Abstract
Context:
Loss of prokineticin 2 (PROK2) signaling in mice disrupts circadian rhythms, but the role of PROK2 signaling in the regulation of circadian rhythms in humans is undetermined.
Objective:
The aim of the study was to examine the circadian rhythms of humans with a complete loss-of-function PROK2 mutation using an inpatient constant routine (CR) protocol.
Design and Setting:
We conducted a case study in an academic medical center.
Subjects and Methods:
Two siblings (one male and one female, ages 67 and 62 y, respectively) with isolated GnRH deficiency (IGD) due to a biallelic loss-of-function PROK2 mutation were studied using an inpatient CR protocol. Historical data from inpatient CR protocols conducted in healthy controls (ages 65–81 y) were used for comparison.
Main Outcome Measures:
We measured circadian phase markers (melatonin, cortisol, and core body temperature) and neurobehavioral performance (psychomotor vigilance task [PVT] and subjective alertness scale).
Results:
Circadian waveforms of melatonin and cortisol did not differ between the IGD participants with PROK2 mutation and controls. In both IGD participants, neurobehavioral testing with PVT showed disproportionate worsening of PVT lapses and median reaction time in the second half of the CR.
Conclusions:
Humans with loss of PROK2 signaling lack abnormalities in circadian phase markers, indicating intact central circadian pacemaker activity in these patients. These results suggest that PROK2 signaling in humans is not required for central circadian pacemaker function. However, impaired PVT in the PROK2-null participants despite preserved endocrine rhythms suggests that PROK2 may transmit circadian timing information to some neurobehavioral neural networks.
In humans, observed rhythms of sleep-wake cycles, locomotor activity, thermoregulation, and circulating rhythms of hormones (eg, cortisol, TSH, melatonin) are evidence of endogenous circadian (∼24-h) rhythmicity (1). These rhythmic circadian signals are known to directly influence the function of the hypothalamo-pituitary-gonadal axis in mammals (2). The master circadian clock residing within the suprachiasmatic nucleus (SCN) of the hypothalamus is constituted from complex autoregulatory transcriptional and translational feedback loops consisting of both activating and repressive pathways (3). Despite the advances in our understanding of the core clock mechanisms within the SCN, very little is known about SCN output signals, including the link between the circadian clock network and hypothalamic control of reproduction. Both synaptic and humoral mechanisms are known to transmit SCN signals to adjacent brain areas. A three-stage synaptic network involving the subparaventricular zone and the dorsomedial nucleus of the hypothalamus has been proposed as the key neuroanatomical infrastructure for the conduit of circadian signals to some SCN target regions (4). Multiple molecules including vasopressin, TGF-α, prokineticin 2 (PROK2), and cardiotrophin-like cytokine have been proposed as SCN output signals (5), but their relative roles in the regulation of circadian rhythms in humans are not known.
Of the putative circadian output molecules, PROK2, a unique cysteine-rich secreted peptide, represents a logical candidate to mediate the SCN output because it fulfills several criteria for such a molecule in that it: 1) is highly expressed in the SCN; 2) oscillates with a circadian periodicity (6); 3) is activated by core clock genes clock and bmal1 (6); 4) has its cognate receptor, PROKR2, expressed in key SCN target areas (6) with PROK2-expressing neurons projecting directly to those SCN areas; 5) inhibits nocturnal locomotor activity in mice after its intracerebroventricular injection (6); and 6) is associated with attenuated circadian rhythms of nocturnal locomotor activity, thermoregulation, sleep-wake cycle, and circulating corticosteroid and glucose levels when either Prok2 (7) or its receptor Prokr2 (8) is deleted in mice. Secondly, although the oscillation of core clockwork genes within the SCN is unaltered in Prok2−/− mice (5), recent evidence suggests that PROK2 alters spontaneous firing rates within SCN neurons and therefore may directly alter this circadian pacemaker (9). Finally, PROK2-expressing neurons have been shown to project to key areas within the hypothalamus that may influence various neuroendocrine circadian rhythms (10, 11).
In addition to an attenuated circadian phenotype, Prok2−/− (12) and Prokr2−/− (13) mice also display hypogonadotropic hypogonadism and olfactory bulb agenesis, a phenotypic combination characteristic of Kallmann syndrome (KS) in humans. KS is characterized by isolated GnRH deficiency (IGD), a reproductive disorder characterized by pubertal failure and hypogonadism secondary to hypothalamic GnRH deficiency. Recently, we and others have described naturally occurring homozygous and heterozygous loss-of-function (LOF) mutations in PROK2 and PROKR2 in humans with KS (12, 14–16) as well as the normosmic form of IGD (normosmic idiopathic hypogonadotropic hypogonadism) (15, 16). This remarkable association of a severe reproductive phenotype seen in both mice and humans harboring mutations in a gene encoding a putative circadian output signal immediately raises the possibility that PROK2 signaling may be a key link between circadian rhythms and reproduction. The current study was thus undertaken to test the hypothesis that human participants with complete LOF PROK2 mutations would display attenuated circadian rhythms.
Subjects and Methods
Participants
Two Portuguese siblings, a brother and sister, harboring a complete LOF biallelic frame-shift mutation (p.[I55fsX1]+[I55fsX1]) in PROK2 as documented in our previous report (12) were recruited for this study. Both participants were nonsmokers in good health who had no history of thyroid disease, liver disease, or sleep disorders apart from their reproductive phenotypes and had otherwise normal physical examinations with normal complete blood count and TSH levels. Hormone replacement therapy, if indicated, was continued throughout the study. The study was approved by the Institutional Review Board at Partners' Healthcare, and written informed consent was obtained from each subject before participation.
Experimental protocol
A validated inpatient protocol was undertaken to distinguish between endogenous circadian and diurnal (evoked) rhythms (17). Briefly, for 2 weeks before circadian assessment, while in Portugal, both participants wore a wrist activity monitor to record their daily activity and maintained a sleep/wake cycle log. Then, both participants were flown to Boston, Massachusetts, and admitted to the Intensive Physiologic Monitoring (IPM) Unit of the Center for Clinical Investigation at Brigham and Women's Hospital. The IPM has specialized suites with conditions that minimize external time cues, including outside lighting. The first 2 days (d 1–2) in the IPM were devoted to acclimating to the new experimental conditions. To avoid time-zone shifting, all events (activity, meals, sleep, and wake) during the inpatient study were scheduled at each participant's habitual Portugal time, as determined during the outpatient monitoring.
Indwelling iv catheters were placed in a forearm vein on day 1, and serial blood collection was commenced on day 1 for melatonin (hourly) and cortisol (every 20 min). Core body temperature (CBT) was measured every minute using a rectal temperature sensor. Melatonin, cortisol, and CBT are three validated markers of human circadian pacemaker amplitude and phase (1). For the neurobehavioral testing, participants rated their subjective alertness (alert-sleepy) using a 100-mm non-numeric linear scale (18) several times per hour and performed tests of calculation (19) and the psychomotor vigilance task (PVT) several times per day (20). All sleep episodes were polysomnographically recorded and scored using standard methods (21). After awakening on day 3, the participants remained awake in constant routine (CR) conditions for the next 40 hours; these conditions include the participant being continuously recumbent, awake, in dim light levels, and ingesting small meals hourly to minimize or evenly spread any evoked perturbations that affect measurement of endogenous circadian rhythms. During this time, blood sampling for melatonin and cortisol continued. After the 40-hour CR ended, each participant slept at their habitual Portugal time. After awakening, the participant was discharged.
Data analysis and comparisons
Serum melatonin and cortisol levels were measured as previously described (22). Data from the two IGD participants were compared with data from healthy older people from two different inpatient protocols that included CR in the same IPM facility under the same screening and inpatient procedures (23, 24). All of these control participants were healthy by history, physical examination, laboratory results on blood and urine, electrocardiogram, and psychological screening. None were using any prescription medication, over-the-counter medicines, caffeine, tobacco, and alcohol in the 3 weeks before and throughout their inpatient study. For melatonin and CBT, data were available from 23 (17 females, 6 males) healthy older participants ages 65–81 years whose CR lasted approximately 40 hours. Cortisol data were available in a subset of 16 of those individuals (23). For the neurobehavioral performance testing, data were available from seven healthy older participants (three females, four males) ages 60–71 years whose CR lasted 28 or 52 hours (24). Data were averaged by 2-hour bins relative to the start of the CR for each control and IGD individual. These data were then averaged across all healthy control individuals but were reported separately for the two IGD individuals. For data collected during CR conditions: 1) CBT data were analyzed using a two-harmonic regression analysis with autocorrelation (25) for circadian phase and amplitude; 2) cortisol data were analyzed by three-harmonic regression analysis (26); and 3) melatonin data were analyzed using a method that includes two-harmonic regression (26). Because this study included only two IGD participants, summary statistical analysis was not performed on their data.
Results
Clinical summary of study participants
IGD participant 1, a 67-year-old man, had severe KS (cross-cultural University of Pennsylvania Smell Identification test score, 4/12) and had presented with microphallus and complete absence of puberty. At the time of his study, he was otherwise well and his only medication was T replacement. IGD participant 2 was the younger sibling of participant 1 and was a 62-year-old woman. She had normosmic idiopathic hypogonadotropic hypogonadism (cross-cultural University of Pennsylvania Smell Identification test score, 11/12) and had presented with primary amenorrhea and complete absence of puberty at the age of 18. At the time of the current study, she was on no medications and was otherwise healthy. Detailed reproductive phenotypes and genetic analysis of both IGD participants have been reported previously (12).
Circadian rhythms in melatonin, cortisol, CBT, and sleep
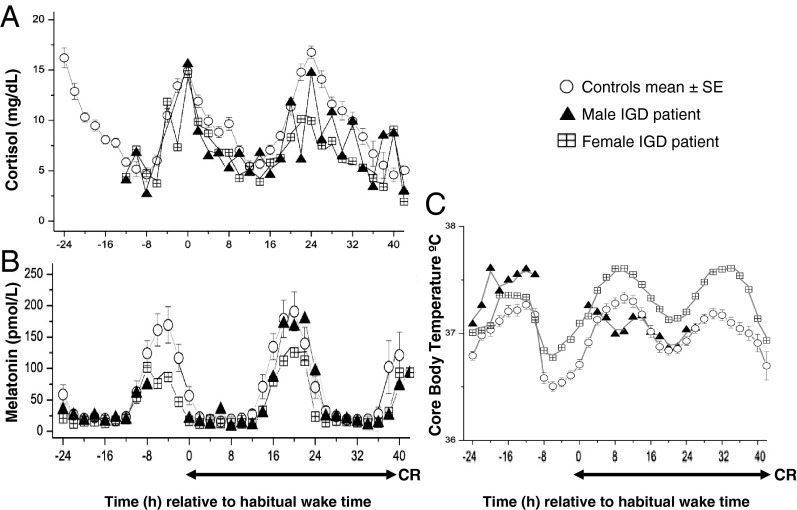
The timing and amplitude of the peak and troughs as well as the individual circadian waveforms of cortisol (Figure 1A) and melatonin (Figure 1B) in both IGD participants were similar to controls. CBT during the CR was similar to control participants in the female IGD participant. CBT data in the male IGD participant were similar to controls at the beginning of the study, but data points were unreliable during the end of the CR due to failure of the rectal temperature probe, and these data points are not shown (Figure 1C). Total sleep time and slow-wave sleep parameters in both IGD participants were similar to control participants (data not shown).
Figure 1.
Melatonin (A) and cortisol (B) levels and CBT (C) referenced to each participant's habitual wake time. The duration of the CR is also shown. Data for individual IGD participants are shown as triangles (male IGD participant) and checkered squares (female IGD participant), and each data point represents averaged 2-hour bins. CBT data for the male IGD participant (C, triangles) is incomplete due to failure of the temperature probe. In the healthy controls, data of each participant were averaged across 2-hour bins and then averaged across all control participants. The mean ± SEM of averaged 2-hour bins for the entire control group is shown in circles.
Neurobehavioral performance testing
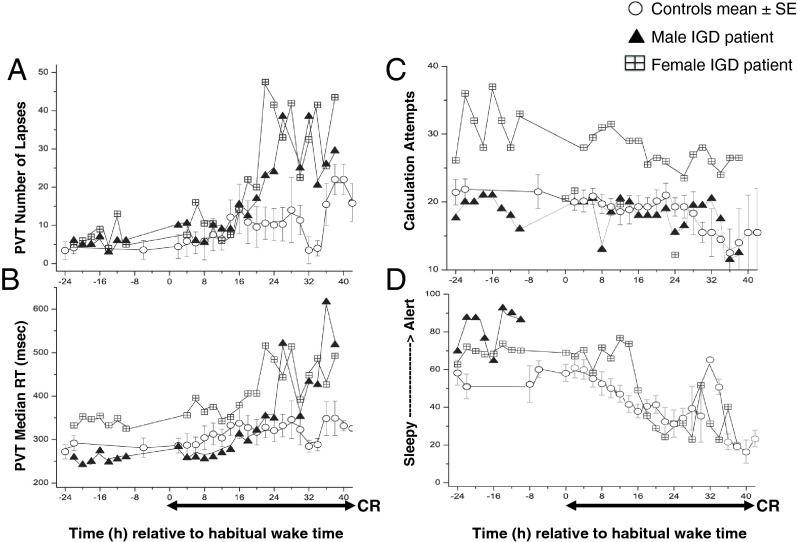
In both IGD participants, PVT lapses (Figure 2A) and median reaction time (Figure 2B) showed a trend for disproportionate worsening in the second half of the CR. The number of calculation attempts in the male IGD participant was similar to controls. The female IGD participant showed a consistently higher number of calculation attempts both before and during the CR; however, the circadian rhythmicity was maintained (Figure 2C). Complete subjective alertness measures were available only for the female IGD participant and were similar to controls (Figure 2D).
Figure 2.
PVT lapses (A), median reaction time (RT) (B), calculation attempts (C), and alertness scale (alert —> sleepy) (D) referenced to each participant's habitual wake time. The duration of the CR is also shown. Data for individual IGD participants are shown as triangles (male IGD participant) and checkered squares (female IGD participant), and each data point represents averaged 2-hour bins. In the healthy controls, data for each participant were averaged across 2-hour bins and then averaged across all control participants. The mean ± SEM of averaged 2-hour bins for the entire control group is shown in circles.
Discussion
Using a CR protocol that controls for known masking effects on circadian markers, we show that central rhythms of validated circadian phase markers (melatonin, cortisol, and CBT) are intact in humans with LOF PROK2 mutations, suggesting that PROK2 signaling is not a major determinant of central circadian pacemaker activity in humans. However, circadian neurobehavioral function is impaired in these participants, suggesting that PROK2 may be involved in the transmission of circadian timing information to at least some neurobehavioral neural networks. To date, causative mutations in core clock genes or their transcriptional machinery have been identified in a few Mendelian families with circadian rhythm sleep disorders (27). Likewise, genome-wide association studies have identified some core clockwork genes as contributors to selected circadian phenotypes (28). Thus far, SCN output genes have not been linked to any monogenic or polygenic circadian phenotypes. This study demonstrates that the lack of PROK2, a putative SCN output molecule, does not produce any central circadian abnormalities.
The preserved central circadian core pacemaker rhythms in humans with PROK2 mutations in contrast to attenuated rhythms in Prok2−/− mice warrant further consideration of potential mechanisms. It is possible that either induction of PROK1 during development or stochastic developmental/redundant adaptations in other SCN output pathways (eg, vasopressin/TGF-α) may compensate for the failure of PROK2 signaling, at least for some SCN output pathways. Moreover, accessory feedback loops are known to confer stability and robustness to the circadian system in other mammals (29), and similar networks may operate among the SCN output molecules, thus compensating for the loss of the PROK2 signaling. Species-specific differences between mice and humans may also explain the absence of central circadian abnormalities in humans. Mice with homozygous null alleles in Prok2 (12) and Prokr2 (13) show fully penetrant hypogonadotropic hypogonadism and olfactory bulb aplasia. In contrast, although most homozygous mutations in PROK2 and PROKR2 produce a severe KS phenotype (30), the female IGD participant in this study carrying a homozygous LOF PROK2 mutation is normosmic, suggesting variable penetrance of the olfactory phenotype in humans, as has been shown in several PROK2/PROKR2 pedigrees (16, 30). This variable expressivity of phenotypes may partly explain some of the discordance seen in the circadian phenotypes in mice compared to humans.
In humans, the PVT shows robust performance changes in response to sleep loss and adverse circadian phase (31) and predicts impairment in other tasks requiring sustained attention such as simulated driving (32). Despite a progressive increase in homeostatic sleep pressure with each cumulative hour awake, performance tends to stabilize or improve in healthy controls at approximately 10 hours and again 34 hours after habitual wake time, a time when the circadian pacemaker maximally promotes arousal (33). The blunting of this protective effect of the circadian arousal signal on neurobehavioral performance in the PROK2-null participants (Figure 2, A and B) despite preserved melatonin and cortisol rhythms suggests that PROK2 may be important in transmitting circadian timing information to at least some neural networks involved in neurobehavioral function.
The current study is limited by a small sample size (two participants). However, given the highly significant molecular defect in both participants and the comprehensive circadian phase evaluation using the CR, the results demonstrate important qualitative information regarding the PROK2 signaling system. In keeping with our observations on cortisol levels, Sarfati et al (30) also demonstrated normal cortisol levels collected every 4 hours over 24 hours in homozygous (n = 1) and as well heterozygous participants (n = 4) with PROK2/PROKR2 mutations, thus providing further validation to the current study. Although the current study results suggest that PROK2 may regulate some neurobehavioral circadian rhythms, it is possible that the neurobehavioral abnormalities seen in this study either relate to impaired homeostatic (ie, noncircadian) regulation of sleep or wakefulness or are secondary to the overseas travel undertaken by the IGD participants. However, the studies in both the domestic control participants and the IGD patients were timed relative to each participant's habitual sleep time to minimize circadian misalignment from overseas travel. Additionally, the IGD participants' flights to Boston occurred during their habitual wake time, and the CR started after two nocturnal sleep episodes at their habitual time. Therefore, homeostatic influences of the travel schedule were kept to a minimum. Finally, in this study, both IGD participants were over 60 years old, and studying younger PROK2/PROKR2 participants may uncover circadian defects during other developmental time points (eg, during adolescence).
In conclusion, we show that humans with homozygous complete LOF mutations in PROK2 do not display discernible circadian phase and amplitude abnormalities in melatonin, cortisol, or CBT, indicating that PROK2 signaling is not required for an intact central circadian pacemaker activity in the SCN. However, neurobehavioral testing of the PROK2-null participants reveals blunting of the protective effect of the circadian arousal signal on neurobehavioral performance, suggesting that PROK2 signaling may be important in the transmission of circadian timing to some neurobehavioral networks.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 HD15788 and U54 HD028138 (to W.F.C.), K02-HD045459 (to E.B.K.), P01-AG009975, RC2-HL101340, K24-HL105664 (to E.B.K.), T32-HLO701–10 (to D.A.C.), and M01-RR-02635.
Current address for D.A.C.: Department of Neurology, Sentara Medical Group, Norfolk, VA 23507. Current address for A.A.D. and N.P.: Endocrine, Diabetes, and Metabolism Service, Centre Hospitalier Universitaire Vaudois, 1011 Lausanne, Switzerland.
Disclosure Summary: W.F.C. is a consultant for Quest and Athena Diagnostics. E.B.K. is supported for clinical work but is not the site-responsible investigator on a multi-center trial supported by Vanda Pharmaceuticals. C.A.C. has received consulting fees from or served as a paid member of scientific advisory boards for: Bombardier, Inc.; Boston Red Sox; Boston Celtics; Cephalon, Inc. (acquired by Teva Pharmaceutical Industries Ltd. October 2011); Michael Jackson's mother and children; Koninklijke Philips Electronics, N.V.; Novartis; United Parcel Service (UPS); and Vanda Pharmaceuticals, Inc.; and Zeo Inc. C.A.C. owns an equity interest in Lifetrac, Inc.; Somnus Therapeutics, Inc.; and Vanda Pharmaceuticals, Inc., and received royalties from McGraw Hill, Penguin Press/Houghton Mifflin Harcourt, and Philips Respironics, Inc. C.A.C. has also received research support from Cephalon, National Football League Charities, ResMed and Philips Respironics. C.A.C. is the incumbent of an endowed professorship provided to Harvard University by Cephalon, Inc. and holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker). Since 1985, C.A.C. has also served as an expert witness on various legal cases related to sleep and/or circadian rhythms. The other authors have nothing to declare.
Footnotes
- CBT
- core body temperature
- CR
- constant routine
- IGD
- isolated GnRH deficiency
- KS
- Kallmann syndrome
- LOF
- loss-of-function
- PROK2
- prokineticin 2
- PROKR2
- PROK2 receptor
- PVT
- psychomotor vigilance task
- SCN
- suprachiasmatic nucleus.
References
- 1. Czeisler CA, Klerman EB. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res. 1999;54:97–130; discussion 130–132 [PubMed] [Google Scholar]
- 2. Boden MJ, Kennaway DJ. Circadian rhythms and reproduction. Reproduction. 2006;132:379–392 [DOI] [PubMed] [Google Scholar]
- 3. Lowrey PL, Takahashi JS. Genetics of circadian rhythms in mammalian model organisms. Adv Genet. 2011;74:175–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437:1257–1263 [DOI] [PubMed] [Google Scholar]
- 5. Li JD, Hu WP, Boehmer L, et al. Attenuated circadian rhythms in mice lacking the prokineticin 2 gene. J Neurosci. 2006;26:11615–11623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cheng MY, Bullock CM, Li C, et al. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature. 2002;417:405–410 [DOI] [PubMed] [Google Scholar]
- 7. Hu WP, Li JD, Zhang C, Boehmer L, Siegel JM, Zhou QY. Altered circadian and homeostatic sleep regulation in prokineticin 2-deficient mice. Sleep. 2007;30:247–256 [PMC free article] [PubMed] [Google Scholar]
- 8. Prosser HM, Bradley A, Chesham JE, Ebling FJ, Hastings MH, Maywood ES. Prokineticin receptor 2 (Prokr2) is essential for the regulation of circadian behavior by the suprachiasmatic nuclei. Proc Natl Acad Sci USA. 2007;104:648–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ren P, Zhang H, Qiu F, et al. Prokineticin 2 regulates the electrical activity of rat suprachiasmatic nuclei neurons. PLoS One. 2011;6:e20263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang C, Truong KK, Zhou QY. Efferent projections of prokineticin 2 expressing neurons in the mouse suprachiasmatic nucleus. PLoS One. 2009;4:e7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yuill EA, Hoyda TD, Ferri CC, Zhou QY, Ferguson AV. Prokineticin 2 depolarizes paraventricular nucleus magnocellular and parvocellular neurons. Eur J Neurosci. 2007;25:425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pitteloud N, Zhang C, Pignatelli D, et al. Loss-of-function mutation in the prokineticin 2 gene causes Kallmann syndrome and normosmic idiopathic hypogonadotropic hypogonadism. Proc Natl Acad Sci USA. 2007;104:17447–17452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsumoto S, Yamazaki C, Masumoto KH, et al. Abnormal development of the olfactory bulb and reproductive system in mice lacking prokineticin receptor PKR2. Proc Natl Acad Sci USA. 2006;103:4140–4145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dodé C, Teixeira L, Levilliers J, et al. Kallmann syndrome: mutations in the genes encoding prokineticin-2 and prokineticin receptor-2. PLoS Genet. 2006;2:e175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cole LW, Sidis Y, Zhang C, et al. Mutations in prokineticin 2 and prokineticin receptor 2 genes in human gonadotrophin-releasing hormone deficiency: molecular genetics and clinical spectrum. J Clin Endocrinol Metab. 2008;93:3551–3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Martin C, Balasubramanian R, Dwyer AA, et al. The role of the prokineticin 2 pathway in human reproduction: evidence from the study of human and murine gene mutations. Endocr Rev. 2011;32:225–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines? J Biol Rhythms. 2002;17:4–13 [DOI] [PubMed] [Google Scholar]
- 18. Bond AJ, Lader MH. The use of analogue scales in rating subjective feelings. Br J Med Psychol. 1974;47:211–218 [Google Scholar]
- 19. Klein KE, Wegmann HM, Brüner H. Circadian rhythm in indices of human performance, physical fitness and stress resistance. Aerosp Med. 1968;39:512–518 [PubMed] [Google Scholar]
- 20. Dinges DF, Powell JW. Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Behav Res Methods Instrum Comput. 1985;17:652–655 [Google Scholar]
- 21. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques, and Scoring System for Sleep Stages of Human Subjects. Washington, DC: Public Health Service, US Government Printing Service; 1968 [Google Scholar]
- 22. Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythms. 2002;17:181–193 [DOI] [PubMed] [Google Scholar]
- 23. Klerman EB, Duffy JF, Dijk DJ, Czeisler CA. Circadian phase resetting in older people by ocular bright light exposure. J Investig Med. 2001;49:30–40 [DOI] [PubMed] [Google Scholar]
- 24. Klerman EB, Dijk DJ. Age-related reduction in the maximal capacity for sleep—implications for insomnia. Curr Biol. 2008;18:1118–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7:177–202 [DOI] [PubMed] [Google Scholar]
- 26. Czeisler CA, Duffy JF, Shanahan TL, et al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181 [DOI] [PubMed] [Google Scholar]
- 27. Jones CR, Huang AL, Ptáček LJ, Fu YH. Genetic basis of human circadian rhythm disorders. Exp Neurol. 2013;243:28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang L, Jones CR, Ptacek LJ, Fu YH. The genetics of the human circadian clock. Adv Genet. 2011;74:231–247 [DOI] [PubMed] [Google Scholar]
- 29. Baggs JE, Price TS, DiTacchio L, Panda S, Fitzgerald GA, Hogenesch JB. Network features of the mammalian circadian clock. PLoS Biol. 2009;7:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sarfati J, Guiochon-Mantel A, Rondard P, et al. A comparative phenotypic study of Kallmann syndrome patients carrying monoallelic and biallelic mutations in the prokineticin 2 or prokineticin receptor 2 genes. J Clin Endocrinol Metab. 2010;95:659–669 [DOI] [PubMed] [Google Scholar]
- 31. Cohen DA, Wang W, Wyatt JK, et al. Uncovering residual effects of chronic sleep loss on human performance. Sci Transl Med. 2010;2:14ra13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Arnedt JT, Owens J, Crouch M, Stahl J, Carskadon MA. Neurobehavioral performance of residents after heavy night call vs after alcohol ingestion. JAMA. 2005;294:1025–1033 [DOI] [PubMed] [Google Scholar]
- 33. Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–3538 [DOI] [PMC free article] [PubMed] [Google Scholar]