaluation and whether subjects underwent trials of stopping treatment for their IHH. The lower bound for the age at which reversal could have occurred is set by the last assessment at which the patient was still deemed to have IHH (indicated by the left brackets in Figure 1). For men, the average earliest age at which reversal could have occurred was 23.8 years (median 21.5 y, range 18–41.5 y); for women, this was 24.0 years (median 20.5 y, range 18–35 y). The upper bound for the age at which reversal could have occurred is set by the first time when the patient was noted to have reversed (as shown by right brackets in Figure 1). For men, this was a mean age of 28.3 years (median 24.0 y, range 19.1–53.6 y), and for women 26.5 years (median 26.5 y, range 19.5–35.5 y).
Figure 1.
Clinical timelines for IHH patients who underwent reversal (39 men, subjects 1–39, and 5 women, subjects 1F–5F). Horizontal bars indicate duration of treatment of various types: blue, sex steroids; red, GnRH; green: gonadotropins; black: clomiphene; gray, type of treatment unknown. Question marks signify periods when the precise timing of initiation or cessation of therapy was unknown. Brackets delimit time intervals during which reversal could have occurred, with left brackets indicating the last documentation of hypogonadotropic status, and right brackets indicating the time at which reversal was discovered. The finding demonstrating occurrence of reversal is given to the right: cyclicity, regular menstrual periods in the absence of treatment; fertility, fertility in the absence of treatment; neuroendocrine, neuroendocrine profile with normal LH pulse frequency and amplitude; T, testosterone >250 ng/dL; TV, testicular volume increase. The duration between stopping therapy and the time at which reversal was discovered is also noted to the right, if applicable. Inset, incidence of reversal with age.
Because some patients who had not been found to reverse may in fact achieve reversal in the future, a time-to-event analysis was performed to model the incidence of reversal, using the average of the two bounds as an estimate of the actual time of reversal. This analysis revealed a lifetime incidence of 22% (Figure 1, inset). Similar analyses using the lower and upper bounds for when reversal occurred produced lifetime incidence rates of 19% and 27%, respectively. Using the stricter set of criteria (testosterone, menstrual cyclicity, fertility), the lifetime incidence of reversal was estimated to be 15% (13%–23%).
IHH patients are classically subdivided into those with anosmia (ie, with KS) and those with normal senses of smell (normosmic IHH). Given the importance of the olfactory system in GnRH neuronal development, we anticipated that the overwhelming majority of patients with reversal would have intact olfactory systems. Against our expectations, 13 of the 44 patients who reversed (30%) were anosmic. Three of these patients with KS had undergone magnetic resonance imaging scans with dedicated imaging of the olfactory bulbs. One patient with self-reported anosmia had no detectable olfactory bulbs (Supplemental Figure 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org), and one patient with a smell-test score 2/12 indicating anosmia was also reported to have olfactory bulb agenesis. A third individual with a smell-test score 26/40 indicating hyposmia had normal olfactory bulbs and sulci. Furthermore, one normosmic patient (smell-test score 32/40) with unilateral cryptorchidism had an undetectable left olfactory bulb and sulcus (data not shown).
Phenotypic characteristics
Men
Men who achieved reversal had a broad range of clinical characteristics on their initial presentation for IHH. Testicular volumes before any treatment ranged from 2 to 15 mL (mean 6.9 mL, median 5 mL), with 31% (5/16) having prepubertal testicular volumes of <4 mL. Micropenis and cryptorchidism are markers of inadequate activity of the reproductive endocrine axis during fetal development (38). Of patients with reversal, 27% had a history of micropenis (9 of 33 patients for whom phallic length was documented, all normosmic), comparable with a previously reported rate of micropenis in IHH patients in general of 16% (36). Only 15% of all reversal patients had cryptorchidism (6 of 39 patients for whom testicular descent was documented, 5 normosmic IHH, and 1 KS), less than what has previously been reported for GnRH deficiency (29% [36]), although this difference was not statistically significant (P = .06).
Of the four criteria used to define reversal, testicular growth in the absence of GnRH/gonadotropin therapy, a marker of endogenous gonadotropin secretion, was the most frequently observed sign of reproductive endocrine activity. It was seen in all men with reversal, of whom 29 had never received GnRH/gonadotropin therapy, and 10 who had cryptorchidism or prepubertal testicular volumes before reversal. In these men, the mean ± SD increase in testicular volume was 6.6 ± 3.7 mL, from 7.4 ± 4.8 mL to 14.0 ± 6.0 mL (Table 2, Figure 2A) (36). Patients with reversal continued to experience increases in their testicular size, such that the average testicular volume at the last recorded follow-up was 15.3 ± 5.7 mL (Table 2, Figure 2A).
Figure 2.
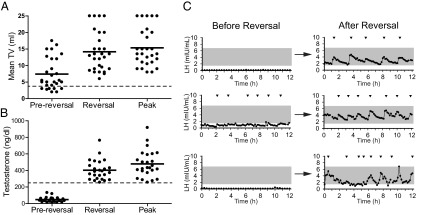
A, Testicular volume (TV) measurements of 29 men who demonstrated testicular growth without the use of GnRH or gonadotropins: at the time of presentation for reproductive endocrine evaluation, at the time at which reversal of IHH was first noted, and highest TV recorded after reversal. Bars, means. The dashed line represents the pubertal threshold for testicular volume (4 mL). B, Serum testosterone of 27 men who achieved serum testosterone levels of ≥250 ng/dL (dashed line) off therapy. Testosterone measurements are shown at the time of presentation for reproductive endocrine evaluation, time at which reversal of IHH was first noted, and highest testosterone recorded after reversal. Bars, means. C, Neuroendocrine profiles of three men before and after reversal of IHH. Arrowheads signify LH pulses and the shaded region represents the normal range of LH for healthy men (26).
The second most frequently observed sign of reversal was an increase in serum testosterone in the absence of treatment. Of 38 men who had testosterone measurements off treatment, 25 (66%) achieved testosterone levels >250 ng/dL without exogenous therapy (prereversal 48 ± 31 ng/dL, range 13–132 ng/dL; postreversal 402 ± 120 ng/dL, range 256–764 ng/dL; Table 2, Figure 2B). As with testicular growth, testosterone levels continued to rise, reaching a peak average value of 479 ± 162 ng/dL, range 257–918 ng/dL (Table 2, Figure 2B).
Of the 27 men who participated in frequent blood sampling studies to chart pulsatile LH secretion, 25 (93%) displayed normal patterns of LH secretion after reversal (Table 2, Figure 2C). Of these 25 men, 11 had previously undergone similar neuroendocrine evaluation before reversal, and 5 of these had complete absence of LH pulses on these prior studies. Of the men who did not have prior neuroendocrine studies, all met at least one of the other criteria for reversal.
Seven men achieved the most striking evidence of reversal: fertility in the absence of concurrent or prior gonadotropin/GnRH therapy. Genetic paternity was demonstrated in one of these cases. All of these men also met at least one of the other criteria for reversal.
Women
Five women from three families underwent reversal of IHH (Tables 1 and 2, Figure 1). Before reversal, two women had partial pubertal development with breast development in the absence of exogenous treatment, occurring late, at ages 15 to 16 years; two women had no breast development by 13 years and 15 years, respectively, at which time estrogen therapy was started. No information on breast development is available for the fifth woman. Two women demonstrated reversal by spontaneously conceiving, one in the absence of any therapy for 3 months and the other after stopping therapy for an unknown duration of time. Three women (60%) achieved spontaneous menstrual cyclicity after remaining off treatment for 9 to 12 months. Of four women who underwent neuroendocrine studies, one had a pattern of normal LH secretion within normal limits demonstrating reversal (LH pulse amplitude 1.5 IU/L; frequency 10 pulses/12 h).
Genetic characteristics
Thirty-eight unrelated probands who underwent reversal participated in genetic studies. Seventeen probands harbored RSVs in at least one gene associated with IHH (Supplemental Table 1). Deleterious RSVs were identified in FGFR1 (n = 5, 13%), GNRHR (n = 3, 8%), HS6ST1 (n = 1, 3%), PROKR2 (n = 2, 5%), TAC3 (n = 1, 3%), and TACR3 (n = 3, 8%). Of the 12 deleterious variants identified in these individuals, 4 led to premature termination (TAC3 p.A20fsX39; TACR3 p.W275X x2; FGFR1 p.C603X and p.R622X), 7 were missense changes previously demonstrated to be loss-of-function mutations in vitro (TACR3 p.Y256H; FGFR1 p.A671P and p.Y99C; GNRHR p.Q106R x3; PROKR2 p.R85C and p.V331M; HS6ST1 p.M404V) (15, 16, 28–30), and 1 variant (FGFR1 p.G687R) was predicted to be damaging by five in silico programs. Three variants were not predicted to be deleterious (FGFR1 p.V427L; TACR3 p.V98V; NELF p.R196H). Heterozygous mutations in TACR3 and GNRHR were considered to contribute to the pathogenesis of IHH, as heterozygous mutations in these genes are seen more frequently in patients with IHH than in control populations (15, 37).
Notably, the genetics of reversal favored genes in the neurokinin B pathway, with 11% (4/38) of reversal probands carrying deleterious RSVs in TAC3 or TACR3 vs 3% (39/1156) of a large population of IHH probands who had no known evidence for reversal (P = .044). There were two families in which multiple siblings with IHH demonstrated reversal; all affected individuals had mutations in either TAC3 or TACR3. In contrast, mutations in FGFR1, GNRHR, and PROKR2 were seen at frequencies comparable with those seen in IHH patients in general: FGFR1 13% (n = 5/38) vs 8% (n = 98/1156, P = .4); GNRHR 8% (n = 3/38) vs 4% (n = 43/1156, P = .2); PROKR2 5% (n = 2/38) vs 4% (n = 51/1156, P = .7). No mutations in the X-linked gene KAL1 were seen in men with reversal vs 4% (n = 36/855) in IHH men in general (P = .4). There were no significant differences in clinical characteristics (eg, anosmia, testicular volume) between patients with TAC3/TACR3 mutations and patients who did not have mutations in the neurokinin B pathway.
Relapse after reversal
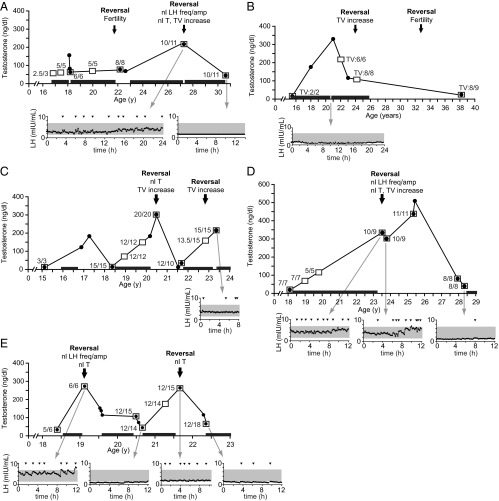
A subset of patients who reversed did not sustain their reversal. Five men (13% of those who reversed) reverted to a hypogonadal state (testosterone range = 12–82 ng/dL) an average of 3.7 years after reversal (range = 0.7–13.7 y) (Figure 3). A common feature among these five men was emotional, metabolic, and/or psychiatric stress before relapse: relationship and unemployment stress in the subject depicted in Figure 3A, work-related stress (Figure 3B), anxiety treated with citalopram (Figure 3C), depression (Figure 3D), and weight loss of 7 kg (Figure 3E). One of the five patients once again recovered reproductive function: the subject depicted in Figure 3E achieved a testosterone level of 257 ng/dL.
Figure 3.
Clinical courses of five men who reversed their IHH but relapsed to hypogonadotropism, with panels A–E showing data for subjects 4, 17, 14, 5, and 7, respectively. Times at which reversal occurred are marked by arrows, with labels indicating the findings demonstrating reversal (LH freq/amp, LH frequency and amplitude; nl, normal; T, testosterone; TV, testicular volume). Testosterone measurements are indicated by black circles joined by black lines; numbers adjacent to open squares indicate measurements of testicular volume (in milliliters), and black bars above the X-axis indicate periods of testosterone therapy. Gray arrows point to LH secretory profiles at the indicated times; arrows mark LH pulses, and shaded regions indicate the normal male range for LH.
Discussion
This examination of the largest cohort of patients with reversal of IHH to date revealed a lifetime incidence of reversal of 22% and a phenotypic and genotypic diversity that underscores the dynamic nature of the reproductive endocrine system. On the one hand, the reproductive endocrine axis demonstrates remarkable resilience, such that reversal can occur even in patients with severe GnRH deficiency. On the other hand, the axis exhibits an ongoing susceptibility to insult, as reversal was followed by relapse in several cases.
The time-to-event analysis in this study estimated a 22% lifetime incidence of reversal, a remarkably high rate. Even performing the time-to-event analysis using only patients who achieved normal testosterone, menstrual cyclicity, and/or fertility, the lifetime incidence remains 15%. Biases of referral may have influenced the detection of reversal. For instance, patients may have been referred specifically because of the possibility of reversal. Alternatively, patients who reversed may have elected not to follow-up, and so the exact effects of referral bias are difficult to predict. The retrospective nature of this study also lends uncertainty, because not all information was available for all subjects. Other potential limitations include intraobserver variation in measurement of testicular volume, lack of available confirmation of paternity, and variability in frequency of follow-up. Despite these limitations, our results suggest that reversal of GnRH deficiency is a more common phenomenon than previously appreciated.
Reversal was not restricted to patients with “mild” GnRH deficiency. Several features can be used to judge the severity of a patient's GnRH deficiency. Because testosterone is required for descent of the testicles and growth of the penis during male fetal development, hypogonadism during the fetal period results in cryptorchidism and micropenis (38). Similarly, complete absence of reproductive endocrine activity in adolescence results in failure to achieve pubertal hallmarks such as increases in testicular volume or breast development. A more direct measure of reproductive endocrine activity can be achieved by examination of LH secretory patterns, which in patients with IHH range from complete absence of pulses to seemingly normal patterns (albeit inappropriately normal in light of the loss of negative sex-steroid feedback) (39). The fact that reversal occurred in patients with cryptorchidism, micropenis, absence of pubertal development, and/or absence of pulsatile LH secretion indicates that reversal can occur even in the context of severe GnRH deficiency. Thus, at least in these cases, reversal represents a transformation of a magnitude on par with that of the awakening of the reproductive endocrine axis at puberty.
A notable example of reversal was seen in a patient who had no olfactory bulbs. During development, GnRH neurons reach their final destinations in the forebrain by migrating along the axonal projections from the olfactory neurons to the olfactory bulb, and thus absence of olfactory bulbs should leave the GnRH neurons stranded outside the central nervous system. The finding that reproductive endocrine activity can be achieved by individuals without olfactory bulbs, as demonstrated by this report and others as well as a report of normal reproductive function in patients with isolated congenital anosmia and undetectable olfactory bulbs (14, 17, 40), suggests that human GnRH neurons may not be wholly dependent on an intact olfactory system to reach their target regions in the forebrain. Alternatively, it is possible that the olfactory structures were present early in development, allowing GnRH neuronal migration to proceed normally, and then degenerated later.
Findings from animal models suggest mechanisms by which proper GnRH neuronal migration could occur in the absence of an intact olfactory system. In chicks and zebrafish, GnRH neurons appear to have origins outside the olfactory system in the anterior pituitary placode and cranial neural crest (4, 41). In addition, hypothalamic neurons can arise during neurogenesis in adult mammalian life, raising the possibility that there may be ongoing generation of GnRH neurons rather than a fixed complement of GnRH neurons at birth (42). When differentiated GnRH neurons are implanted into GnRH-deficient hpg mice, they project fibers to the median eminence, demonstrating the ability of GnRH neurons to form new functional connections in adulthood (43). Finally, only a fraction of the already small complement of GnRH neurons is necessary for normal reproductive function. In male mice merely 12% of the GnRH neuronal population is enough to maintain pulsatile gonadotropin secretion and onset of sexual maturation (43, 44). Thus, only a relatively small number of GnRH neurons need to be properly positioned in the hypothalamus to achieve normal gonadotropin stimulation.
Just as patients with reversal exhibited a range of phenotypes that mirrored that of patients with IHH as a whole, the genetic spectrum of reversal also largely mirrored that of IHH in general, with comparable frequencies of mutations in FGFR1, PROKR2, and GNRHR. There were two notable exceptions to this, however. One was the absence of mutations in KAL1 in reversal patients—a conspicuous absence as KAL1 is one of the most commonly mutated genes in IHH (45). Patients with mutations in KAL1 generally exhibit complete absence of pubertal development and lack of pulsatile LH secretion (46, 47), although there are some exceptions; this stands in contrast to the variable penetrance and expressivity seen with mutations in other commonly mutated IHH genes such as FGFR1 and PROKR2. Thus, most KAL1 mutations appear to cause severe IHH, perhaps so severe that the potential for reversal is diminished. However, even this is not absolute, because reversal has been reported in a patient with a deleterious mutation in KAL1 (12).
The other notable genetic feature of reversal was a significant enrichment for mutations in the genes encoding neurokinin B and its receptor (TAC3 and TACR3, respectively), strengthening the association between the neurokinin B pathway and reversal that we have reported previously (15). Furthermore, all familial cases of reversal were associated with mutations in TAC3/TACR3. The fact that patients with these mutations developed IHH demonstrates the critical importance for neurokinin B signaling at puberty, but the strong association with reversal suggests that other factors—possibly environmental or genetic—can compensate for defects in neurokinin B signaling later in life. However, this compensation can be incomplete, as one patient with a mutation in TACR3 who reversed later relapsed.
Indeed, cases of relapse after reversal both in our cohort and in other recent reports (17, 19, 48) indicate an ongoing fragility of the reproductive endocrine system that counterbalances the resilience of the system demonstrated by reversal itself. The five patients in our cohort who reversed and then relapsed were similar to the reversal cohort as a whole in displaying a broad range of clinical characteristics on initial presentation—with both prepubertal and pubertal testicular volumes, presence and absence of micropenis, and presence and absence of anosmia—as well as in their genetic profiles, with mutations in FGFR1 and TACR3 found in one patient each. The patients' histories all suggest possible explanations for their relapse, with emotional, metabolic, or psychiatric stress before relapse. Psychiatric issues, from short-term anxiety to major depression, suppress testosterone levels in men by decreasing LH pulse frequency (49–51). In addition to psychological stress, changes in energy balance, even when minor, can impair male reproductive function. For example, weight loss averaging 5% in college wrestlers results in a 50% drop in testosterone levels (52). It is possible that patients with relapsing-remitting GnRH deficiency have increased vulnerability to metabolic and/or psychological stressors and represent a male equivalent of functional hypothalamic amenorrhea (48).
This study offers several important messages for both clinicians and patients. First, patients with IHH require ongoing clinical monitoring that includes regular assessments of testicular volume, first to look for reversal, then to determine whether reversal is sustained. Second, clinicians should emphasize the need for patients with IHH to take contraceptive precautions despite their apparent infertility. Third, patients with reversal should be counseled regarding the possibility of reverting to hypogonadism and should consider options such as sperm banking for ensuring future fertility.
The reproductive endocrine axis is among the most dynamic of the hormonal systems. The activity of the axis undergoes normal physiologic changes across a broad range of timescales, with day-to-day changes in neuroendocrine dynamics across the menstrual cycle and longer-term changes across the transitions from fetal life to the neonatal period, infancy, childhood, adolescence, and adulthood (1, 53). This report demonstrates that this dynamism extends to pathophysiologic states as well, with reversal of hypogonadotropism occurring even in patients with severe IHH. However, the dynamic nature of the reproductive endocrine axis may also render it susceptible to insults such as negative energy balance and other stresses, and patients who reverse may exhibit a particular sensitivity to these stresses. Further investigations into reversal as well as the newly identified subphenotype of relapsing-remitting IHH may yield important insights into the regulation of the reproductive endocrine axis and may eventually lead to new approaches to the treatment of reproductive endocrine disorders.
Acknowledgments
We thank members of the Massachusetts General Hospital Reproductive Endocrine Unit for discussions and reading of the manuscript, Dr Hang Lee for statistical advice, staff of the Harvard Catalyst Clinical Research Center for assistance with the frequent sampling studies, and all physicians who referred these patients. We also thank the National Heart, Lung, and Blood Institute Grand Opportunity (GO) Exome Sequencing Project and its ongoing studies, which produced and provided exome variant calls for comparison: the Lung GO Sequencing Project (HL-102923), the Women's Health Initiative Sequencing Project (HL-102924), the Broad GO Sequencing Project (HL-102925), the Seattle GO Sequencing Project (HL-102926), and the Heart GO Sequencing Project (HL-103010).
This work was supported by Grants U54 HD028138 and R01 HD015788 from the National Institutes of Health and by the Harvard Catalyst/The Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Awards M01-RR-01066, UL1 RR025758, and UL1TR000170, and financial contributions from Harvard University and its affiliated academic health care centers).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- IHH
- idiopathic hypogonadotropic hypogonadism
- KS
- Kallmann syndrome
- RSV
- rare sequence variants.
References
- 1. Styne DM, Grumbach MM. Puberty: ontogeny, neuroendocrinology, physiology, and disorders. In: Melmed S, Polonsky KS, Larsen PR, Kronenberg HM, eds. Williams Textbook of Endocrinology. 12 ed Philadelphia, PA: Elsevier Saunders; 2011:1054–1201 [Google Scholar]
- 2. Hoffman AR, Crowley WF., Jr Induction of puberty in men by long-term pulsatile administration of low-dose gonadotropin-releasing hormone. N Engl J Med. 1982;307:1237–1241 [DOI] [PubMed] [Google Scholar]
- 3. Dodé C, Hardelin JP. Kallmann syndrome. Eur J Hum Genet. 2009;17:139–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Whitlock KE, Illing N, Brideau NJ, Smith KM, Twomey S. Development of GnRH cells: setting the stage for puberty. Mol Cell Endocrinol. 2006;254–255:39–50 [DOI] [PubMed] [Google Scholar]
- 5. Bauman A. Markedly delayed puberty or Kallmann's syndrome variant. J Androl. 1986;7:224–227 [DOI] [PubMed] [Google Scholar]
- 6. Finkelstein JS, Spratt DI, O'Dea LS, et al. Pulsatile gonadotropin secretion after discontinuation of long term gonadotropin-releasing hormone (GnRH) administration in a subset of GnRH-deficient men. J Clin Endocrinol Metab. 1989;69:377–385 [DOI] [PubMed] [Google Scholar]
- 7. Kadva A, Di WL, Djahanbakhch O, Monson J, Silman R. Evidence for the Bauman variant in Kallmann's syndrome. Clin Endocrinol (Oxf). 1996;44:103–110 [DOI] [PubMed] [Google Scholar]
- 8. Quinton R, Cheow HK, Tymms DJ, Bouloux PM, Wu FC, Jacobs HS. Kallmann's syndrome: is it always for life? Clin Endocrinol (Oxf). 1999;50:481–485 [DOI] [PubMed] [Google Scholar]
- 9. Pitteloud N, Boepple PA, DeCruz S, Valkenburgh SB, Crowley WF, Jr, Hayes FJ. The fertile eunuch variant of idiopathic hypogonadotropic hypogonadism: spontaneous reversal associated with a homozygous mutation in the gonadotropin-releasing hormone receptor. J Clin Endocrinol Metab. 2001;86:2470–2475 [DOI] [PubMed] [Google Scholar]
- 10. Dewailly D, Boucher A, Decanter C, Lagarde JP, Counis R, Kottler ML. Spontaneous pregnancy in a patient who was homozygous for the Q106R mutation in the gonadotropin-releasing hormone receptor gene. Fertil Steril. 2002;77:1288–1291 [DOI] [PubMed] [Google Scholar]
- 11. Pitteloud N, Acierno JS, Jr, Meysing AU, Dwyer AA, Hayes FJ, Crowley WF., Jr Reversible Kallmann syndrome, delayed puberty, and isolated anosmia occurring in a single family with a mutation in the fibroblast growth factor receptor 1 gene. J Clin Endocrinol Metab. 2005;90:1317–1322 [DOI] [PubMed] [Google Scholar]
- 12. Ribeiro RS, Vieira TC, Abucham J. Reversible Kallmann syndrome: report of the first case with a KAL1 mutation and literature review. Eur J Endocrinol. 2007;156:285–290 [DOI] [PubMed] [Google Scholar]
- 13. Raivio T, Falardeau J, Dwyer A, et al. Reversal of idiopathic hypogonadotropic hypogonadism. N Engl J Med. 2007;357:863–873 [DOI] [PubMed] [Google Scholar]
- 14. Sinisi AA, Asci R, Bellastella G, et al. Homozygous mutation in the prokineticin-receptor2 gene (Val274Asp) presenting as reversible Kallmann syndrome and persistent oligozoospermia: case report. Hum Reprod. 2008;23:2380–2384 [DOI] [PubMed] [Google Scholar]
- 15. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tornberg J, Sykiotis GP, Keefe K, et al. Heparan sulfate 6-O-sulfotransferase 1, a gene involved in extracellular sugar modifications, is mutated in patients with idiopathic hypogonadotrophic hypogonadism. Proc Natl Acad Sci USA. 2011;108:11524–11529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Laitinen EM, Tommiska J, Sane T, Vaaralahti K, Toppari J, Raivio T. Reversible congenital hypogonadotropic hypogonadism in patients with CHD7, FGFR1 or GNRHR mutations. PLoS One. 2012;7:e39450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kulshreshtha B, Khadgawat R, Gupta N, Ammini A. Progression of puberty after initiation of androgen therapy in patients with idiopathic hypogonadotropic hypogonadism. Indian J Endocrinol Metab. 2013;17:851–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tommiska J, Jorgensen N, Christiansen P, Juul A, Raivio T. A homozygous R262Q mutation in the gonadotropin-releasing hormone receptor presenting as reversal of hypogonadotropic hypogonadism and late-onset hypogonadism. Clin Endocrinol (Oxf). 2013;78:316–317 [DOI] [PubMed] [Google Scholar]
- 20. Doty RL, Shaman P, Kimmelman CP, Dann MS. University of Pennsylvania Smell Identification Test: a rapid quantitative olfactory function test for the clinic. Laryngoscope. 1984;94:176–178 [DOI] [PubMed] [Google Scholar]
- 21. Lewkowitz-Shpuntoff HM, Hughes VA, Plummer L, et al. Olfactory phenotypic spectrum in idiopathic hypogonadotropic hypogonadism: pathophysiological and genetic implications. J Clin Endocrinol Metab. 2012;97:E136–E144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shaw ND, Seminara SB, Welt CK, et al. Expanding the phenotype and genotype of female GnRH deficiency. J Clin Endocrinol Metab. 2011;96:E566–E576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dwyer AA, Hayes FJ, Plummer L, Pitteloud N, Crowley WF., Jr The long-term clinical follow-up and natural history of men with adult-onset idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2010;95:4235–4243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man: pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab. 1999;84:1028–1036 [DOI] [PubMed] [Google Scholar]
- 26. Hayes FJ, DeCruz S, Seminara SB, Boepple PA, Crowley WF., Jr Differential regulation of gonadotropin secretion by testosterone in the human male: absence of a negative feedback effect of testosterone on follicle-stimulating hormone secretion. J Clin Endocrinol Metab. 2001;86:53–58 [DOI] [PubMed] [Google Scholar]
- 27. Exome Variant Server NHLBI GO Exome Sequencing Project (ESP). Seattle, WA [Google Scholar]
- 28. Raivio T, Sidis Y, Plummer L, et al. Impaired fibroblast growth factor receptor 1 signaling as a cause of normosmic idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2009;94:4380–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bedecarrats GY, Linher KD, Kaiser UB. Two common naturally occurring mutations in the human gonadotropin-releasing hormone (GnRH) receptor have differential effects on gonadotropin gene expression and on GnRH-mediated signal transduction. J Clin Endocrinol Metab. 2003;88:834–843 [DOI] [PubMed] [Google Scholar]
- 30. Cole LW, Sidis Y, Zhang C, et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab. 2008;93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. González-Perez A, López-Bigas N. Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. Am J Hum Genet. 2011;88:440–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sunyaev S, Ramensky V, Koch I, Lathe W, 3rd, Kondrashov AS, Bork P. Prediction of deleterious human alleles. Hum Mol Genet. 2001;10:591–597 [DOI] [PubMed] [Google Scholar]
- 33. Ng PC, Henikoff S. SIFT: predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ferrer-Costa C, Gelpí JL, Zamakola L, Parraga I, de la Cruz X, Orozco M. PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics. 2005;21:3176–3178 [DOI] [PubMed] [Google Scholar]
- 35. Schwarz JM, Rödelsperger C, Schuelke M, Seelow D. MutationTaster evaluates disease-causing potential of sequence alterations. Nat Methods. 2010;7:575–576 [DOI] [PubMed] [Google Scholar]
- 36. Pitteloud N, Hayes FJ, Boepple PA, et al. The role of prior pubertal development, biochemical markers of testicular maturation, and genetics in elucidating the phenotypic heterogeneity of idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2002;87:152–160 [DOI] [PubMed] [Google Scholar]
- 37. Gianetti E, Hall JE, Au MG, et al. When genetic load does not correlate with phenotypic spectrum: lessons from the GnRH receptor (GNRHR). J Clin Endocrinol Metab. 2012;97:E1798–E1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Seminara SB, Hayes FJ, Crowley WF., Jr Gonadotropin-releasing hormone deficiency in the human (idiopathic hypogonadotropic hypogonadism and Kallmann's syndrome): pathophysiological and genetic considerations. Endocr Rev. 1998;19:521–539 [DOI] [PubMed] [Google Scholar]
- 39. Spratt DI, Carr DB, Merriam GR, Scully RE, Rao PN, Crowley WF., Jr The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J Clin Endocrinol Metab. 1987;64:283–291 [DOI] [PubMed] [Google Scholar]
- 40. Moya-Plana A, Villanueva C, Laccourreye O, Bonfils P, De Roux N. PROKR2 and PROK2 mutations cause isolated congenital anosmia without gonadotropic deficiency. Eur J Endocrinol. 2012;168(1):31–37 [DOI] [PubMed] [Google Scholar]
- 41. Whitlock KE. Origin and development of GnRH neurons. Trends Endocrinol Metab. 2005;16:145–151 [DOI] [PubMed] [Google Scholar]
- 42. Lee DA, Bedont JL, Pak T, et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat Neurosci. 2012;15:700–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Silverman AJ, Gibson M. Hypothalamic transplantation repair of reproductive defects in hypogonadal mice. Trends Endocrinol Metab. 1990;1:403–408 [DOI] [PubMed] [Google Scholar]
- 44. Herbison AE, Porteous R, Pape JR, Mora JM, Hurst PR. Gonadotropin-releasing hormone neuron requirements for puberty, ovulation, and fertility. Endocrinology. 2008;149:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Costa-Barbosa FA, Balasubramanian R, Keefe KW, et al. Prioritizing genetic testing in patients with Kallmann syndrome using clinical phenotypes. J Clin Endocrinol Metab. 2013;98:E943–E953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Oliveira LM, Seminara SB, Beranova M, et al. The importance of autosomal genes in Kallmann syndrome: genotype-phenotype correlations and neuroendocrine characteristics. J Clin Endocrinol Metab. 2001;86:1532–1538 [DOI] [PubMed] [Google Scholar]
- 47. Beranova M, Oliveira LM, Bédécarrats GY, et al. Prevalence, phenotypic spectrum, and modes of inheritance of gonadotropin-releasing hormone receptor mutations in idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2001;86:1580–1588 [DOI] [PubMed] [Google Scholar]
- 48. Santhakumar A, Balasubramaniam R, Miller M, Quinton R. Reversal of Isolated Hypogonadotropic Hypogonadism: long-term integrity of hypothalamo-pituitary-testicular axis in two men is dependent on intermittent androgen exposure. Clin Endocrinol (Oxf). 2013 [DOI] [PubMed] [Google Scholar]
- 49. Rose RM, Bourne PG, Poe RO, Mougey EH, Collins DR, Mason JW. Androgen responses to stress. II. Excretion of testosterone, epitestosterone, androsterone and etiocholanolone during basic combat training and under threat of attack. Psychosom Med. 1969;31:418–436 [DOI] [PubMed] [Google Scholar]
- 50. Kreuz LE, Rose RM, Jennings JR. Suppression of plasma testosterone levels and psychological stress. A longitudinal study of young men in Officer Candidate School. Arch Gen Psychiatry. 1972;26:479–482 [DOI] [PubMed] [Google Scholar]
- 51. Schweiger U, Deuschle M, Weber B, et al. Testosterone, gonadotropin, and cortisol secretion in male patients with major depression. Psychosom Med. 1999;61:292–296 [DOI] [PubMed] [Google Scholar]
- 52. Strauss RH, Lanese RR, Malarkey WB. Weight loss in amateur wrestlers and its effect on serum testosterone levels. JAMA. 1985;254:3337–3338 [PubMed] [Google Scholar]
- 53. Hall JE. Neuroendocrine control of the menstrual cycle. In: Strauss JF, III, Barbieri RL, eds. Yen and Jaffe's Reproductive Endocrinology. 6th ed Philadelphia, PA: Elsevier Saunders; 2009:139–154 [Google Scholar]