Abstract
Context:
Denosumab is a humanized monoclonal antibody to receptor activator of nuclear factor-κB ligand used primarily for postmenopausal osteoporosis and skeletal-related events from metastatic cancer. Its safety in children has not been established.
Objective:
The objective of the study was to investigate the effects of denosumab treatment on skeletal growth and histology.
Design:
This was an observational case report with radiological and histopathological analyses.
Setting:
The study was conducted at a clinical research center.
Patients:
A 9-year-old boy with fibrous dysplasia treated with a 7-month course of denosumab participated in the study.
Intervention:
Histological analyses were performed and compared on growth plates from limbs that had been amputated before and 17 months after denosumab treatment.
Main Outcome Measures:
Skeletal radiographs and bone histopathology from before and after treatment were measured.
Results:
After initiating denosumab, sclerotic metaphyseal bands appeared on radiographs. Posttreatment radiographs revealed migration of the bands away from the growth plates, consistent with continued linear growth. Histologically, the bands were composed of horizontally arranged trabeculae containing calcified cartilage. This cartilage appeared to derive from unresorbed primary spongiosa as a result of osteoclast inhibition by denosumab, similar to what has been observed with bisphosphonates. By 17 months after treatment, active bone resorption and formation had returned, as evidenced by the presence of active osteoclasts in resorption pits and osteoid surfaces.
Conclusions:
Further studies are needed to determine the safety of denosumab on the growing skeleton. However, in this child there was continued epiphyseal activity both during and after treatment and reversal of bone turnover suppression after treatment discontinuation, suggesting that denosumab did not have significant adverse effects on growth.
Denosumab is a novel antiresorptive agent that targets osteoclasts through the inhibition of receptor activator of nuclear factor-κB ligand (RANKL), an osteogenic factor essential for osteoclast formation, function, and survival (1). In postmenopausal women, denosumab leads to increased bone mineral density and reduced fracture risk (2). Denosumab has also shown efficacy in the treatment of giant cell tumors (3) and adults with bone metastases (4). More recently, its use has been reported in children with fibrous dysplasia (FD) (5), osteogenesis imperfecta type IV (6), hypercalcemia (7), and giant cell tumor (8). In adults, denosumab appears to be relatively well tolerated, with a safety profile similar to bisphosphonates (9, 10). Its safety in children has not been established.
In children, denosumab carries additional theoretical risks to linear growth and bone modeling. Bisphosphonates, which also target osteoclasts, have been used in children for many years for conditions including osteogenesis imperfecta (11), FD (12), juvenile osteoporosis (13), and other disorders associated with low bone density. Although the long-term safety of bisphosphonate use in children has not been established, short-term data suggest they are well tolerated without significant effects on skeletal growth (14). Despite sharing similar cellular targets, bisphosphonates and denosumab have widely different mechanisms of action. Bisphosphonates are synthetic analogs of inorganic pyrophosphate that act by binding to the mineral component of bone and incorporating into osteoclast metabolism, inhibiting osteoclast activity and recruitment, and inducing osteoclast apoptosis. In contrast, denosumab specifically inhibits the RANKL/receptor activator of nuclear factor-κB pathway, inhibiting osteoclast formation and function (15). A key difference between the two drugs is the tissue half-life. Bisphosphonates have a half-life on the order of 10 years (16), whereas the half-life of denosumab is significantly shorter at less than 30 days (17).
Our group previously reported the use of denosumab in a child with FD (5). In this report, we describe the skeletal effects of denosumab treatment and discontinuation in this child.
Clinical Patient and Methods
Clinical history
A 9-year-old boy presented with rapidly expanding FD in the left femur. Of note, the patient's right leg had previously been amputated due to a similarly expansive lesion. Additional details are available in a previously published report (5). In brief, denosumab was initiated in an attempt to slow expansion and prevent the amputation of his remaining lower extremity. Significant reductions in bone turnover markers, FD expansion, and FD-related bone pain were observed. The patient received treatment at monthly intervals, for a total of three doses at 1 mg/kg, three doses at 1.25 mg/kg, and one dose at 1.5 mg/kg, dosing that was modified from studies in giant cell tumors (3). The patient developed secondary hyperparathyroidism and hypophosphatemia during the treatment period. While on treatment, the patient sustained a left femoral fracture after falling out of bed, and denosumab was halted after the seventh dose. Severe hypercalcemia to 4.5 mmol/L (18 mg/dL) occurred after denosumab cessation. He was treated with pamidronate 3 mg/kg divided into four doses, and a single dose of zoledronic acid 0.075 mg/kg, over a total period of 9 weeks, after which the hypercalcemia resolved. Denosumab thus demonstrated potential efficacy in treatment of FD but was associated with significant adverse effects on mineral metabolism both during and after treatment.
After resolution of hypercalcemia, the patient had no further electrolyte imbalances. Bone turnover markers declined and remained near baseline. The FD lesion in the patient's left femur began to expand rapidly and bone pain returned. Seventeen months after denosumab discontinuation, the family declined further denosumab treatment and instead underwent a disarticulation amputation of his left lower extremity at the femoral-iliac joint. Under an institutional review board-approved protocol, informed consent was obtained to study the bone specimens from the pre- and postdenosumab treatment amputations. This provided a unique opportunity to examine the effects of denosumab on the growing skeleton.
Radiographs
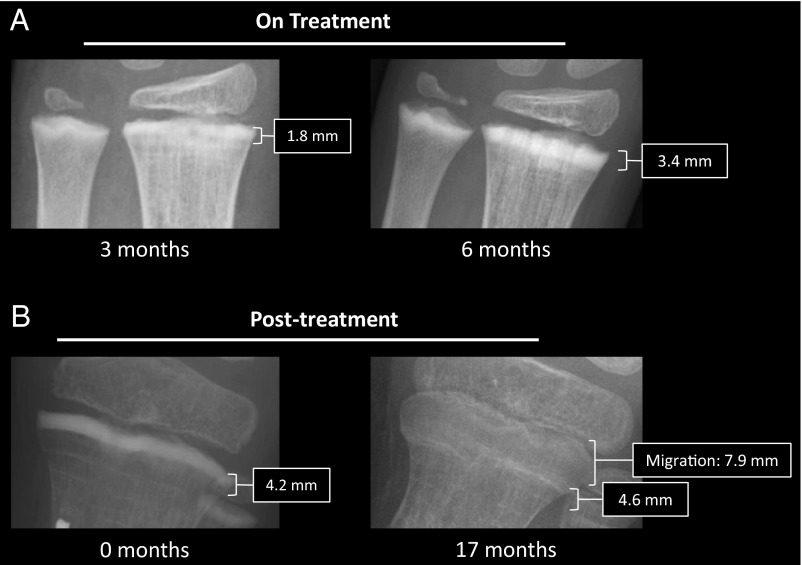
Radiographs taken before, during, and after treatment were studied. Pretreatment radiographs revealed extensive FD involvement in the tibia, fibula, and femur. After initiation of denosumab, thick sclerotic bands developed at the level of the metaphyses (Figure 1A). Seventeen months after stopping denosumab, the bands had migrated toward the diaphysis and appeared more translucent, suggesting that linear growth and remodeling had taken place (Figure 1B). There were no significant changes in bone morphology adjacent to the region of the sclerotic bands.
Figure 1.
Radiographs. A, Radiographic images of the patient's left distal radius and ulna during monthly denosumab treatment. The image on the left was taken after three doses of denosumab, and the image on the right was taken after 6 months of treatment. Thick, sclerotic bands developed adjacent to the growth plate, which increased in width from approximately 1.8–3.4 mm over the course of treatment, indicating ongoing bone formation at the growth plate. B, Posttreatment radiograph of the proximal tibia taken shortly after denosumab discontinuation demonstrates an approximate 4.2-mm sclerotic band (left panel). Seventeen months later the band, now approximately 4.6 mm, has migrated approximately 7.9 mm from the epiphysis and become more radiolucent (right panel), again demonstrating growth over this time period.
Denosumab was discontinued after the patient sustained a midshaft fracture of his left femur near the end of an intramedullary rod. By 8 weeks after the injury and plate fixation, evidence of fracture repair with callus formation was apparent on radiographs. The fracture continued to heal without complication, suggesting that the processes involved in fracture healing, including cartilage resorption and secondary bone remodeling, were not significantly impaired during this period immediately after denosumab cessation.
Histopathology
Surgical specimens from the left (postdenosumab) femur, tibia, and fibula were obtained. Radiographic images of the bone specimens were taken using a Faxitron X-ray machine (Faxitron Bioptics) prior to their fixation in 4% paraformaldehyde. A subset of the specimens were decalcified and embedded in paraffin, whereas the remaining specimens were left undecalcified and embedded in methyl methacrylate.
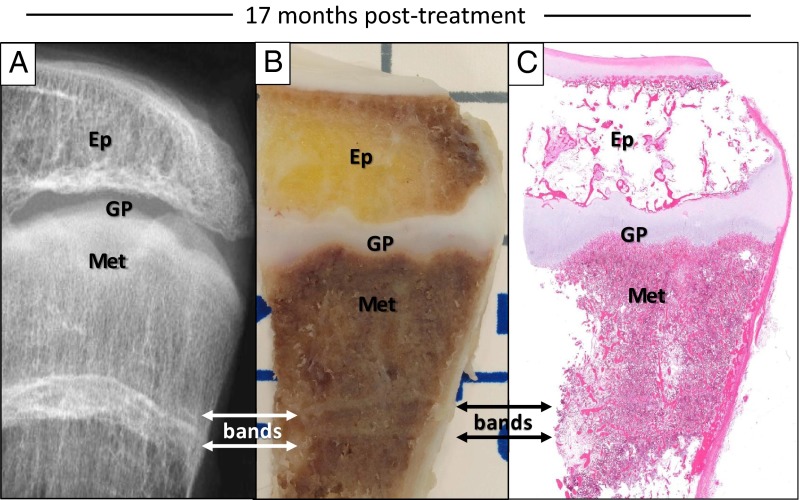
Inspection of the distal tibia at 17 months after denosumab treatment revealed that the sclerotic bands were visible grossly and histologically (Figure 2, A–C). A hematoxylin-eosin-stained section demonstrated that the bands were the result of an increased amount of horizontally arranged trabeculae (Figure 2C), consistent with the radiodense appearance.
Figure 2.
Sclerotic bands. A, A radiographic image of the surgically removed distal tibia 17 months after discontinuation of denosumab taken with a Faxitron X-ray machine demonstrates a transverse, sclerotic band (bands with arrows) located in the metaphysis (Met). Ep, epiphysis; GP, growth plate. B, The band on the radiograph was also visible on a photograph of a gross specimen taken from the distal tibia as two distinct bands located approximately 2 cm away from the growth plate. C, In an hematoxylin-eosin-stained section from the same specimen in panel B, transverse sclerotic bands were composed of increased amounts of trabeculae, organized into a horizontally oriented pattern.
In a bone specimen from the first amputation (prior to denosumab treatment), calcified cartilage was found only within the primary spongiosa located adjacent to the growth plate (Figure 3, A–D). In contrast, after denosumab treatment, substantial amounts of calcified cartilage were present in the primary spongiosa and the region of the bands, which was greater than 2 cm from the epiphysis (Figure 4, A–D). This retained cartilage surrounded by normal-appearing trabecular bone in the bands region can be clearly visualized on Goldner's trichrome-stained section (Figure 5, A and B) and was further confirmed to be cartilage by toluidine blue staining for proteoglycans (Figure 5, C and D). Von Kossa staining of an undecalcified section revealed complete mineralization of the trabeculae making up the bands, including the areas corresponding to the embedded cartilage (Figure 5, E and F). This confirmed that the retained cartilage was calcified.
Figure 3.
Predenosumab histopathology. A, An hematoxylin-eosin-stained section of the patient's growth plate prior to the initiation of denosumab at the time of his first amputation showed a region with a normal-appearing growth plate (GP), trabecular bone (TB), and bone marrow (M). Calcified cartilage is present only within the primary spongiosa (PS) at the osteochondral junction. Fibrous tissue (FT) can be seen in this specimen, consistent with extensive fibrous dysplasia involvement. B, Higher magnification of the osteochondral junction (upper box in panel A) demonstrated the presence of tongues of calcified cartilage (CC) within the primary spongiosa and active osteoclasts (yellow arrow). C, Higher magnification of a region away from the growth plate (lower box in panel A) revealed normal trabecular bone (TB) with no evidence of calcified cartilage. D, High-power view of osteoclasts (yellow arrows) actively resorbing primary spongiosa.
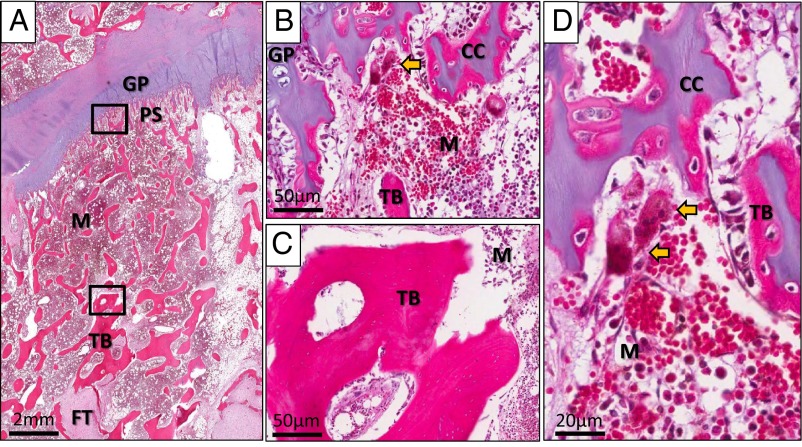
Figure 4.
Postdenosumab histopathology. A, An hematoxylin-eosin-stained section of the patient's growth plate taken 17 months after discontinuation of denosumab at the time of his second amputation showed sclerotic bands (bands with arrows) distant from the growth plate (GP). Calcified cartilage was present not only in the primary spongiosa (PS) but also within trabeculae in the region of the sclerotic bands. B, On high magnification of the osteochondral junction (upper box in panel A), calcified cartilage (CC) was found within the primary spongiosa. Also present were actively resorbing osteoclasts (yellow arrows) located adjacent to blood vessels (BV). C, Higher magnification of the bands (lower box in panel A) revealed islands of retained calcified cartilage (CC) within the trabecular bone (TB). An active osteoclast within a resorption pit can be seen in this area as well (yellow arrow). A, adipose tissue; M, normal marrow. D, A Goldner's trichrome-stained section taken from the same specimen at the osteochondral junction (upper box in panel A) again demonstrated the presence of calcified cartilage (CC) within the primary spongiosa. It also revealed actively resorbing osteoclasts (yellow arrows) and the presence of osteoid surfaces (black arrow), suggesting active bone formation and resorption.
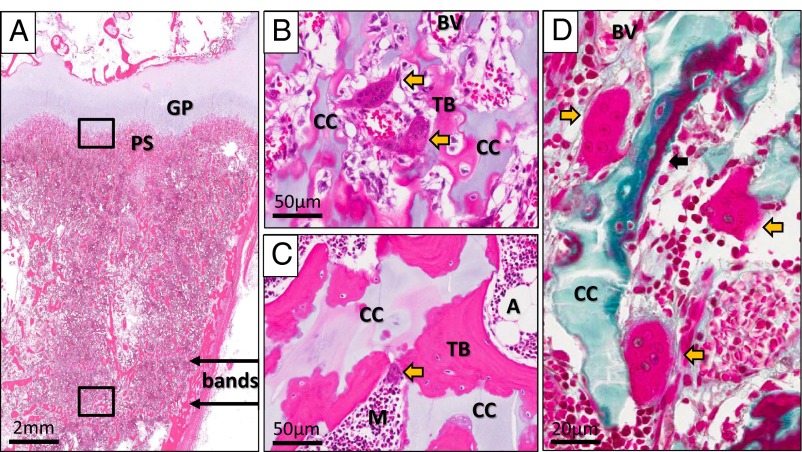
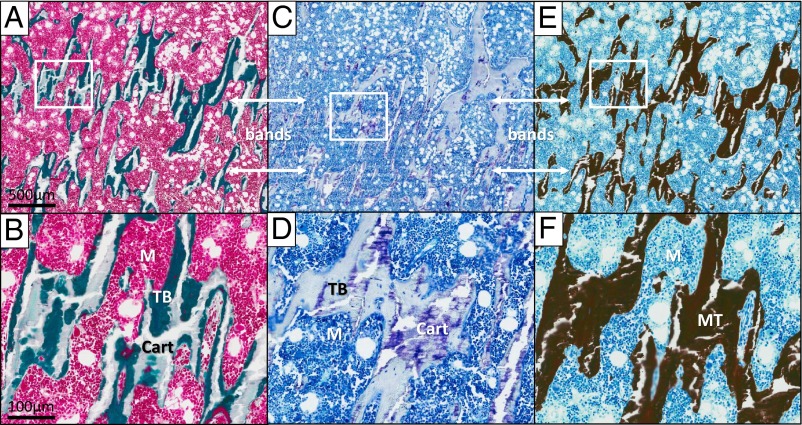
Figure 5.
Histopathology of the bands. A and B, Goldner's trichrome staining of a nondecalcified section of the patient's left distal tibia. A, Low magnification of a region containing the bands (bands with arrows). B, Higher magnification revealed islands of cartilage (Cart) stained light green embedded within trabecular bone (TB) stained dark green that make up the bands. M, normal bone marrow. C and D, Toluidine blue staining of a nondecalcified section from the same specimen. C, Low magnification of the bands region demonstrated retained cartilage (stained purple) within the bands. D, Positive staining for aggrecans (purple), a major component of cartilage, can be clearly visualized within the trabecular bone. E and F, Von Kossa staining of an adjacent section from the same specimen. E, Low magnification demonstrated that the bands are composed of mineralized tissue (stained black). F, Higher magnification showed that the previously detected islands of cartilage within the trabecular bone were also stained black, suggesting that they are calcified cartilage. MT, mineralized tissue.
The retained calcified cartilage most likely represents remnants of unresorbed primary spongiosa. During endochondral ossification, primary spongiosa is typically removed via osteoclast resorption near the osteochondral junction. This process can be seen in the pretreatment sections (Figure 3D). Although previous studies have reported that osteoclasts were essentially absent during denosumab treatment (18), abundant osteoclasts with normal morphology were present near the osteochondral junction by 17 months after denosumab cessation (Figure 4B). Osteoclasts were also present near the trabeculae at the bands and may be acting to resorb the retained cartilage (Figure 4C). Additionally, the presence of osteoid surface suggests active bone formation (Figure 4D).
Discussion
Seven months of denosumab treatment in a child with FD led to the radiographic appearance of transverse sclerotic bands parallel to the growth plates, similar to what has been observed with bisphosphonate use (19). In children treated with bisphosphonates, histological analyses of growth plates revealed retention of calcified cartilage, which is hypothesized to represent horizontal trabeculae formed during the temporary inhibition of epiphyseal activity (20). Rauch et al (20) noted that bands in a child treated with pamidronate contained decreasing amounts of calcified cartilage as they progressed farther from the growth plate. In a larger analysis, these transverse lines persisted for an average of 4 years before disappearing on radiographs, demonstrating that the bands undergo remodeling over time (21).
In our patient, denosumab treatment led to a similar skeletal phenotype with sclerotic bands parallel to the growth plate and retained primary spongiosa. Because denosumab and bisphosphonates both inhibit osteoclast function, the same underlying mechanism may explain the observed skeletal effects with denosumab. The bands in our patient were uninterrupted and lengthened during treatment. This is similar to what is seen in children treated with continuous oral bisphosphonates and is likely reflective of the relatively short interval between doses, which was adapted from the protocol used to treat giant cell tumors (3). Whether these effects have significant clinical implications is uncertain. Experience with bisphosphonate use in children is more extensive, and few complications related to the sclerotic lines have been reported. However, the consequences of long-term bone turnover suppression on the growing skeleton have not been determined. Abnormalities in bone modeling at the distal radius have been reported after bisphosphonate discontinuation in children with osteogenesis imperfecta (22). Concern has been raised that bisphosphonate discontinuation may lead to an increased fracture risk at the interface between newly formed bone and the denser transverse bands; however, this remains largely theoretical (22). Osteopetrosis has been reported with the use of long-term, high-dose bisphosphonate therapy in children (23), which has persisted for years after discontinuation (24). Although raising caution for the use of antiresorptive therapy in children, this adverse effect has been limited to very high dosages, well above the level recommended for most indications.
Effects on linear growth are an important consideration in any bone targeting agent used in children. Bisphosphonate therapy has not been reported to adversely affect linear growth, even with long-term use (14). There are no published reports on the effect of denosumab on linear growth in children, and the growth effects of RANKL inhibition have not been determined. In an animal model, RANKL knockout mice developed severe osteopetrosis with growth retardation (25). Monitoring linear growth in our patient was technically challenging; we are thus unable to definitively determine the effect of denosumab on linear growth in this child. There was no increase in measured arm span over a 6-month period, which may represent impaired linear growth as a complication of treatment. However, 6 months is possibly an insufficient period of time for determination of growth velocity, and measurement of arm span in this patient was difficult due to pain and impaired mobility. Migration of the transverse bands away from the growth plate after the cessation of therapy is evidence that growth plates are active and that linear growth continued during this period. Additional studies in larger numbers of children are needed to quantify the effects of denosumab on linear growth, however, these findings that denosumab may not have significant inhibitory growth effects are encouraging. The presence of FD in this child may potentially impact the generalizability of these findings. However, given that FD is a mosaic disease and the areas of the metaphyses analyzed represent non-FD bone, the histological changes are unlikely to be significantly impacted by his underlying skeletal disease.
Osteoclasts play an important role in callus formation and fracture healing. Because of a theoretical risk of impaired fracture healing, denosumab treatment was halted in our patient after he sustained a femoral fracture. His fracture healed without complications, suggesting that prior denosumab treatment did not negatively impact fracture healing. This observation is consistent with a preclinical study of murine facture healing, in which osteoprotegerin, the endogenous molecule that denosumab mimics, caused delayed resorption of cartilage but did not compromise biomechanical properties of the healing fractures (26). A large, more recent analysis found no increase in fracture healing complications in patients who had received denosumab for osteoporosis (27). The rapid reversal of the denosumab effects after treatment cessation likely also contributed to our patient's unimpaired fracture healing.
Denosumab and bisphosphonates have very different durations of action. Bisphosphonates embed and accumulate in bone, and effects persist long after treatment is discontinued. In contrast, denosumab remains in the extracellular fluid and has a more rapid offset of action. Histomorphometrical analysis of iliac crest biopsies from a large number of patients treated with denosumab demonstrated a significant reduction in the number of osteoclasts, eroded surface, and osteoid surface while on treatment (18). In our patient, 17 months after stopping denosumab, active bone resorption and formation were present on histological analysis, signaling the reversal of denosumab inhibition of bone turnover. Bone turnover markers were elevated above baseline shortly after denosumab discontinuation and returned to baseline after 9 weeks and bisphosphonate administration (5). This is consistent with previous reports of a rebound in bone turnover after denosumab discontinuation (28) and suggests that denosumab-induced bone turnover suppression can be reversed rapidly after treatment is stopped.
Our patient received two doses of pamidronate at age 4 years (0.5 and 1 mg/kg, respectively, given 3 months apart) and a short course of bisphosphonates for treatment of hypercalcemia after denosumab discontinuation. It is unclear to what extent this proportionately lower dose of bisphosphonates contributed to the observed epiphyseal changes. Given denosumab's considerably shorter half-life, its skeletal effects are expected to be shorter in duration compared with bisphosphonates. This is supported by preclinical data, in which cynomolgus monkeys treated with doses of denosumab 50 times greater than the highest doses used in clinical protocols developed only minor growth plate changes, which resolved after treatment cessation (29, 30). The continued epiphyseal activity observed in our patient is consistent with this preclinical data. If the patient's bisphosphonate exposure had significantly impacted his growth plate histology, it would conceivably have attenuated the observed reversal of bone turnover suppression.
Conclusion
In this child with FD, denosumab treatment led to retention of primary spongiosa and development of transverse sclerotic metaphyseal lines. Similar findings have been observed with bisphosphonate use in children without significant clinical implications. Active fracture healing and epiphyseal activity occurred during and after discontinuation of treatment, suggesting that denosumab effects on linear growth may not be significant. Furthermore, resumption of normal remodeling of the primary spongiosa by at least 17 months after treatment demonstrates that the effects of denosumab on the growth plate can be reversed relatively quickly upon cessation of therapy. Although additional studies are needed to determine its safety in the pediatric population, denosumab holds promise as a treatment for skeletal diseases in children.
Acknowledgments
The author contributions were as follows: study design, H.D.W., A.M.B., J.Y.T., A.A.M., and M.T.C.; study conduct, H.D.W., A.M.B., J.Y.T., R.I.G., F.A.F., J.Z.K.-V., A.A.M., and M.T.C.; data collection, H.D.W., A.M.B., J.Y.T., A.A.M., and M.T.C.; data analysis, H.D.W., A.M.B., A.A.M., and M.T.C.; data interpretation, H.D.W., A.M.B., A.A.M., and M.T.C.; drafting the manuscript, H.D.W., A.M.B., and M.T.C.; revising the manuscript content, H.D.W., A.M.B., A.A.M., and M.T.C.; approving the final version of the manuscript, H.D.W., A.M.B., J.Y.T., R.I.G., F.A.F., J.Z.K.-V., A.A.M., and M.T.C. M.T.C. takes responsibility for the integrity of the data analyses.
This work was supported by the intramural research program of the National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services, and was additionally supported in part by the Medical Research Scholars Program, a public-private partnership supported jointly by the National Institutes of Health and generous contributions to the Foundation for the National Institutes of Health from Pfizer Inc, The Leona M. and Harry B. Helmsley Charitable Trust, and the Howard Hughes Medical Institute as well as other private donors.
Disclosure Summary: The authors have nothing to declare.
Footnotes
- FD
- fibrous dysplasia
- RANKL
- receptor activator of nuclear factor-κB ligand.
References
- 1. Lacey DL, Boyle WJ, Simonet WS, et al. Bench to bedside: elucidation of the OPG-RANK-RANKL pathway and the development of denosumab. Nat Rev Drug Discov. 2012;11:401–419 [DOI] [PubMed] [Google Scholar]
- 2. Cummings SR, San Martin J, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361:756–765 [DOI] [PubMed] [Google Scholar]
- 3. Thomas D, Henshaw R, Skubitz K, et al. Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol. 2010;11:275–280 [DOI] [PubMed] [Google Scholar]
- 4. Fizazi K, Lipton A, Mariette X, et al. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571 [DOI] [PubMed] [Google Scholar]
- 5. Boyce AM, Chong WH, Yao J, et al. Denosumab treatment for fibrous dysplasia. J Bone Miner Res. 2012;27:1462–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Semler O, Netzer C, Hoyer-Kuhn H, Becker J, Eysel P, Schoenau E. First use of the RANKL antibody denosumab in osteogenesis imperfecta type VI. J Musculoskelet Neuronal Interact. 2012;12:183–188 [PubMed] [Google Scholar]
- 7. Shroff R, Beringer O, Rao K, Hofbauer LC, Schulz A. Denosumab for post-transplantation hypercalcemia in osteopetrosis. N Engl J Med. 2012;367:1766–1767 [DOI] [PubMed] [Google Scholar]
- 8. Karras NA, Polgreen LE, Ogilvie C, Manivel JC, Skubitz KM, Lipsitz E. Denosumab treatment of metastatic giant-cell tumor of bone in a 10-year-old girl. J Clin Oncol. 2013;31:e200–e202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brown JP, Prince RL, Deal C, et al. Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24:153–161 [DOI] [PubMed] [Google Scholar]
- 10. Papapoulos S, Chapurlat R, Libanati C, et al. Five years of denosumab exposure in women with postmenopausal osteoporosis: results from the first two years of the FREEDOM extension. J Bone Miner Res. 2012;27:694–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Glorieux FH, Bishop NJ, Plotkin H, Chabot G, Lanoue G, Travers R. Cyclic administration of pamidronate in children with severe osteogenesis imperfecta. N Engl J Med. 1998;339:947–952 [DOI] [PubMed] [Google Scholar]
- 12. Matarazzo P, Lala R, Masi G, Andreo M, Altare F, de Sanctis C. Pamidronate treatment in bone fibrous dysplasia in children and adolescents with McCune-Albright syndrome. J Pediatr Endocrinol Metab. 2002;15(suppl 3):929–937 [PubMed] [Google Scholar]
- 13. Hoekman K, Papapoulos SE, Peters AC, Bijvoet OL. Characteristics and bisphosphonate treatment of a patient with juvenile osteoporosis. J Clin Endocrinol Metab. 1985;61:952–956 [DOI] [PubMed] [Google Scholar]
- 14. Zeitlin L, Rauch F, Plotkin H, Glorieux FH. Height and weight development during four years of therapy with cyclical intravenous pamidronate in children and adolescents with osteogenesis imperfecta types I, III, and IV. Pediatrics. 2003;111:1030–1036 [DOI] [PubMed] [Google Scholar]
- 15. Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48:677–692 [DOI] [PubMed] [Google Scholar]
- 16. Cremers SC, Pillai G, Papapoulos SE. Pharmacokinetics/pharmacodynamics of bisphosphonates: use for optimisation of intermittent therapy for osteoporosis. Clin Pharmacokinet. 2005;44:551–570 [DOI] [PubMed] [Google Scholar]
- 17. Yonemori K, Fujiwara Y, Minami H, et al. Phase 1 trial of denosumab safety, pharmacokinetics, and pharmacodynamics in Japanese women with breast cancer-related bone metastases. Cancer Sci. 2008;99:1237–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reid IR, Miller PD, Brown JP, et al. Effects of denosumab on bone histomorphometry: the FREEDOM and STAND studies. J Bone Miner Res. 2010;25:2256–2265 [DOI] [PubMed] [Google Scholar]
- 19. van Persijn van Meerten EL, Kroon HM, Papapoulos SE. Epi- and metaphyseal changes in children caused by administration of bisphosphonates. Radiology. 1992;184:249–254 [DOI] [PubMed] [Google Scholar]
- 20. Rauch F, Travers R, Munns C, Glorieux FH. Sclerotic metaphyseal lines in a child treated with pamidronate: histomorphometric analysis. J Bone Miner Res. 2004;19:1191–1193 [DOI] [PubMed] [Google Scholar]
- 21. Land C, Rauch F, Glorieux FH. Cyclical intravenous pamidronate treatment affects metaphyseal modeling in growing patients with osteogenesis imperfecta. J Bone Miner Res. 2006;21:374–379 [DOI] [PubMed] [Google Scholar]
- 22. Rauch F, Cornibert S, Cheung M, Glorieux FH. Long-bone changes after pamidronate discontinuation in children and adolescents with osteogenesis imperfecta. Bone. 2007;40:821–827 [DOI] [PubMed] [Google Scholar]
- 23. Whyte MP, Wenkert D, Clements KL, McAlister WH, Mumm S. Bisphosphonate-induced osteopetrosis. N Engl J Med. 2003;349:457–463 [DOI] [PubMed] [Google Scholar]
- 24. Whyte MP, McAlister WH, Novack DV, Clements KL, Schoenecker PL, Wenkert D. Bisphosphonate-induced osteopetrosis: novel bone modeling defects, metaphyseal osteopenia, and osteosclerosis fractures after drug exposure ceases. J Bone Miner Res. 2008;23:1698–1707 [DOI] [PubMed] [Google Scholar]
- 25. Kong YY, Yoshida H, Sarosi I, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323 [DOI] [PubMed] [Google Scholar]
- 26. Gerstenfeld LC, Sacks DJ, Pelis M, et al. Comparison of effects of the bisphosphonate alendronate versus the RANKL inhibitor denosumab on murine fracture healing. J Bone Miner Res. 2009;24:196–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adami S, Libanati C, Adachi J, et al. Denosumab administration is not associated with fracture healing complications in postmenopausal women with osteoporosis: results from the FREEDOM trial. J Bone Miner Res. 2010;25:MO0405 [Google Scholar]
- 28. Bone HG, Bolognese MA, Yuen CK, et al. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972–980 [DOI] [PubMed] [Google Scholar]
- 29. Bussiere J BR, Pyrah I, Branstetter D, et al. Enhanced pre-postnatal toxicity study of AMG 162 administered by SC injection to pregnant cynomolgus monkeys with up to 6-months postnatal evaluation. Paper presented at: 52nd Annual Meeting of the Society of Toxicology; 2013; San Antonio, TX (abstract) [Google Scholar]
- 30. Bussiere JL. A 6/12-month toxicity study of denosumab in cynomolgus monkeys. J Bone Miner Res. 2012;27(suppl 1). http://wwwasbmrorg/meetings/annualmeeting/abstractdetailaspx?aid=cac1502d-a2de-4466-b0c2-7b8c61685eee Accessed June 29, 2013 [Google Scholar]