Abstract
To examine whether a decline in follicular oocyte maturation inhibitor (OMI) is associated with attainment of oocyte maturation and fertilizability, OMI was measured in follicular fluid (FF) of 39 follicles of 20 normal women given human menopausal gonadotrophin and human chorionic gonadotrophin to induce follicular growth and maturation. Oocytes were aspirated per laparoscope, the fluid was saved, and the egg was observed, incubated, and inseminated with the husband's sperm. Concepti that developed to the 4- to 8-cell stage were transferred to the uterus and the women were followed for pregnancy. OMI activity in each FF was measured by using cultured cumulus-enclosed porcine oocytes (30-40 oocytes per FF sample). Estrogen, progesterone, oocytes (30-40 oocytes per FF sample). Estrogen, progesterone, and delta 4-androstenedione were measured in FF by radioimmunoassay. The FF of 13 preovulatory follicles yielding oocytes that were mature and fertilizable had significantly less OMI activity (mean +/- SEM) (0.58 +/- 0.10 unit/ml) compared to follicles yielding immature oocytes (2.8 +/- 0.56 units/ml; n = 9), atretic oocytes (5.5 +/- 2.5 units/ml; n = 7), or preovulatory oocytes with fractured zonae (1.9 +/- 0.63 units/ml; n = 7). The estrogen concentration (mean +/- SEM) of preovulatory follicles yielding mature fertilizable eggs or mature eggs with fractured zonae was greater (396 +/- 34 ng/ml; n = 20) compared to follicles yielding immature or atretic eggs (203 +/- 59 ng/ml; n = 9 and 97 +/- 47 ng/ml; n = 7, respectively; P less than 0.05). Progesterone concentration (mean +/- SEM; ng/ml) of FF was generally elevated in all preovulatory follicles (635 +/- 53) compared to immature or atretic follicles (230 +/- 64 and 76 +/- 17, respectively; P less than 0.05). It may be concluded that in normal follicle maturation there is a decline in OMI in the follicle containing an oocyte that becomes mature and fertilizable. There is also an increase in estrogen, progesterone, and follicle size. It is also possible to have an abnormal follicle maturation when there is an increase in size as well as FF, estrogen, and progesterone, but withut a decline in OMI--a situation which can lead to production of a nonfertilizable oocyte.
Full text
PDF
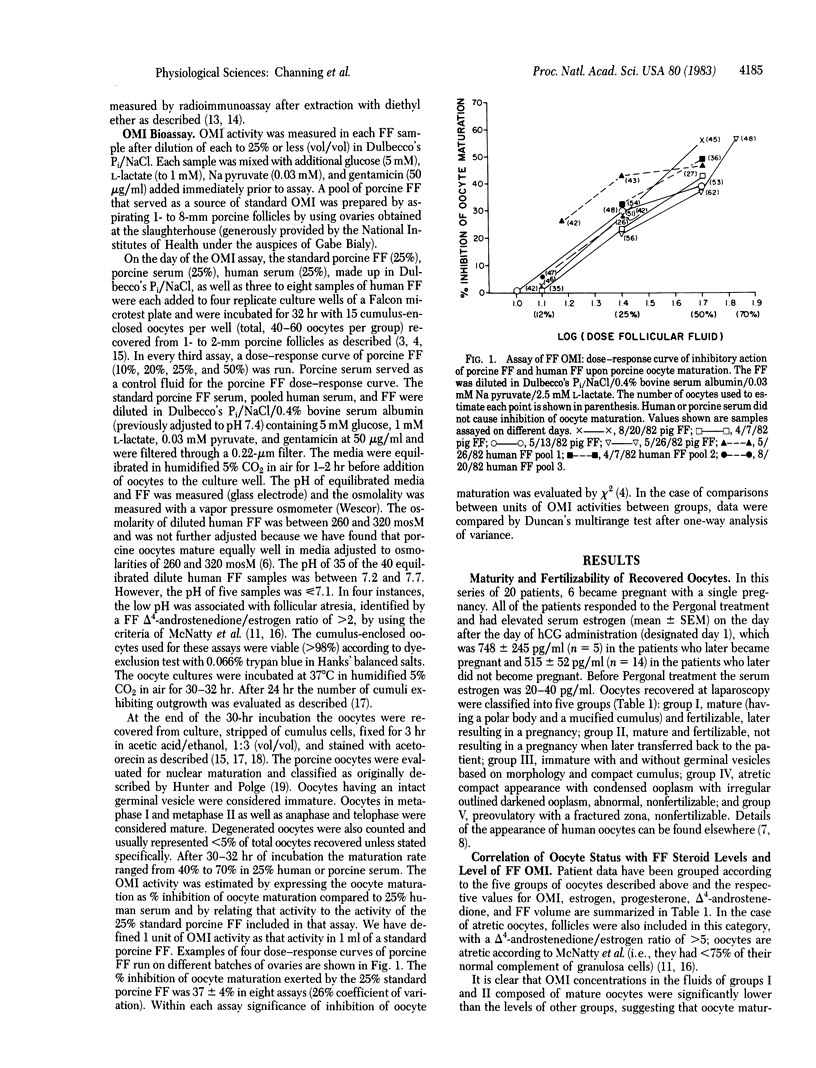
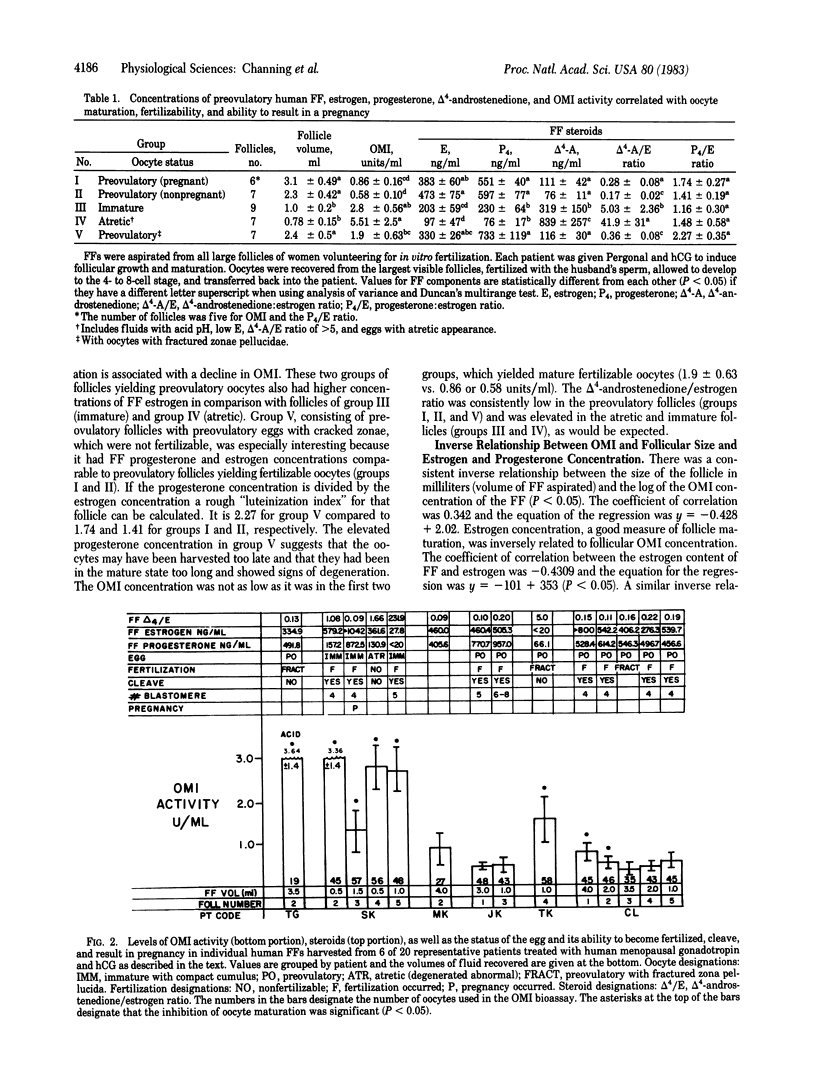
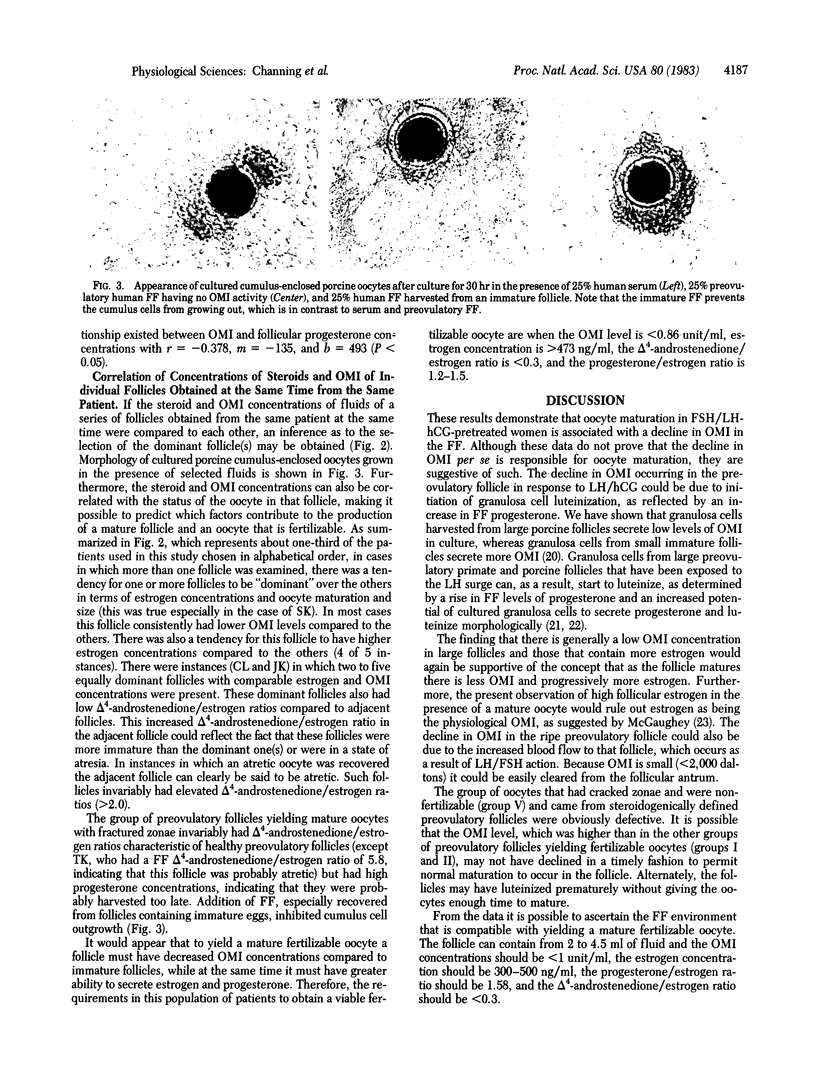

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brailly S., Gougeon A., Milgrom E., Bomsel-Helmreich O., Papiernik E. Androgens and progestins in the human ovarian follicle: differences in the evolution of preovulatory, healthy nonovulatory, and atretic follicles. J Clin Endocrinol Metab. 1981 Jul;53(1):128–134. doi: 10.1210/jcem-53-1-128. [DOI] [PubMed] [Google Scholar]
- Carson R. S., Trounson A. O., Findlay J. K. Successful fertilisation of human oocytes in vitro: concentration of estradiol-17 beta, progesterone and androstenedione in the antral fluid of donor follicles. J Clin Endocrinol Metab. 1982 Oct;55(4):798–800. doi: 10.1210/jcem-55-4-798. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Anderson L. D., Hoover D. J., Kolena J., Osteen K. G., Pomerantz S. H., Tanabe K. The role of nonsteroidal regulators in control of oocyte and follicular maturation. Recent Prog Horm Res. 1982;38:331–408. doi: 10.1016/b978-0-12-571138-8.50014-7. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Brinkley H. J., Young E. P. Relationship between serum luteinizing hormone levels and the ability of porcine granulosa cells to luteinize and respond to exogenous luteinizing hormone in culture. Endocrinology. 1980 Jan;106(1):317–322. doi: 10.1210/endo-106-1-317. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Coudert S. P. Contribution of granulosa cells and follicular fluid to ovarian estrogen secretion in rhesus monkey in vivo. Endocrinology. 1976 Mar;98(3):590–597. doi: 10.1210/endo-98-3-590. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Gagliano P., Hoover D. J., Tanabe K., Batta S. K., Sulewski J., Lebech P. Relationship between human follicular fluid inhibin F activity and steroid content. J Clin Endocrinol Metab. 1981 Jun;52(6):1193–1198. doi: 10.1210/jcem-52-6-1193. [DOI] [PubMed] [Google Scholar]
- Channing C. P. Progesterone and estrogen secretion by cultured monkey ovarian cell types: influences of follicular size, serum luteinizing hormone levels, and follicular fluid estrogen levels. Endocrinology. 1980 Jul;107(1):342–352. doi: 10.1210/endo-107-1-342. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Tsafriri A. Mechanism of action of luteinizing hormone and follicle-stimulating hormone on the ovary in vitro. Metabolism. 1977 Apr;26(4):413–468. doi: 10.1016/0026-0495(77)90108-1. [DOI] [PubMed] [Google Scholar]
- Channing C. P., Tsai V., Sachs D. Role of insulin, thyroxin and cortisol in luteinization of porcine granulosa cells grown in chemically defined media. Biol Reprod. 1976 Sep;15(2):235–247. doi: 10.1095/biolreprod15.2.235. [DOI] [PubMed] [Google Scholar]
- Edwards R. G., Steptoe P. C., Purdy J. M. Fertilization and cleavage in vitro of preovulator human oocytes. Nature. 1970 Sep 26;227(5265):1307–1309. doi: 10.1038/2271307a0. [DOI] [PubMed] [Google Scholar]
- Garcia J. E., Jones G. S., Wright G. L., Jr Prediction of the time of ovulation. Fertil Steril. 1981 Sep;36(3):308–315. [PubMed] [Google Scholar]
- Hillensjö T., Batta S. K., Schwartz-Kripner A., Wentz A. C., Sulewski J., Channing C. P. Inhibitory effect of human follicular fluid upon the maturation of porcine oocytes in culture. J Clin Endocrinol Metab. 1978 Dec;47(6):1332–1335. doi: 10.1210/jcem-47-6-1332. [DOI] [PubMed] [Google Scholar]
- Hillensjö T., Pomerantz S. H., Schwartz-Kripner A., Anderson L. D., Channing C. P. Inhibition of cumulus cell progesterone secretion by low molecular weight fractions of porcine follicular fluid which also inhibit oocyte maturation. Endocrinology. 1980 Feb;106(2):584–591. doi: 10.1210/endo-106-2-584. [DOI] [PubMed] [Google Scholar]
- Hunter R. H., Polge C. Maturation of follicular oocytes in the pig after injection of human chorionic gonadotrophin. J Reprod Fertil. 1966 Dec;12(3):525–531. doi: 10.1530/jrf.0.0120525. [DOI] [PubMed] [Google Scholar]
- Jones H. W., Jr, Acosta A. A., Garcia J. A technique for the aspiration of oocytes from human ovarian follicles. Fertil Steril. 1982 Jan;37(1):26–29. doi: 10.1016/s0015-0282(16)45971-6. [DOI] [PubMed] [Google Scholar]
- Jones H. W., Jr, Jones G. S., Andrews M. C., Acosta A., Bundren C., Garcia J., Sandow B., Veeck L., Wilkes C., Witmyer J. The program for in vitro fertilization at Norfolk. Fertil Steril. 1982 Jul;38(1):14–21. [PubMed] [Google Scholar]
- Kraiem Z., Druker B., Lunefeld B. Inhibitory action of human follicular fluid on the ovarian accumulation of cyclic AMP. J Endocrinol. 1978 Jul;78(1):161–162. doi: 10.1677/joe.0.0780161. [DOI] [PubMed] [Google Scholar]
- Ledwitz-Rigby F., Rigby B. W. Follicular fluid stimulation of steroidogenesis in immature granulosa cells in vitro. Mol Cell Endocrinol. 1979 Apr;14(1):73–79. doi: 10.1016/0303-7207(79)90059-5. [DOI] [PubMed] [Google Scholar]
- McGaughey R. W. The maturation of porcine oocytes in minimal, defined culture media with varied macromolecular supplements and varied osmolarity. Exp Cell Res. 1977 Oct 1;109(1):25–30. doi: 10.1016/0014-4827(77)90040-4. [DOI] [PubMed] [Google Scholar]
- McNatty K. P. Hormonal correlates of follicular development in the human ovary. Aust J Biol Sci. 1981;34(3):249–268. doi: 10.1071/bi9810249. [DOI] [PubMed] [Google Scholar]
- McNatty K. P., Smith D. M., Makris A., Osathanondh R., Ryan K. J. The microenvironment of the human antral follicle: interrelationships among the steroid levels in antral fluid, the population of granulosa cells, and the status of the oocyte in vivo and in vitro. J Clin Endocrinol Metab. 1979 Dec;49(6):851–860. doi: 10.1210/jcem-49-6-851. [DOI] [PubMed] [Google Scholar]
- Moor R. M., Warnes G. M. Regulation of meiosis in mammalian oocytes. Br Med Bull. 1979 May;35(2):99–103. doi: 10.1093/oxfordjournals.bmb.a071578. [DOI] [PubMed] [Google Scholar]
- Seibel M. M., Smith D. M., Levesque L., Borten M., Taymor M. L. The temporal relationship between the luteinizing hormone surge and human oocyte maturation. Am J Obstet Gynecol. 1982 Mar 1;142(5):568–572. doi: 10.1016/0002-9378(82)90763-3. [DOI] [PubMed] [Google Scholar]
- Stone S. L., Pomerantz S. H., Schwartz-Kripner A., Channing C. P. Inhibitor of oocyte maturation from porcine follicular fluid: further purification and evidence for reversible action. Biol Reprod. 1978 Oct;19(3):585–592. doi: 10.1095/biolreprod19.3.585. [DOI] [PubMed] [Google Scholar]
- Trounson A. O., Mohr L. R., Wood C., Leeton J. F. Effect of delayed insemination on in-vitro fertilization, culture and transfer of human embryos. J Reprod Fertil. 1982 Mar;64(2):285–294. doi: 10.1530/jrf.0.0640285. [DOI] [PubMed] [Google Scholar]
- Tsafriri A., Channing C. P. An inhibitory influence of granulosa cells and follicular fluid upon porcine oocyte meiosis in vitro. Endocrinology. 1975 Apr;96(4):922–927. doi: 10.1210/endo-96-4-922. [DOI] [PubMed] [Google Scholar]
- Tsafriri A., Channing C. P. Influence of follicular maturation and culture conditions on the meiosis of pig oocytes in vitro. J Reprod Fertil. 1975 Apr;43(1):149–152. doi: 10.1530/jrf.0.0430149. [DOI] [PubMed] [Google Scholar]
- Tsafriri A., Pomerantz S. H., Channing C. P. Inhibition of oocyte maturation by porcine follicular fluid: partial characterization of the inhibitor. Biol Reprod. 1976 Jun;14(5):511–516. doi: 10.1095/biolreprod14.5.511. [DOI] [PubMed] [Google Scholar]