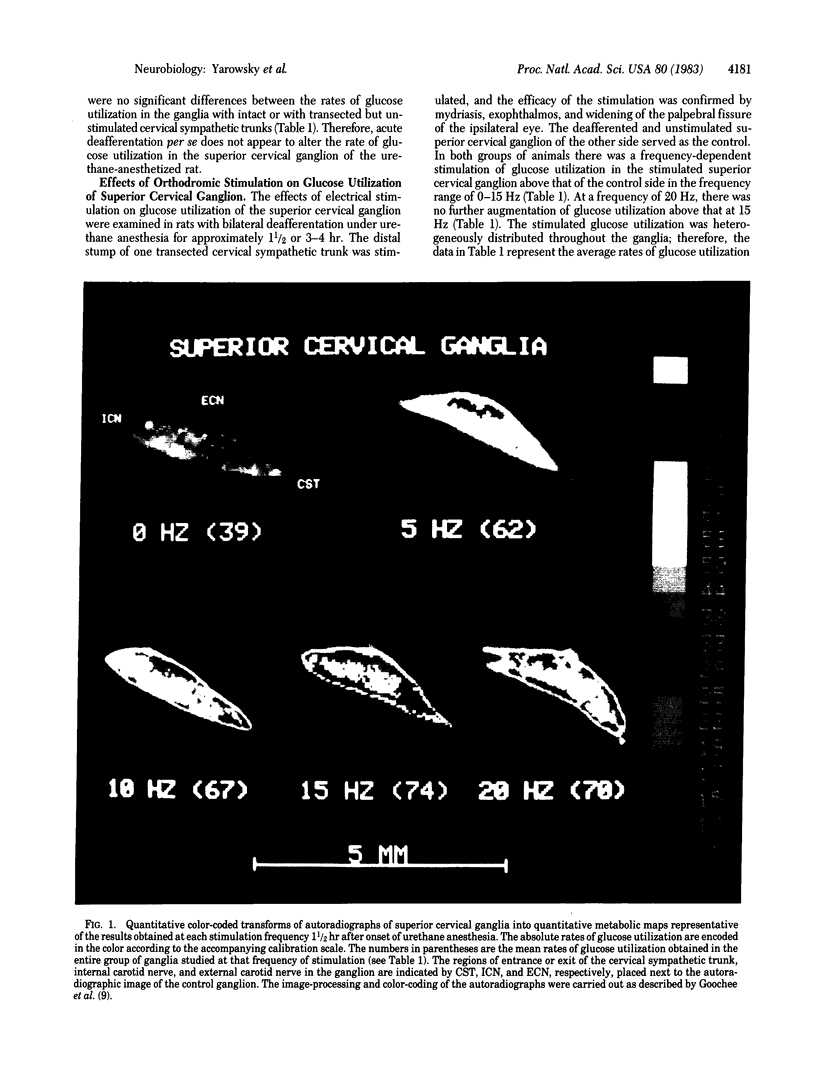
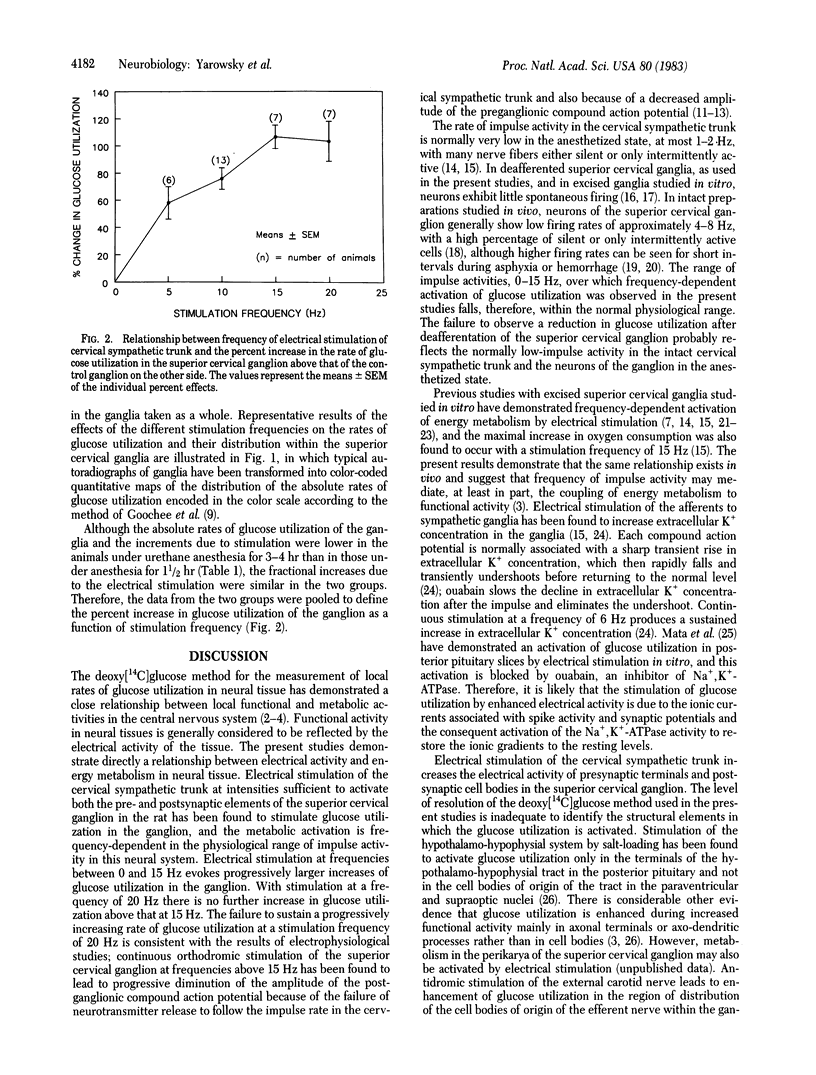
Abstract
Electrical stimulation of the distal stump of the transected cervical sympathetic trunk produces a frequency-dependent activation of glucose utilization, measured by the deoxy[14C]glucose method, in the superior cervical ganglion of the urethane-anesthetized rat. The frequency dependence falls between 0-15 Hz; at 20 Hz the activation of glucose utilization is no greater than at 15 Hz. Deafferentation of the superior cervical ganglion is transection of the cervical sympathetic trunk does not diminish the rate of glucose utilization in the ganglion in the urethane-anesthetized rat. These results indicate that the rate of energy metabolism in an innervated neural structure is, at least in part, regulated by the impulse frequency of the electrical input to the structure, and this regulation may be an essential component of the mechanism of the coupling of metabolic activity to functional activity in the nervous system.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birks R. I. A long-lasting potentiation of transmitter release related to an increase in transmitter stores in a sympathetic ganglion. J Physiol. 1977 Oct;271(3):847–862. doi: 10.1113/jphysiol.1977.sp012028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers C. W., Zigmond R. E. Localization of neurons in the rat superior cervical ganglion that project into different postganglionic trunks. J Comp Neurol. 1979 May 15;185(2):381–391. doi: 10.1002/cne.901850211. [DOI] [PubMed] [Google Scholar]
- CHUNGCHAROEN D., DE BURGH DALY M., SCHWEITZER A. The blood supply of the superior cervical sympathetic and the nodose ganglia in cats, dogs and rabbits. J Physiol. 1952 Dec;118(4):528–536. doi: 10.1113/jphysiol.1952.sp004814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOLIVO M., LARRABEE M. G. Metabolism of glucose and oxygen in a mammalian sympathetic ganglion at reduced temperature and varied pH. J Neurochem. 1958 Oct;3(1):72–88. doi: 10.1111/j.1471-4159.1958.tb12611.x. [DOI] [PubMed] [Google Scholar]
- Galvan M., Bruggencate G. T., Senekowitsch R. The effects of neuronal stimulation and ouabain upon extracellular K+ and Ca2+ levels in rat isolated sympathetic ganglia. Brain Res. 1979 Jan 19;160(3):544–548. doi: 10.1016/0006-8993(79)91084-9. [DOI] [PubMed] [Google Scholar]
- Goochee C., Rasband W., Sokoloff L. Computerized densitometry and color coding of [14C] deoxyglucose autoradiographs. Ann Neurol. 1980 Apr;7(4):359–370. doi: 10.1002/ana.410070414. [DOI] [PubMed] [Google Scholar]
- Green J. H., Heffron P. F. Studies upon patterns of activity in single post-ganglionic sympathetic fibres. Arch Int Pharmacodyn Ther. 1968 May;173(1):232–243. [PubMed] [Google Scholar]
- Härkönen M. H., Passonneau J. V., Lowry O. H. Relationships between energy reserves and function in rat superior cervical ganglion. J Neurochem. 1969 Oct;16(10):1439–1450. doi: 10.1111/j.1471-4159.1969.tb09896.x. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G. Oxygen consumption of excised sympathetic ganglia at rest and in activity. J Neurochem. 1958;2(2-3):81–101. doi: 10.1111/j.1471-4159.1958.tb12355.x. [DOI] [PubMed] [Google Scholar]
- LARRABEE M. G., POSTERNAK J. M. Selective action of anesthetics on synapses and axons in mammalian sympathetic ganglia. J Neurophysiol. 1952 Mar;15(2):91–114. doi: 10.1152/jn.1952.15.2.91. [DOI] [PubMed] [Google Scholar]
- Mata M., Fink D. J., Gainer H., Smith C. B., Davidsen L., Savaki H., Schwartz W. J., Sokoloff L. Activity-dependent energy metabolism in rat posterior pituitary primarily reflects sodium pump activity. J Neurochem. 1980 Jan;34(1):213–215. doi: 10.1111/j.1471-4159.1980.tb04643.x. [DOI] [PubMed] [Google Scholar]
- McAfee D. A., Yarowsky P. J. Calcium-dependent potentials in the mammalian sympathetic neurone. J Physiol. 1979 May;290(2):507–523. doi: 10.1113/jphysiol.1979.sp012787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Casella C. Nervous transmission in the superior cervical ganglion of the thiamine-deficient rat. Q J Exp Physiol Cogn Med Sci. 1970 Jan;55(1):25–35. doi: 10.1113/expphysiol.1970.sp002047. [DOI] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Casella C. Synaptically mediated potentials elicited by the stimulation of post-ganglionic trunks in the guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):55–67. doi: 10.1007/BF00587046. [DOI] [PubMed] [Google Scholar]
- Polosa C. Spontaneous activity of sympathetic preganglionic neurons. Can J Physiol Pharmacol. 1968 Nov;46(6):887–896. doi: 10.1139/y68-138. [DOI] [PubMed] [Google Scholar]
- Schwartz W. J., Smith C. B., Davidsen L., Savaki H., Sokoloff L., Mata M., Fink D. J., Gainer H. Metabolic mapping of functional activity in the hypothalamo-neurohypophysial system of the rat. Science. 1979 Aug 17;205(4407):723–725. doi: 10.1126/science.462184. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Localization of functional activity in the central nervous system by measurement of glucose utilization with radioactive deoxyglucose. J Cereb Blood Flow Metab. 1981;1(1):7–36. doi: 10.1038/jcbfm.1981.4. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. Relation between physiological function and energy metabolism in the central nervous system. J Neurochem. 1977 Jul;29(1):13–26. doi: 10.1111/j.1471-4159.1977.tb03919.x. [DOI] [PubMed] [Google Scholar]
- Sokoloff L. The F.O. Schmitt Lecture in Neuroscience 1980. The relationship between function and energy metabolism: its use in the localization of functional activity in the nervous system. Neurosci Res Program Bull. 1981 May;19(2):159–207. [PubMed] [Google Scholar]
- Toga A. W., Collins R. C. Metabolic response to optic centers to visual stimuli in the albino rat: anatomical and physiological considerations. J Comp Neurol. 1981 Jul 10;199(4):443–464. doi: 10.1002/cne.901990402. [DOI] [PubMed] [Google Scholar]
- Zigmond R. E., Chalazonitis A. Long-term effects of preganglionic nerve stimulation on tyrosine hydroxylase activity in the rat superior cervical ganglion. Brain Res. 1979 Mar 23;164:137–152. doi: 10.1016/0006-8993(79)90011-8. [DOI] [PubMed] [Google Scholar]