Abstract
The intestinal epithelium forms a stable barrier protecting underlying tissues from pathogens in the gut lumen. This is achieved by specialized integral membrane structures such as tight and adherens junctions that connect neighboring cells and provide stabilizing links to the cytoskeleton. Junctions are constantly remodeled to respond to extracellular stimuli. Assembly and disassembly of junctions is regulated by interplay of actin remodeling, endocytotic recycling of junctional proteins, and various signaling pathways. Accumulating evidence implicate small G proteins of the Ras superfamily as important signaling molecules for the regulation of epithelial junctions. They function as molecular switches circling between an inactive GDP-bound and an active GTP-bound state. Once activated, they bind different effector molecules to control cellular processes required for correct junction assembly, maintenance and remodelling. Here, we review recent advances in understanding how GTPases of the Rho, Ras, Rab and Arf families contribute to intestinal epithelial homeostasis.
Keywords: Rho, Rac, cdc42, Ras, Rap, Arf, inflammatory bowel disease, colitis, tight junction, adherens junction, actin cytoskeleton
Introduction
The intestinal epithelium separates intestinal tissues from the environment including the intestinal luminal microflora. It is a highly specialized cell layer characterized by tight intercellular contacts that prevent unwanted fluid leakage and penetration of pathogens.1 Disruption of this cellular barrier can lead to diarrhea or acute colitis and is also a hallmark of chronic inflammatory disorders of the intestines such as Crohn’s disease (CD) or ulcerative colitis (UC), also referred to as inflammatory bowel disease (IBD).2 The stability of the epithelial barrier is ensured by transmembrane adhesion molecules organized into structures called tight junctions, adherens junctions and desmosomes (Fig. 1).3,4 These structures also guarantee a strict polarization of the epithelium into apical and basal compartments by blocking intramembranal diffusion of plasma membrane components. The most apically localized junction is the tight junction followed immediately by adherens junctions. Together they are referred to as the apical junctional complex (AJC).5 Tight junctions (TJ) are comprised of transmembrane adhesion molecules of various protein families such as the claudins, junctional adhesion molecules (JAM) or occludin that are intracellularly linked to the actin cytoskeleton via various adaptor molecules such as members of the zonula occludens (ZO) family.6 Adherens junctions (AJ) are comprised mainly of E-cadherin that is connected to the stabilizing circumferential actin belt via adaptor molecules of the catenin family. Moreover, nectins are components of AJ.7 Desmosomes are found beneath AJ, are comprised of the desmosomal cadherins desmoglein and desmocollin and connected intracellularly to the keratin intermediate filament cytoskeleton via the adaptor molecules desmoplakin, plakophillin and plakoglobin.4 TJs are known to seal the paracellular pathway, whereas AJ and desmosomes rather provide the mechanical adhesive strength to maintain the cellular contacts and allow TJ assembly.8,9 The connection of junctional structures to the actin cytoskeleton is of vital importance for the stability of the epithelial barrier.10,11 Thus, the regulation not only of these structures themselves but of the interaction between them is crucial for the maintenance of epithelial integrity. Disruption of both junctions and cytoskeleton are key events during infection and inflammation leading to a breakdown of barrier functionality.12-14 Important signaling molecules involved in the regulation of actin dynamics and AJC stability are small GTPases.15,16 GTPases are molecular switches that cycle between an inactive GDP-bound state and an active GTP-bound state (Fig. 2). GTP binding leads to conformational changes allowing for the interaction of GTPases with their downstream effector molecules to regulate various important cellular processes.17 The activation loop of GTPases is regulated by three different classes of proteins: guanylate exchange factors (GEF) regulate the exchange of GDP for GTP and thus activate small GTPases; guanylate activating proteins (GAP) activate the intrinsic GTPase function to hydrolyze bound GTP and thus deactivate small GTPases; guanylate dissociation inhibitors sequester GDP-bound small GTPases and prevent them from being activated but also from being degraded.18,19 Misregulation of GTPase activation is associated with both junctional and cytoskeletal dysfunction. Since this can be observed in many pathological conditions including IBD, GTPases are important pharmacological targets.20,21 Activated GTPases bind to their effector molecules to stimulate cellular processes such as epithelial polarization and formation of junctions that have been reviewed elsewhere.15,22-25 Additionally, the maintenance of intestinal epithelial barrier integrity also relies on a GTPase-mediated fine-tuning of junctional and cytoskeletal dynamics.10,25 Impressive progress has been made recently in identifying mechanisms by which GTPases regulate intestinal epithelial barrier functions. In this review, we summarize common and unique mechanisms by which different small GTPases contribute to the regulation of intestinal barrier homeostasis and how dysregulation during infection or inflammation evokes pathologic conditions.
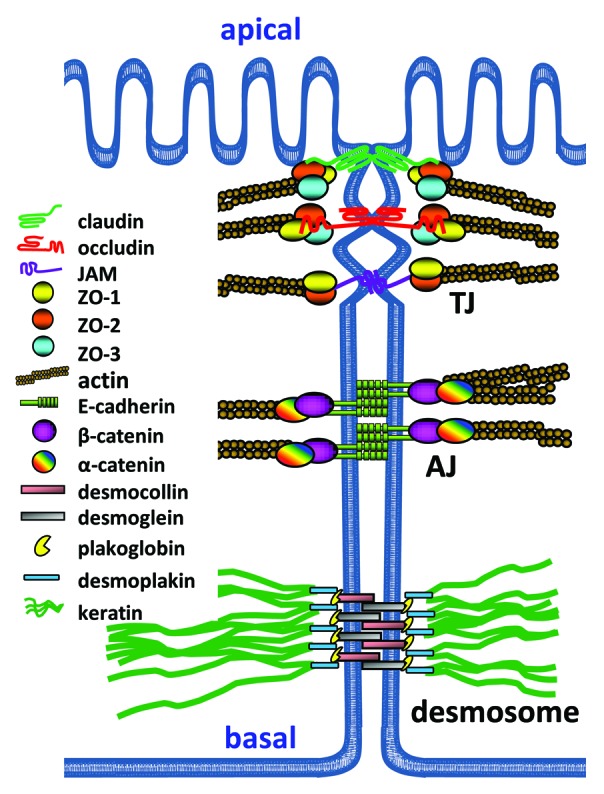
Figure 1. Polarized intestinal epithelium with intercellular junctions. The apical junctional complex, consisting of TJ and AJ, allows the establishment of cell polarization into apical and basaloteral compartments. The most apically localized junction is the TJ followed by AJ and desmosomes along the lateral cell-cell contacts. These structures have the same organization: 1) integral membrane proteins with heterophilic or homophilic interaction in cis (interaction with neighboring molecules within the same membrane) and in trans (interaction with proteins of the neighboring cell to form the actual cell contacts); 2) scaffold molecules which connect the integral membrane proteins with actin or intermediate filaments, and signaling molecules such as small GTPases and their GEFs and GAPs; and 3) the actin cytoskeleton or intermediate filaments that provide mechanical strength for the junctions.
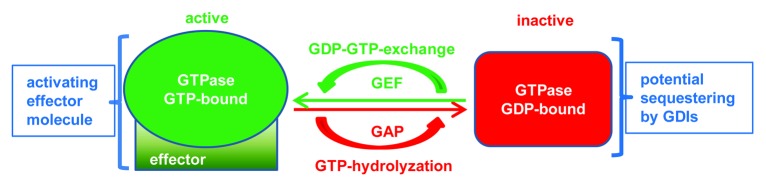
Figure 2. Regulation of small GTPases. Small GTPases of the Ras superfamily are mostly regulated via a cycle of GDP-for-GTP exchange regulated by GEFs and hydrolysis of bound GTP facilitated by GAPs. GDP-bound GTPases can be sequestered by GDIs to prevent their activation. Active GTPases interact and activate a plethora of different effector molecules to transduce the activating signal.
The Ras superfamily of small GTPases
The Ras superfamily of small GTPases consists of 6 subfamilies, namely Ras, Rho, Arf, Ran, Rab and Rheb.26 This superfamily of now more than 150 members is named after its protagonist Ras and plays a role in several crucial cellular processes. Most members of this superfamily share the ability of acting as molecular switches that circle between a quiescent GDP-bound state and an active GTP-bound state. However, some members of the Rho subfamily such as RhoE and RhoH do not possess intrinsic GTPase activity, are therefore constitutively GTP-bound and thought to be regulated by modulation of levels of expression or phosphorylation.27,28 The general domain structure of virtually all Ras superfamily GTPases consists of a guanine nucleotide binding site responsible for binding GDP and GTP, two flexible “switch” regions (SW1 and SW2) that undergo conformational changes to allow for binding to downstream effector proteins, and unique C-terminal domains that can be post-translationally modified by farnesylation or geranyl-geranylation to regulate the subcellular localization of the GTPase.18 By contrast, Arf GTPases contain a unique N-terminal helical domain that is target for post-(or co-)translational myristoylation that is required for membrane localization of Arf members throughout the cell.22
While the different subfamilies within the Ras superfamily are known to regulate very different processes in many different cell types, a lot of functional overlaps have also been observed.18,29,30 The Rho subfamily is best known for translating extracellular stimuli to the maintenance and reorganization of the actin cytoskeleton thus driving cell movement and changes in morphology. Moreover, Rho GTPases regulate cell-cycle progression, and MAPK-dependent gene expression.31 Members of the Ras subfamily are known to regulate cell polarization, proliferation and survival.23 By contrast, the Arf and Rab subfamilies are best known for regulating vesicular trafficking,22,32 the Ran subfamily mainly regulates nuclear transport and Rheb GTPases regulate mTOR activation.33,34 However, some Ras and Arf GTPases have also been shown to participate in cytoskeletal remodeling. Accumulating evidence suggests a crucial role for members of the Rho, Ras, Arf and Rab subfamilies in the regulation of intestinal epithelial homeostasis as discussed below starting with the Rho family because it is the best studied in this field.
We provide two tables that give overviews of GTPase functions on various aspects of intestinal epithelial homeostasis and barrier integrity: Table 1 summarizes stabilizing and destabilizing effects that GTPase-mediated signaling pathways have on the intestinal epithelial barrier after various pro- or anti-inflammatory stimuli or infectious agents, respectively. In this table, we also correlate the described GTPase mechanisms to the diseases, animal or cellular models in which the effects had been studied. Some GTPases exert synergistic effects on the intestinal epithelial barrier, while others have rather unique functions. Table 2 displays common and unique mechanisms that GTPases transduce to regulate intestinal epithelial homeostasis and barrier functionality. Moreover, we provide cartoons to visualize the many pathways by which small GTPases influence intestinal epithelial barrier integrity (Figs. 3–8)
Table 1. Overview of small GTPases and their effects on intestinal epithelial barrier function.
| Small GTPase | Regulation via | Mode of action | Model | Barrier effect | Reference |
|---|---|---|---|---|---|
| Rho family | |||||
| RhoA | Myo9b | Regulates activity of RhoA via its GAP domain and RhoA localization to control MLC phosphorylation. Supports wound healing and correct TJ composition. | Caco-2 | ↑ | 62 |
| p114RhoGEF | Induces spatially restricted RhoA activation and ROCKII recruitment to support early cell contact formation. | Caco-2, HCE | ↑ | 47 | |
| Myo9a | Regulates RhoA activity, cell differentiation and TJ assembly. | Myo9a-KO Caco-2 | ↑ | 65 | |
| CXCL12/ CXCR4 |
Increases RhoA and F-actin localization at the leading edge. Increases MLC phosphorylation during wound healing to regulate cell migration and restitution. | IEC-6, T84 | ↑ | 50 | |
| ARHGEF11 | Activates RhoA and MLC at cell-cell contact sites to promote contraction of the actomyosin cytoskeleton, polarization, TJ assembly and epithelial barrier formation. | EpH4 | ↑ | 48 | |
| TcdA | Induces RhoA glycosylation and reorganization of the actin cytoskeleton leading to activation of caspase 3 and apoptosis. | HT-29 | ↓ | 72,73 | |
| IFN-γ | Induces endocytosis of TJ proteins via RhoA in confluent cells. | T84 | ↓ | 60 | |
| ROCK1 | Contributes to radiation-induced inflammation and barrier dysfunction. | C57Bl/6 | ↓ | 61 | |
| RhoB | miRNA21 | Less degradation of RhoB ameliorates DSS-induced intestinal epithelial apoptosis and permeability. | miRNA21 KO mice, UC patients |
↑ | 81, 82 |
| Rac1 | FPR/PI3K | Mediates activation of Rac1 and cdc42 to trigger epithelial cell restitution during wound healing. | SK-CO15 | ↑ | 84 |
| PTP-PEST | Suppresses activation of Rac1 at AJ to control activation of RhoA and AJ assembly during cell contact formation. | IEC-6, KM 12, DLD-1, KM20 | ↑ | 88 | |
| TNF-α | Increases activation of Rac1 leading to JNK and caspase activation to promote apoptosis. | IEC-6 | ↓ | 91 | |
| Rac2 | C. rodentium | Increases lymphocyte responsiveness, leukocyte recruitment, inflammation and epithelial crypt hyperplasia. | Rac2-KO | ↑ | 97 |
| Cdc42 | Tuba | Activates cdc42 for correct spindle orientation via Par6B and aPKC during cell division to control correct formation of cell-cell junctions and crypts. | Caco-2 | ↑ | 99–101 |
| SH3BP1 | Regulates spatio-temporal cdc42 activity and actin remodeling to ensure proper junction formation. | A431, HCE, Caco-2 | ↑ | 105 | |
| AMP-18 | Promotes TJ formation by recruitment of the polarity complex PKCϛ/Par6/Par3 via active cdc42. Protects from DSS colitits. | Caco-2 | ↑ | 107 | |
| Cdc42-KO, Rab8a | Increases permeability by impaired polarity, proliferation and increased apoptosis. | Cdc42-KO MVID biopsies | ↓ | 103,104 | |
| SopE | Mimicks a eukaryotic GEF to activate cdc42 inducing caspase-1-mediated secretion of Il-1 and Il-18 to trigger gut inflammation in vivo. | Caspase-1-KO, IL-1R-KO, IL18-KO | ↓ | 108 | |
| C. jejuni, CadF | Triggers activation of cdc42 and Rac1 to facilitate invasion. | INT-407 | ↓ | 109 | |
| Ras family | |||||
|---|---|---|---|---|---|
| Rap1 | Afadin | Controls epithelial permeability probably by regulation of PI3K/Akt and correct nectin localization at AJ. | Afadinvil−/−-KO | ↑ | 128 |
| Rap2A | PDZ-GEF | Activates TNIK for recruitment of MST4 and phosphorylation of Ezrin. Regulates polarization and brush border formation. | Ls174T-W4 | ↑ | 131 |
| Rap2C | PDZ-GEF1 | JAM-A and afadin-dependent recruitment of PDZ-GEF1 and activation of Rap2C inhibits RhoA-mediated actin contraction. | SK-CO15 | ↑ | 127 |
| Ras | Inflammation: TNF-α, DSS, TNBS | Increases levels of proinflammatory cytokines, epithelial apoptosis and susceptibility to colitis via disturbed Raf/MEK/ERK signaling. | DSS/TNBS colitis in mice and rat; KSR1-KO | ↓ | 119–121 |
| EphrinB1/EprhinB2R | Increases RAS activity and ERK phosphorylation and decreases Rap1 activity to induce actin cytoskeleton contractility. | DLD-1 | ↓ | 129 | |
| Rab family | |||||
|---|---|---|---|---|---|
| Rab5 | Ca-depletion | Internalization of junction proteins for a transient opening of cell contacts. | T84 | ↓ | 135 |
| Coxsackie virus infection | Activation of Rab5 and 34 cause specific internalization of occludin and the virus. | Caco-2 | ↓ | 136 | |
| Rab4/11 | IFN-γ | Macropinocytosis of TJ proteins into recycling endosomes. | T84 | ↓ | 137 |
| Rab11 | MVID | Redistribution to apical cytoplasmic regions. | MVID biopsies | ↓ | 138 |
| Rab13 | Ca-depletion, CD |
Redistribution together with other TJ molecules into the cytosol. | Caco-2, Human CD biopsies | ↓ | 139, 140 |
| Arf family | |||||
|---|---|---|---|---|---|
| Arf1 | GBF1 | Mediates vesicular Golgi-to-plasma membrane transport of junctional proteins. | SK-CO15 | ↑ | 149 |
| Arfrp1 | - | Promotes trans-Golgi-to-plasma membrane trafficking of E-cadherin and E-cadherin mediated adhesion. | Arfrp1vil−/−-KO, Hela, Ltk-Ecad |
↑ | 155 |
| Arf6 | IFN-γ | Activates an ERK1/2-MEK-PI3K-Fyn-ARF6 cascade to facilitate transcytosis of bacteria across gut epithelia. | T84 | ↓ | 146 |
| Arf1, Arf6 | CT | Induces ADP-ribosylation of Gαs, clathrin-mediated CT-uptake and intracellular vesicular trafficking. | H4, T84 | ↓ | 148 |
Table 2. Overview of common and unique mechanisms by which small GTPases affect intestinal epithelial barrier function.
| Mechanism | Mode of action | Small GTPases involved |
Model | Effect on barrier | Reference |
|---|---|---|---|---|---|
| common | |||||
| Cell contact stability | Increased formation of cortical actin and TJ strengthening | Rac1, cdc42, Rap1 | Afadinvil−/−-KO | ↑ | 21, 58, 128 |
| Cell contact stability | Inhibition of RhoA-mediated actin contractiliy | Rac1, Rap2C | IEC-6. KM 12, DLD-1, KM20, SK-CO15 | ↑ | 88, 127 |
| AJ stabilization | SH3BP1-mediated stabilization of the junctional actin belt | Rac1, cdc42 | A431, HCE, Caco-2 | ↑ | 105 |
| Wound healing | Regulation of proper actin dynamics and formation of junctions | RhoA, Rac1, cdc42, Rap1 | Caco-2, SK-CO15, A431, HCE, IEC-6, T84 | ↑ | 50, 62, 105, 157 |
| Vesiclular transport | Correct transport of E-cadherin from Golgi to plama membrane | Arfrp1 | Arfrp1vil−/−-KO, Hela, Ltk-Ecad |
↑ | 155 |
| Induction of apoptosis | Activation of caspases after proinflammatory stimuli | RhoA, Rac1 | HT-29, IEC-6 |
↓ | 72, 91 |
| TJ disassembly | Stimuli-dependent transient endocytosis of TJ molecules | Rab5, Rab11, Rab13 | T84, Caco-2, human biopses | ↓ | 135–137, 139, 140 |
| Facilitated pathogen invasion | GTPase activation by toxins that trigger inflammation, pathogen endocytosis or transcytosis | RhoA, cdc42, Arf1, Arf6 | HT-29, Caco-2, Casp-1-KO, IL-1R-KO, IL18-KO, T84, H4 |
↓ | 72–74, 108, 109, 146, 148 |
| unique | |||||
|---|---|---|---|---|---|
| Cortical actin formation | Rac1 and cdc42 activation | Rap1 | SK-CO15, IEC-6 |
↑ | 83, 84 |
| Brush border formation | Activation of ezrin | Rap2A | Ls174T-W4 | ↑ | 131 |
| TJ stabilization by accumulation of ZOs | Recruitment of the polarity complex PKCϛ/Par6/cdc42/Par3 | cdc42 | Caco-2 | ↑ | 107 |
| Mitotic spindle orientation | Correct single-lumen crypt formation | cdc42 | Caco-2 | ↑ | 99 |
| TJ protein endocytosis | IFNγ-induced formation of vacuolar apical compartments via ROCK1 and myosin II | RhoA | T84 | ↓ | 60 |
| Contractile stress fibers; Erk, p38 and MLCphosphorylation | ROCK1 and mDia1 activation by proinflammatory cytokines | RhoA | Caco-2 | ↓ | 52 |
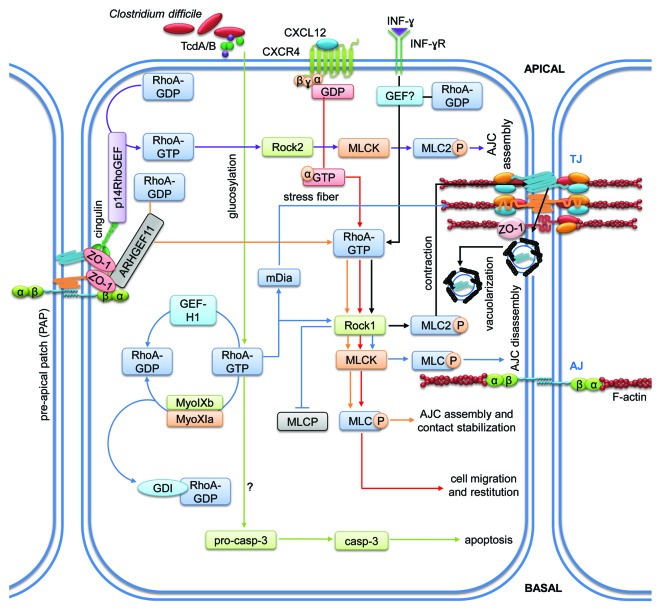
Figure 3. RhoA-mediated signaling pathways regulating intestinal epithelial homeostasis. Cartoon depicting molecular mechanisms employed by RhoA to control intestinal epithelial barrier integrity. Arrows indicate activation and lines with bars inhibition. Each color represents a separate pathway. Question marks indicate known outcomes by yet undefined mechanisms. For details see text.
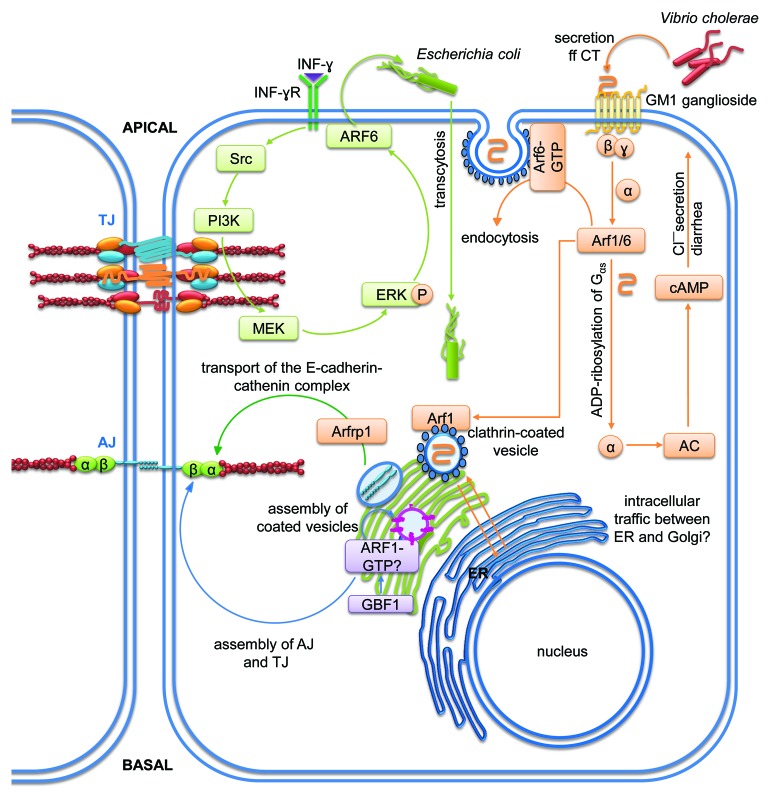
Figure 8. Signaling pathways of members of the Arf subfamily regulating membrane trafficking for the control of intestinal epithelial homeostasis. Cartoons depicting molecular mechanisms employed by the Arf subfamily (B) to control intestinal epithelial barrier integrity. Arrows indicate activation and lines with bars inhibition. Each color represents a separate pathway. Question marks indicate known outcomes by yet undefined mechanisms. For details see text.
Rho family
The mammalian Rho family is comprised of 20 members with RhoA, Rac1 and cdc42 being the best characterized members.15 The classical Rho family members (activated by GTP binding) are divided into different subgroups including Rho, Rac, cdc42, RhoF and RhoD. The atypical Rho GTPases (activated by different mechanisms) are divided into the RhoBTB, Rnd, RhoU and RhoV subgroups.35 Rho GTPases are found in all eukaryotic species, are highly conserved and phylogenetic analyses revealed at least one Rho gen as common eukaryotic ancestor.36 More than 100 targets have so far been identified for Rho GTPases including kinases, scaffolding molecules and other GTPases.37 However, Rho GTPases are best known for their ability to control actin dynamics and thus cell morphology, motility and polarity. For example, Rac1 activation is associated with lamellipodium formation and membrane ruffling, whereas cdc42 controls actin microspike and filopodium formation. By contrast, RhoA activity is correlated with increased actin stress fiber formation via mDia1 and actomyosin contractility via ROCK1.38 Besides actin dynamics, Rho GTPases also control intracellular signaling pathways involving PI3K, MAPK, and NF-κB.31 On top of the target abundancy, the activation cycle of Rho family GTPases is regulated by a plethora of GEFs and GAPs.18 For example, one GEF can in some cases activate several GTPases, and in other cases one GTPase can be activated by several GEFs. While this redundancy is obviously necessary to ensure a strict spatio-temporal regulation of GTPase signaling, it renders the study of Rho signaling pathways challenging. In the context of tissue barriers, plenty of studies can be found in the literature describing important roles for Rho GTPases during the formation of epithelial cell contacts and the establishment of the AJC. The interested reader is referred to some excellent recent reviews covering these aspects of Rho GTPase-mediated signaling.25,39-41 Given the mentioned complexity, it is impossible to cover all aspects of Rho GTPase signaling in one review; hence we will focus on recent discoveries on Rho GTPase signaling in regulating intestinal epithelial homeostasis (summarized in Tables 1 and 2, and Fig. 3).
Rho A
RhoA is essential during both barrier formation and disruption making the spatio-temporal control of its activation and effector specificty the most critical aspects that control the outcome of RhoA activation during intestinal epithelial homeostasis. RhoA-mediated signaling pathways are summarized in Figure 3 and Tables 1 and 2.
GEFs regulate spatio-temporal control of RhoA activation to control junction assembly
The spatio-temporal regulation of Rho activation is crucial for several aspects of Rho signaling and has long been known to occur in various cell types during various processes. In particular, a single Rho GTPase may need to be activated in one part of a cell while inactivation may be required simultaneously in another to achieve the aim of a given signal. Rho GTPase signaling thus requires mechanisms that regulate their activity.42 However, the mechanisms regulating this orchestration in space and time have remained elusive. In particular, RhoA needs to be activated to support initial cell–cell adhesion but later be inactivated to prevent cell spreading and proliferation via formation of contractile stress-fibers.43,44 Binding of GEF-H1, a GEF for RhoA, to cingulin at TJ led to the inactivation of GEF-H1, thus providing a means to regulate Rho-mediated signaling during TJ formation.45 Interestingly, siRNA-mediated depletion of GEF-H1 inhibited RhoA/ROCK-II-mediated AJC disassembly in colonic SK-CO15 cells.46 Thus, GEF-H1 may play a central role to achieve a spatio-temporal regulation of RhoA.
Recently, another GEF has been identified to play a major role during RhoA regulation in Caco-2 cells.47 p114RhoGEF is required for RhoA activation at cell–cell junctions. Importantly, depletion of p114RhoGEF led to a loss of RhoA activity at junctions but stimulated Rho signaling in basal cellular regions to induce MLC activation. At TJ, p114RhoGEF formed a complex with myosin II, Rock II and the junctional adaptor cingulin to trigger activation of RhoA at TJ to regulate junction formation and epithelial morphogenesis in a spatially restricted manner.
Another mechanism of RhoA contribution to TJ formation and epithelial barrier functionality is the recruitment of ARHGEF11, a GEF for RhoA, first to newly formed AJ and later to establishing TJ, where it interacts directly with ZO-1. At cell contacts, ARHGEF11 activates RhoA and MLC to trigger the organization of the AJC and the perijunctional actomyosin ring that stabilize epithelial contacts.48,49 Thus, accumulating evidence suggests that GEFs are critical regulators of RhoA activation in space and time in order to regulate the formation and maintenance of the AJC. If other GEFs, GAPs or RhoGDIs also contribute to this spatio-temporal regulation of intestinal epithelial homeostasis via RhoA needs to be investigated in future studies. Moreover, the physiological stimuli that induce regulation of these GEFs and/or GAPs during physiological, pathological and developmental processes still remain elusive.
RhoA regulates enterocyte migration
A pathway by which RhoA/ROCK1 signaling regulates intestinal epithelial wound healing is the activation of CXCR4 by its ligand, the chemokine CXCL12, to trigger RhoA-GTP and F-actin recruitment to the leading edge of wounded IEC-6 and T84 cell monolayers where MLC gets activated to induce enterocyte migration. The authors concluded that this Rho-dependent signaling cascade is needed for the intestinal epithelial barrier to resist injury and mediate healing.50 This is an important finding since it demonstrates that chemokines do not only contribute to epithelial damage during inflammation via excessive recruitment of neutrophils,51 but are also important mediators of epithelial reconstitution. Additionally, RhoA and its effectors ROCK1 and mDia1 are required for proper Caco-2 migration in wound healing assays.52 Inhibition or downregulation of either protein reduced p38, MLC and nuclear Erk phosphorylation leading to significantly reduced migration.
RhoA inactivation by cAMP signaling ameliorates colitis but inhibits wound healing
The use of cAMP-elevating agents in the treatment of IBD has been discussed because adrenomedullin, for example, ameliorated DSS colitis by reducing levels of proinflammatory cytokines.53,54 However, another recent study revealed that other cAMP-inducing compounds (forskolin/rolipram) induced PKA activation, RhoA inactivation together with reduced actin turnover and inhibition of intestinal epithelial wound healing in vitro and in vivo.55 Clearly, more studies are needed to clarify the mode of action of cAMP-elevating compounds before therapeutic application is warranted.
Myosin-dependent RhoA signaling contributes to junction disassembly
RhoA is a well-known regulator of the actin cytoskeleton via its effectors ROCK1 that controls actomyosin contractility via MLC phosphorylation and mDia1 that is required for the assembly of linear actin filaments to form stress fibers. By regulating actomyosin contractility, RhoA has profound effects on epithelial barrier function.10,11,56-58 In particular, intestinal epithelial barrier disruption in response to proinflammatory cytokines such as IFN-γ is mediated by endocytosis of TJ proteins in T84 cells that is dependent on the activation of the RhoA/ROCK1 pathway and myosin II.59,60 Interestingly, ROCK1 plays an important role during the onset of radiation-induced barrier dysfunction because inhibition of ROCK1 in vivo significantly reduced chemokine production and intestinal permeability to 4 kDa FITC-dextran.61 Misregulation of RhoA/ROCK1-mediated MLC phosphorylation after loss of myosin IXb (myo9b) has been reported. Downregulation of myo9b, which contains a GAP domain specific for RhoA, resulted in constant phosphorylation of MLC and increased actomyosin contraction affecting intestinal epithelial wound healing and permeability to 3 kDa TRITC-dextran in Caco-2 cells.62 Moreover, loss of myo9b was accompanied by aberrant localization of the TJ molecules ZO1, claudin-1 and occludin. This may have implications for the pathogenesis of IBD, because myo9b polymorphisms have been associated to development of IBD.63,64 Downregulation of myosin XIa, which also contains a Rho-GAP domain, in Caco-2 cells led to disturbed junction formation via increased RhoA signaling.65
Alcohol abuse leads to a RhoA-dependent increase in intestinal epithelial permeability
Another interesting mechanism of barrier disruption has been reported after excessive alcohol consumption that not only causes cirrhosis and other liver diseases but also increased intestinal epithelial permeability via redistribution of TJ molecules and increased oxidative stress.66-68 Mechanistically, RhoA contributed to ethanol-induced increases in intestinal epithelial permeability; and shRNA-mediated downregulation of RhoA partially inhibited ethanol-induced decreases in transepithelial electrical resistance (TER), occludin internalization and increases in RhoA and MLC activity.69
Pathogen-induced RhoA activation leads to apoptosis and barrier dysfunction
Apoptosis during inflammation or infection is another reason for epithelial barrier dysfunction,70 and this can also be mediated by RhoA. For example, toxins A and B, the main pathogenic factors of Clostridium difficile, a pathogen that causes diarrhea and colitis, are known to glycosylate RhoA and thereby inactivate it.71 This inactivation was associated with altered TJ protein localization and induction of caspase-dependent apoptosis in HT-29 and T84 cells.12,72 RhoA glycosylation was indeed required to induce intestinal epithelial apoptosis since a mutant form of Clostridium difficile toxin A (TcdA) lacking glucosyltransferase functionality could not induce apoptosis. By contrast, actin disruption alone, another well-known consequence of TcdA, by latrunculin A was not sufficient to mimick the observed apoptotic effects.72 TcdA-induced apoptosis could be reversed by co-incubation with glutamine or alanyl-glutamine in IEC-6 cells that was accompanied by increased RhoA expression.73 Additionally, other pathogens such as Escherichia coli, Mycobacterium avium or Salmonella typhimurium have been shown to exploit mechanisms involving RhoA activation to facilitate infection.74-77 Thus, Rho GTPases play a major role not only for the formation and homeostasis of the intestinal barrier but also as targets of pathogens to induce barrier dysfunction, and to trigger invasion. How these pathogen-induced changes in Rho GTPase activity are recognized by cells to start counteracting the infection has been a mystery. A very recent study identified nucleotide-binding oligomerization domain protein 1 (NOD1) as the molecule that sensed aberrant Rho GTPase activation upon infection. Activation of the NOD1 pathway induced NF-κB-dependent inflammatory responses to induce pathogen clearance.78
Rho B
In addition to known Rho family functions such as actin remodeling and cell migration, RhoB, unlike RhoA and RhoC, also acts as gastric and colorectal tumor suppressor by regulating cell cycle and apoptosis.79,80 Very recently, RhoB has been implicated in the regulation of intestinal epithelial barrier function. In mice deficient for micro-RNA-21 (miR-21) an increased expression of RhoB has been reported after seven days treatment with 3.5% DSS compared with WT, while the expression levels of cdc42 were decreased. This increased expression of RhoB was accompanied by an amelioration of DSS-induced intestinal epithelial apoptosis and permeability to 4 kDa FITC-dextran.81 Furthermore, in biopsies of colon tissues of individuals suffering from colitis, an accumulation of miR-21 has been observed which was accompanied by reduced expression of RhoB. The authors concluded that miR-21 degrades RhoB mRNA, leading to depletion of RhoB protein, dysfunction of TJ and decreased TER suggesting an important role for both miR-21 and RhoB for epithelial barrier homeostasis.82 However, the exact mechanism how RhoB contributes to TJ and thus epithelial barrier stability still needs to be investigated in more detail.
Rac1
Rac1 is the forming member of the Rac branch of the Rho family of GTPases and plays important roles in wound healing, bacterial clearance and cell adhesion/migration by regulating actin dynamics. Wound healing, for example, depends on the precise balance of migration and differentiation of epithelial cells which involves Rho GTPase-mediated cytoskeleton remodeling and dissolution and reassembly of cellular junctions.83 Rac1-mediated signaling regulating intestinal epithelial barrier functions are depicted in Figure 4 and summarized in Tables 1 and 2.
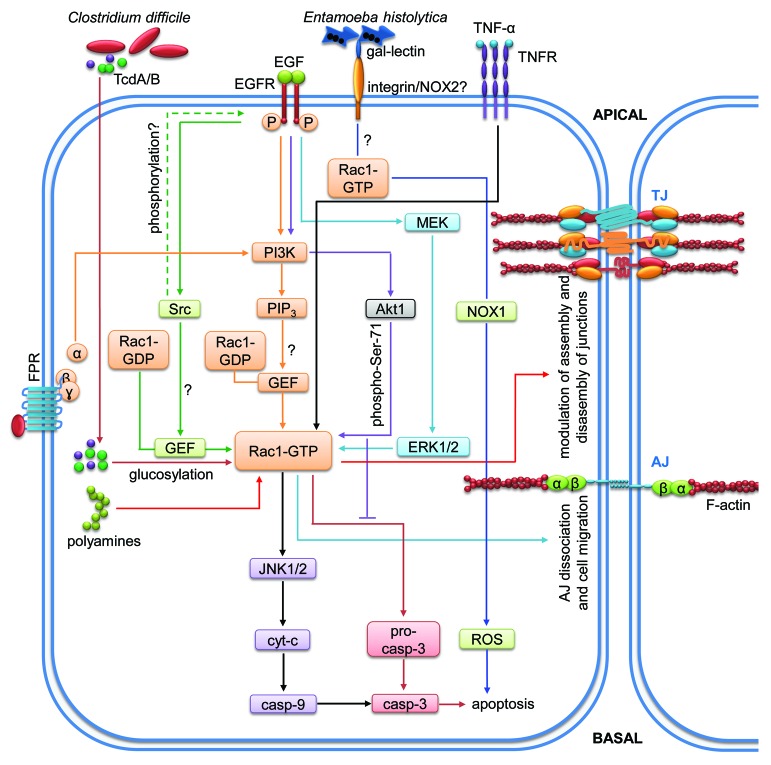
Figure 4. Rac1-mediated signaling pathways regulating intestinal epithelial homeostasis. Cartoon depicting molecular mechanisms employed by Rac1 to control intestinal epithelial barrier integrity. Arrows indicate activation and lines with bars inhibition. Each color represents a separate pathway. Question marks indicate known outcomes by yet undefined mechanisms. For details see text.
Rac1 activation triggers intestinal epithelial cell migration and wound healing
Rac1 together with cdc42 induces intestinal wound closure. This process is mediated by N-formyl peptide receptor (FPR) stimulation leading to enhanced intestinal epithelial cell restitution through PI3K-dependent activation of Rac1 and cdc42.84 Moreover, epidermal growth factor (EGF) stimulation of a mouse colonic epithelial (MCE) cell line induced wound healing mediated by PI3K- and src-mediated Rac-1 activation and membrane translocation to the leading edge where Rac1 promotes lamellipodial extension.85 Specific polyamine depletion prevented Rac1 activation and inhibited migration of intestinal epithelial cells, an effect that could be rescued by sustained activation of MEK1.86,87
AJ assembly depends on Rac1 activation
Assembly of AJ after calcium switch is also dependent on Rac1. Depletion of PTP-PEST, a tyrosine phosphatase located in AJ, increased Rac1 activity but diminished RhoA activity leading to impaired AJ assembly. Additionally, downregulation of PTP-PEST enhanced the migratory behavior of KM12C colonic cells.88 Thus, PTP-PEST, via Rac1 and RhoA, controls AJ stability and suppresses epithelial migration.
Pathogen-induced Rac1 activation contributes to microbial infection and barrier dysfunction
Many pathogenic bacteria produce virulence factors that target Rho proteins.89 TcdA and B produced by C. difficile were able to glycosylate RhoA and Rac1 leading to apoptosis of HT-29 intestinal epithelial cells. Glycosylation of these GTPases induced reorganization of the actin cytoskeleton and G2/M cell cycle arrest. In parallel, this glycosylation led to the activation of caspases 3, 8 and 9 to trigger apoptosis and barrier dysfunction.72 This activity of TcdA/B was counteracted by EGF, rendering Rac1 less susceptible to glycosylation that was dependent on EGF-mediated serine-71 phosphorylation of Rac1.90 Thus, Ser-71 phosphorylation of Rac1 after EGF treatment might be a protective mechanism against microbial infection and subsequent intestinal epithelial barrier dysfunction.
Inflammation-induced Rac1 activation promotes apoptosis and barrier destabilization
Rac1 is also involved in modulating apoptosis during inflammation. TNF-α-induced apoptosis in IEC-6 cells was prevented by inhibition of Rac1 and subsequent inhibition of caspases 3, 8, and 9.91 Additionally, Rac1 inhibition also prevented TNF-α-induced activation of JNK1/2, a key proapoptotic regulator, while it did not affect TNF-α-induced ERK1/2 and Akt activation in IEC-6 cells. Thus, Rac1 is involved in intestinal epithelial apoptosis during inflammation via JNK, a mechanism that may negatively affect epithelial homeostasis. Infection of HT-29 cells by Entamoeba histolytica also induced caspase-independent cell death that was significantly reduced by siRNA-mediated downregulation of Rac1, thus corroborating a role of Rac1 for epithelial viability during infection/inflammation.92
In genetic studies, two single nucleotide polymorphisms (SNPs) of Rac1 and increased expression of Rac1 in human colon biopsies have been associated to UC, further highlighting the importance of Rac1 in the pathogenesis of IBD.93
Rac2
Rac2 is a member of the Rho family that is almost exclusively expressed in hematopoietic cells where it is part of the NADPH oxidase complex. Interestingly, a genetic association of Rac2 to CD has been reported.94 Rac2 deficiency was accompanied by defective clearance of Aspergillus fumigatus and a dominant-negative Rac2 mutation led to life-threatening bacterial infections in two case reports.95,96 Thus, Rac2 plays an important role in host defense. Recently, the effects of Rac2 deficiency on colitis development have been investigated in a Citrobacter rodentium infection model.97 Even though a delay in bacterial clearance was observed accompanied by epithelial hyperproliferation, barrier dysfunction, increased inflammatory responses of lymphocytes and increased leukocyte recruitment, all mice recovered from the infection. The authors concluded that Rac2-dependent bacterial clearance is dependent on the type of pathogen and that the observed changes in the intestinal epithelium are maybe due to increased lymphocyte responsiveness. These results are interesting because they demonstrate that the functionality of intestinal epithelial cells is also dependent on correct GTPase signaling in immune cells (Table 2).
Cdc42
Cell division cycle 42 (cdc42) is another important member of the Rho family that often acts in concert with Rac1 and thus has similar effects on the intestinal epithelial barrier.58 Cdc42 regulates actin branching via its effector neural Wiskott-Aldrich syndrome protein (N-WASP) and plays important roles in cell adhesion, proliferation, differentiation, migration and polarity.98 Signaling mediated by cdc42 that affects intestinal barrier integrity are summarized in Figure 5 and Tables 1 and 2.
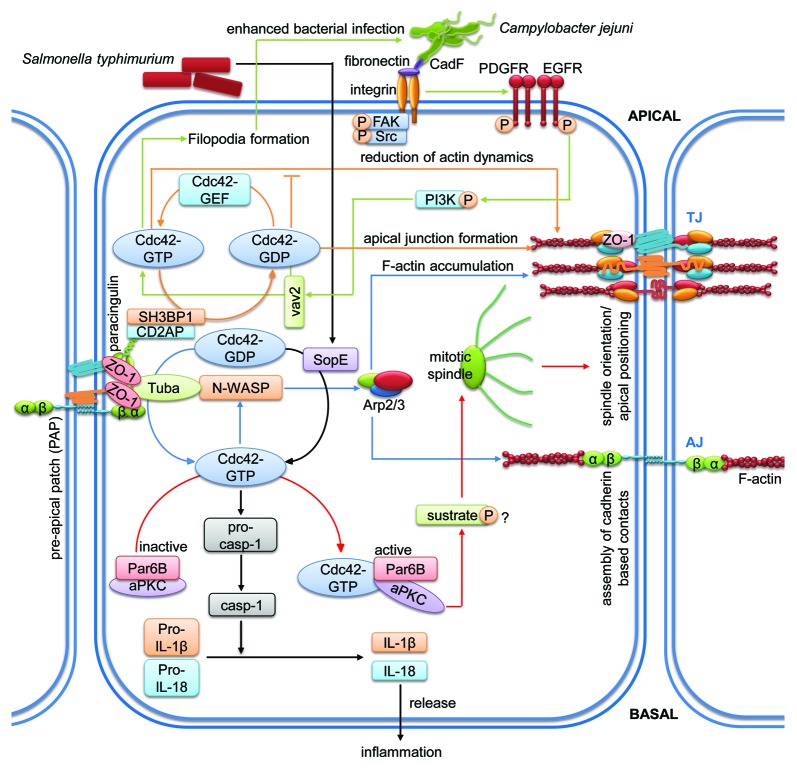
Figure 5. Cdc42-mediated signaling pathways regulating intestinal epithelial homeostasis. Cartoon depicting molecular mechanisms employed by cdc42 to control intestinal epithelial barrier integrity. Arrows indicate activation and lines with bars inhibition. Each color represents a separate pathway. Question marks indicate known outcomes by yet undefined mechanisms. For details see text.
Cdc42 regulates cell polarity and proliferation during barrier formation
Cdc42 is also involved in intestinal epithelial tissue morphogenesis by regulating cell polarity. Depletion of cdc42 in Caco-2 cells growing in a three-dimensional matrix disrupted mitotic spindle orientation during cell division causing compromised location of the apical surfaces within the cyst.99 Additionally, activation of cdc42 by Tuba, a cdc42 GEF, was necessary for correct spindle orientation. This effect seemed to be regulated via Par6B and atypical PKC since downregulation of these molecules phenocopied the observed effects on mitotic spindle orientation seen after loss of cdc42.100 Proper lumen formation in 3D by Caco-2 cells was not only dependent on Tuba but also on N-WASP, a cdc42 effector molecule.101 Downregulation of N-WASP led to similar defects in lumenogenesis as compared with Tuba depletion. Additionally, Tuba depletion caused delayed cell contact reassembly after calcium switch, effects that could be mimicked by cdc42 inhibition or N-WASP depletion.102 These data highlight the importance of a functional Tuba/cdc42/N-WASP axis during epithelial barrier formation.
Crypt morphology and stem cell-mediated self-renewal depends on cdc42
A functional intestinal epithelium depends on constant renewal by differentiation of adult intestinal stem cells to constantly replace dying cells. In intestinal epithelial-specific cdc42 KO mice, the number of proliferating stem cells was increased leading to hyperplasia, crypt enlargement and increased permeability to 4 kDa FITC-dextran.103 In these mice, migration of stem and progenitor cells along the crypt-villus axis was increased. While only a minor defect in junction orientation was observed, epithelial polarity was impaired most likely contributing to increased intestinal permeability. In this study, it has also been demonstrated that the morphology of intestinal epithelial-specific cdc42-KO mice resembles that of MVID patients. Furthermore, cdc42-deficient stem cells showed defects in cell division, a reduced capacity for clonal expansion and increased apoptosis.104 Interestingly, in this study an interplay of cdc42 and Rab8a, another small GTPase of the Rab subfamily, has been demonstrated, since cdc42 deficiency impeded Rab8a activation and subsequent interactions with its effectors, thus preventing Rab8a-dependent vesicle trafficking. Vice versa, Rab8a was required for cdc42-GTP activity in the intestinal epithelium. These data demonstrate that cdc42 is required for a functional intestinal stem cell niche to ensure proper mucosal self-renewal.
GAP-mediated control of cdc42 activity is required for barrier formation
Similar to other small GTPases, cdc42 must be regulated in space and time to ensure first intestinal cell contact formation and then stabilization of the formed barrier. A recent study identified sh3-domain binding protein (SH3BP1), a GAP for cdc42 and Rac1, as a potential mechanism in Caco-2 cells.105 SH3BP1 formed a complex with the TJ adaptor molecules paracingulin and CD2AP to mediate normal cdc42 signaling and junction formation as downregulation of either adaptor molecule caused cdc42 inactivation. Downregulation of SH3BP1 in Caco-2 cells led to altered actin dynamics including reduced junctional actin belt formation and increased basal fiber formation accompanied by altered junctional composition leading to barrier dysfunction. Importantly, SH3BP1 depletion caused basal accumulation of active cdc42, whereas in control cells active cdc42 was mostly located apically. On the other hand, the filamentous actin-capping protein capZ1 also interacted with this SH3BP1 complex and capZ1 depletion led to a failure of junctional actin belt formation. The authors concluded that epithelial morphogenesis requires formation of this complex that controls actin dynamics and junction maturation by regulating spatio-temporal cdc42 signaling.
AMP-18 promotes TJ stability via cdc42
A substance that is known to support the intestinal epithelial barrier in vivo and in vitro is 18 kDa antrum mucosal protein (AMP-18).106 Treatment of Caco-2 cells with this peptide led to activation of both p38 and PKCϛ, accumulation of ZO-1, ZO-2 and JAM-A at TJ and stabilization of junctional actin filaments.107 Via active PKCϛ and cdc42, the polarity complex PKCϛ/Par6/cdc42-GTP/ECT2/Par3 was recruited to TJ where it promoted TJ formation and stabilization by assembly of TJ molecules. Importantly, AMP-18 significantly protected mice from DSS-induced colitis. The authors speculated that AMP-18 may prove beneficial in IBD patients by stabilizing TJ and thus epithelial barrier function.
Cdc42 as target for mucosal pathogens
Cdc42 is also known as a target for toxins produced by pathogens.89 For example, SopE, an effector molecule acting as GEF produced by Salmonella typhimurium, activated both cdc42 and Rac1 leading to caspase-1 activation and increased secretion of the proinflammatory cytokine IL-1β in enterocytes to trigger intestinal inflammation and permeability.108 Cotransfection of SopE with dominant-negative versions of cdc42 and Rac1 interfered with caspase-1 activation and IL-1β secretion, with Rac1 clompletely and cdc42 partially blocking it. If cdc42 then acts on Rac1 rather than on SopE/caspase-1 signaling needs to be verified. However, interfering with RhoA signaling did affect neither caspase-1 activation nor IL-1β secretion, thus proving a specific role of SopE for cdc42 and Rac1.
Additionally, Campylobacter jejuni activates cdc42 to facilitate host cell invasion in the gut.109 Studies using inhibitors, siRNA and KO cells identified a crucial role for the cdc42-GEF vav2 in pathogen-induced cdc42 activation. This activation occurred downstream of fibronectin/β1-integrin, epidermal growth factor receptor (EGFR), platelet-derived growth factor receptor (PDGFR) and PI3K. Activation of cdc42 via this axis caused intestinal epithelial dysfunction, filopodia formation and consequently increased host invasion. The potential pathogenic effector molecule causing activation of this signaling cascade has been suggested to be the fibronectin-binding protein CadF because infection with a CadF mutant strain almost completely abolished cdc42 activation.110
In summary, small GTPases of the Rho family play an important role during host-pathogen interaction (Figs. 3–5; Tables 1 and 2). Aberrant GTPase signaling caused by pathogenic effector molecules causes intestinal epithelial dysfunction and facilitates invasion. Understanding the exact mechanisms how toxins interfere with GTPase signaling may therefore help to develop new strategies for the treatment of diseases with intestinal epithelial barrier malfunctions.
It is important to keep in mind that different members of the Rho subfamily have differential effects on the intestinal epithelial barrier.58 Both activation and deactivation of RhoA by bacterial toxins increased permeability to both ions and 4 kDa FITC-dextran in Caco-2 cells, while only activation of cdc42 and Rac1 decreased permeability and deactivation of all three GTPases increased permeability. These results further demonstrate the importance of a spatio-temporal fine-tuning of Rho GTPase signaling for intestinal epithelial homeostasis.
Ras family
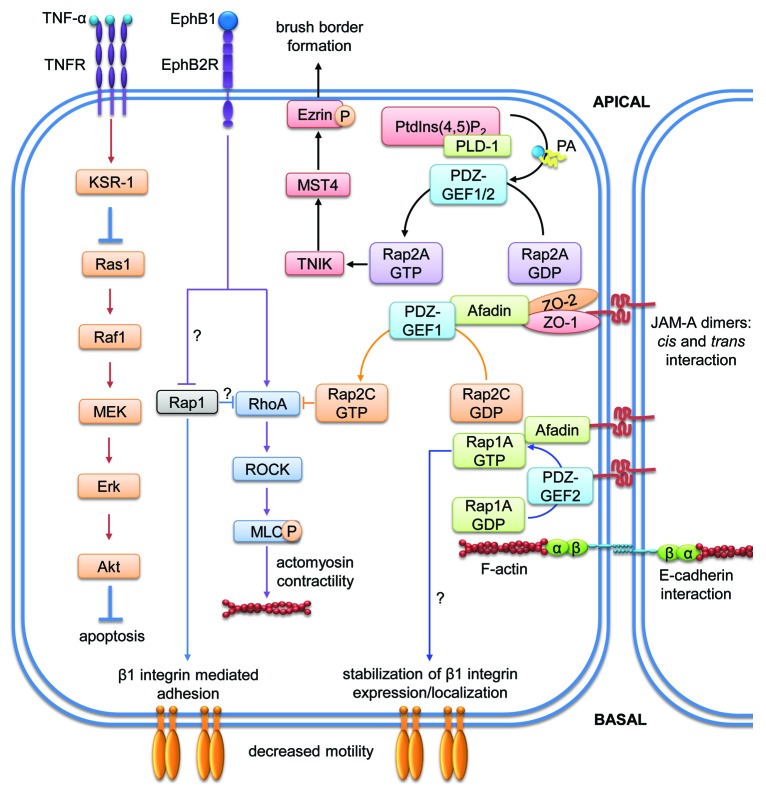
The Ras oncogenes that are now considered the founding members of the Ras family (H-, K-, and N-Ras) were identified around 50 y ago as part of a virus that causes tumor formation in mice.111 In 1981 these genes were identified as mutated forms of cellular proto-oncogenes.112 In 1987 it was clarified that these mutations render the GTPases insensitive to GAPs leading to constitutively active GTPase signaling.113 Ras GTPases are predominantly localized at the plasma membrane from where they convey extracellular signals into multiple intracellular signaling pathway.114 Activated Ras proteins are known to activate major signaling pathways mediated by p38 MAPK, ERK, JNK, PI3K or RalGDS.115 These signals ensure strict regulation of different important cellular processes such as polarization, adhesion and proliferation. The activation of Ras GTPases is regulated by three different groups of Ras GEFs, the mammalian son-of-sevenless (mSos) proteins, Ras guanine nucleotide releasing proteins (RasGRPs) and Ras guanine nucleotide-releasing factors (RasGRFs).116 Mechanistically, spatio-temporal activity of Ras GTPases is mediated by differential post-translational modifications of the C-terminal peptide to guide Ras proteins to distinct cellular membrane compartments, thus ensuring specific interactions with GEFs and effector molecules.115 Compared with the Rho family, not so much is known about the involvement of Ras GTPase signaling in tissue barrier stability. Given its role as oncogene, Ras itself has mostly been studied in the context of cancer. Thus, the best studied members with respect to intestinal epithelial barrier formation are Rap1 and Rap2 (compare Tables 1 and 2, and Figure 6).117
Figure 6. Signaling pathways of members of the Ras subfamily regulating intestinal epithelial homeostasis. Cartoon depicting molecular mechanisms employed by Ras family members to control intestinal epithelial barrier integrity. Arrows indicate activation and lines with bars inhibition. Each color represents a separate pathway. Question marks indicate known outcomes by yet undefined mechanisms. For details see text.
Ras1
Ras1 activation determines severity of experimental colitis
Ras proteins have been extensively studied because 10–20% of all human tumors are characterized by constitutively activated Ras mutants with mutations in K-RAS in around 40% of colorectal cancer cases.118 An involvement of Ras1 in the development of intestinal inflammation and epithelial barrier dysfunction in vivo has been found studying mice deficient for kinase suppressor of Ras1 (KSR1).119 When injected intraperitoneally with TNF-α, these mice are more prone to chronic colitis and show increased colon epithelial apoptosis which is due to a failure to activate an anti-apoptotic pathway involving Raf-1/MEK/ERK, NF-κB and Akt (Table 1). Evidence for a direct role of Ras and not only its suppressor KSR1 came from two recent studies, which showed that Ras expression and activation levels are increased in a DSS colitis mouse model and a TNBS colitis rat model, respectively. DSS colitis was ameliorated by treatment with farnesylthiosalicylic acid (FTS) which was accompanied by decreased levels of active Ras.120 On the other hand, TNBS colitis in rats was ameliorated by moxibustion, a traditional Chinese medical therapy which caused downregulation of inflammation-induced expression of Ras, Raf, MEK and ERK and concomitant suppression of this signaling pathway.121 These data implicate an important proinflammatory role of Ras in the pathogenesis of IBD and warrant further investigation to prove therapeutical potential of Ras inhibitors.
Rap1
Ras-related protein 1 (Rap1) is activated by different extracellular signals and regulatory proteins and participates in a broad range of cellular processes including epithelial cell contact formation (Fig. 6 and Tables 1 and 2).117 Rap1 exists in two different isoforms, Rap1A and Rap1B.122
Rap1 is involved in the regulation of cell adhesion and migration
Rap1 is required for homotypic E-cadherin interactions.123 Afadin, an adaptor protein that binds to different junctional proteins, has been shown to reduce endocytosis of E-cadherin that is not engaged in homophilic interactions in the presence of Rap1.124 Moreover, knockdown of afadin in MDCK cells inhibited recruitment of different tight junction proteins such as claudin-1, occludin, JAM-A and ZO-1 to cell-cell contacts suggesting an activation of afadin via Rap1.125 This mechanism could also be important for intestinal epithelial barrier stabilization by promoting the recruitment of different junctional proteins to cell-cell contacts. Indeed, studies using colonic SK-CO15 cells revealed that JAM-A dimerization is needed for PDZ-GEF2 and afadin interactions with JAM-A.126 Afadin recruited Rap1A in close proximity to PDZ-GEF2 through which it was maintained in an active GTP-bound state to stabilize β1-integrin levels and cell migration. In this study, knockdown of Rap1A, but not Rap1B, reduced both β1-integrin levels and cell migration. However, even though this mechanism affected cell adhesion and migration, it has recently been shown not to affect TER.127
Rap1 contributes to intestinal epithelial barrier stabilization in vivo
By contrast, conditional intestinal-specific afadin KO mice showed lower basal active Rap1 levels and reduced junctional localization of nectins in primary colonic epithelial cells accompanied by increased intestinal permeability to 4 kDa FITC-dextran in vivo.128 These mice were also more susceptible to DSS colitis. Thus, afadin is important for proper Rap1 activation and control of epithelial barrier function under basal and inflammatory conditions in vivo and in vitro. However, the exact mechanism by which Rap1 regulates these processes remains to be elucidated in future studies.
Active Rap1 stabilizes the barrier by dampening acto-myosin contractility
In DLD1 colorectal adenocarcinoma cells, ephrin-B1 stimulation led to activation of RhoA and Ras but inactivation of Rap1 causing actin cytoskeleton remodeling, cell retraction and detachment from the extracellular matrix.129 Moreover, both introduction of constitutively active Rap1 and treatment with the ROCK-specific inhibitor Y-27632 inhibited these processes suggesting a critical role for Rap1 in the regulation of RhoA/ROCK-mediated actin dynamics.
Rap2
Rap2 is closely related to Rap1 with a 60% sequence identity and it has been shown to be regulated by the same GEFs and GAPs as Rap1 although with less efficiency.130 Recent studies elucidated a role of Rap2 activity in intestinal epithelia (Fig. 6 and Tables 1 and 2).131
Rap2A regulates brush border formation and barrier stability
Silencing of Rap2A by shRNA significantly reduced brush border formation in Ls174T-W4 intestinal epithelial cells. Rap2A induced brush border formation after translocation from the cytosol to the apical side of the plasma membrane where it was activated by PDZ-GEF and in turn activated its effectors Traf2- and NCK-interacting protein kinase (TNIK) and mammalian STE20-like protein kinase 4 (MST4) to phosphorylate the actin-binding protein ezrin. Ezrin then triggered actin remodeling for polarization and brush border formation. Moreoever, ezrin is important for cell-cell adhesion by regulating E-cadherin-dependent assembly of AJ via activation of Rac1.132 In normal colon tissue, ezrin localized at cell-cell junctions, whereas it was predominantly found in the cytoplasm in colon tumor tissue. This translocation possibly contributes to the weakening of cell-cell contacts and to an increased invasive potential. Altogether, these data support the idea that Rap2A contributes to a stable intestinal epithelial barrier by promoting the localization of ezrin at the apical side of epithelial cells to trigger cell-cell contact formation and homeostasis.
Rap2 contributes to AJ homeostasis
Analysis of HT-29 cells depleted of Rap1GAP, a negative regulator of Rap1A/B and Rap2A/B/C in mammalian cells, showed increased Rap1 and Rap2 activity accompanied with altered localization of E-cadherin, β-catenin and p-120 catenin.133 This suggests that Rap1GAP is involved in controlling AJ homeostasis in colonic epithelial cells via regulating both Rap1 and Rap2 activity. However, the mechanisms by which Rap2 isoforms affect intestinal epithelial permeability are still poorly understood.
Rap2C and JAM-A are required for TJ stability by controlling acto-myosin contractility
A very recent study showed that Rap2C was activated upon association of the cytoplasmic tail of JAM-A with the second PDZ domain of ZO-2.127 Afadin formed a complex with JAM-A and ZO-2 to recruit PDZ-GEF1 and activate Rap2C in SK-CO15 cells. Downregulation of any of these molecules led to reduced TER suggesting a potential role for this complex in the regulation of intestinal epithelial permeability by controlling the activation of Rap2C. Furthermore, JAM-A also dampened actomyosin contractility via RhoA, since downregulation of JAM-A led to higher levels of active RhoA and MLC phosphorylation.127 These results suggest a potential inhibitory role for JAM-A-activated Rap2C on RhoA that may represent another mechanism by which JAM-A stabilizes the epithelial barrier.
All the mechanisms mentioned before highlight the importance of Rap1 and 2 for the control of epithelial barrier stability by controling different processes such as cell migration, actin dynamics and formation of epithelial junctions.
Rab family
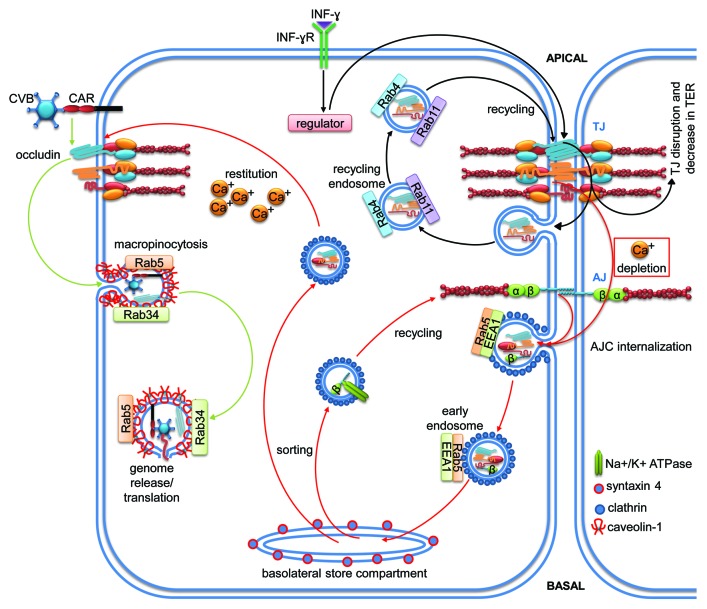
The Rab family of GTPases forms the largest branch within the Ras superfamily. More than 60 members have been identified in humans to date and, while not all have been investigated in detail, several Rab GTPases are master regulators of vesicle formation and trafficking.32 Rab GTPases are structurally similar to other small GTPases with a globular GTP-binding domain, two molecular switch regions, a hypervariable C-terminus that is crucial for correct membrane targeting, and a CAAX motif that can be prenylated or geranylgeranylated to allow membrane association. Additionally, Rabs contain unique conserved stretches of amino acids that are absent in other GTPases and allow classification of Rab subfamilies. These unique stretches are also thought to play a role in defining the specificity of Rabs for certain effectors and membrane localizations. Rabs are delivered to membranes by GDIs in an inactive GDP-bound state where they get activated by appropriate GEFs, a process that is in some cases facilitated by GDI displacement factors (GDF).134 Correct Rab delivery is of utmost importance since the localization of different Rabs on different types of cytosolic membranes is a hallmark of proper intracellular vesicle trafficking. In epithelial cells, newly synthesized components of TJ and AJ are delivered by endosomes via the endoplasmatic reticulum and the Golgi apparatus to the correct final location. This is important because polarized epithelia are characterized by a strict separation of the apical and basolateral plasma membrane compartments. This is achieved by the so called fence function of TJ that prevents membrane components from freely diffusing between these compartments. Among the machinery of regulating molecules, various Rab GTPases can be found that contribute to correct AJC assembly and maintenance in intestinal epithelial cells. Pathways involving members of the Rab family that affect epithelial homeostasis are depicted in Figure 7 and summarized in Tables 1 and 2.
Figure 7. Signaling pathways of members of the Rab subfamily regulating vesicular transport for the control of intestinal epithelial homeostasis. Cartoons depicting molecular mechanisms employed by the Rab subfamily to control intestinal epithelial barrier integrity. Arrows indicate activation and lines with bars inhibition. Each color represents a separate pathway. Question marks indicate known outcomes by yet undefined mechanisms. For details see text.
Rab5 mediates endocytosis and recycling of AJC proteins in calcium-switch assays
Temporal loosening of junctional adhesion can be required in certain pathophysiologic conditions in response to extracellular stimuli. This is usually achieved by internalization of AJC proteins. This process of junction disassembly can be mimicked by calcium depletion in vitro since homophilic adhesive interactions of several junctional transmembrane molecules depend on calcium. In T84 cells, calcium depletion led to rapid clathrin-dependent internalization of several junctional molecules including JAM-A, occludin, ZO-1, E-cadherin and β-catenin.135 Importantly, vesicles containing junctional molecules also stained positive for Rab5, a marker of early endosomes. At later time points (30–60 min), colocalization of junctional markers with Rab5 was lost. However, colocalization with Rab4 or Rab11 (recycling endosomes), Rab7 or Rab9 (late endosomes), LAMP-1 (lysosomes), GM130 and TGN38 (Golgi), or calnexin (endoplasmic reticulum) could neither be observed. By contrast, junctional markers colocalized with syntaxin-4 which had been described as a marker for a basolateral storage compartment. After calcium addition, junctional molecules were retargeted to apical junctions suggesting that the internalized vesicles are recycled. The authors concluded that this recycling mechanism is exploited by intestinal epithelial cells to quickly respond to extracellular stimuli with a transient opening of junctions followed by re-sealing of cell contacts.
Rab5 and Rab34 are required for occludin-mediated Coxsackie virus infection
Interestingly, coxsackievirus invasion of Caco-2 cells required activity of both Rab5 and Rab34.136 Activation of these GTPases promoted specific internalization of occludin from TJ in a macropinocytosis-like fashion that depended also on caveolin. Downregulation of either occludin, Rab5 or Rab34, but not Rab7 or Rab13, prevented virus entry, highlighting the importance of these GTPases for occludin internalization during Coxsackie virus infection. These data demonstrate that a single TJ molecule can be specifically targeted without further affecting TJ composition. Certain viral infections may therefore open new strategies for studying mechanisms of TJ disassembly.
Rab4, Rab11 and Rab13 are involved in epithelial inflammation
In contrast to clathrin-mediated endocytosis after calcium depletion, the proinflammatory cytokine IFN-γ induced internalization of the TJ proteins JAM-A, claudin-1 and occludin via macropinocytosis.137 In this study, colocalization of TJ proteins with the recycling endosome markers Rab4 and Rab11 was detected, whereas no colocalization with late endosomal or lysosomal markers could be detected. Interestingly, internalized TJ proteins did not get degraded but were rather recycled to the apical membrane after removing IFN- γ. This Rab-dependent mechanism of TJ macropinocytosis may thus represent a mechanism by which cell contacts are transiently opened under inflammatory conditions. Since this phenomenon has also been observed in inflamed mucosa in vivo, it is likely that excessive contact to proinflammatory cytokines in IBD contributes to continuous barrier dysfunction due to TJ disassembly mediated by endocytosis.
In another study, Rab11 has been suggested as a diagnostic marker for microvillous inclusion diseases (MVID), an intestinal disorder characterized by epithelial barrier dysfunction and diarrhea.138 While Rab11 is located to apical membrane regions in healthy patients and those with enteritis, it translocates to the cytosol in biopsies of MVID patients. It is tempting to speculate that absence of Rab11 from the AJC causes barrier dysfunction leading to MVID-related diarrhea.
In human intestinal biopsies of healthy individuals, Rab13 and VASP were both located at TJ.139 By contrast, in tissues of CD patients in remission, Rab13 and VASP were redistributed to basolateral regions. However, this was not observed in UC tissues. The authors concluded that constant redistribution of these TJ-associated molecules may constitute a latent risk of inflammatory relapse in CD and that Rab13 may play a role in the pathogenesis of CD.
Rab13 contributes to TJ formation and stability
About 20 y ago, Rab13 was found to co-localize with ZO-1 in forming and established TJ in Caco-2 and mouse intestines.140 Calcium-depletion caused redistribution of Rab13 and ZO-1 into the cytosol. Interestingly, initial E-cadherin-mediated cell-cell contact formation did not lead to Rab13 recruitment to forming AJ suggesting specific functions of Rab13 at TJ. This was confirmed later in MDCK cells by showing that downregulation of Rab13 only affected trafficking of the TJ proteins occludin and claudin-1 but not of E-cadherin. Instead, E-cadherin transport was dependent on the presence of Rab8.141 These results demonstrate that two different Rab GTPases are important for the assembly of TJ (Rab13) and AJ (Rab8).
In conclusion, Rab GTPases do play important roles for intestinal barrier homeostasis, mainly by controlling directed traffic of TJ molecules to and from the AJC (Fig. 7). Nevertheless, other mechanisms such as PKA activation and actin remodeling are regulated by Rabs in non-intestinal epithelial cells.142 If this is the case also in the gut still awaits confirmation.
Arf family
ADP-ribosylation factor (Arf) proteins play a pivotal role in the regulation of membrane traffic, sorting of cargo into vesicles and organelle structure. Besides six mammalian Arf proteins, 20 Arf-like (Arl) proteins and SAR1 belong to the Arf subfamily.22 Arf proteins can also modulate the actin cytoskeleton during formation of membrane ruffles and protrusions through effectors such as phospholipase D and members of the Rho GTPase family. Arfs are also regulated by GEFs and GAPs, but no Arf-specific GDIs have been identified yet. Arf1, Arf2 and Arf3 are active in the Golgi complex as well as in endosomes where they regulate the interaction of actin and actin-binding proteins with the Golgi complex and the assembly of different types of coat complexes into budding vesicles to organize the secretory pathway.143 On the other hand, Arf6 regulates multiple processes such as membrane trafficking, actin remodeling and activation of lipid-modifying enzymes at the plasma membrane such as phosphatidylinositol 4-phosphate 5 kinase (PIP5-kinase) and phospholipase D (PLD), thus controling membrane lipid composition and endocytosis.144,145 In epithelial cells, especially Arf-6 can interact with transmembrane proteins, such as E- cadherin and β1-integrin, and adaptor proteins such as paxillin. Thus, Arf proteins can also play important roles for the formation, stability and functional integrity of epithelial junctions as depicted in Figure 8 and listed in Tables 1 and 2.
Arf6 triggers transcytosis of E. coli induced by IFN-γ
Arf6 is a known mediator of endocytosis and actin cytoeskeletal remodeling. In T84 cells, IFN-γ treatment caused increased transcytosis of non-invasive E. coli.146 This was accompanied by significant increases in ERK1/2 activation and a significant drop in TER. However, the effect of IFN-γ on TER was not associated with reduced expression of occludin or ZO-1 indicating that IFN-γ can stimulate bacterial translocation without significant alteration of TJ composition. The authors also found that bacterial internalization and IFN-γ-stimulated ERK1/2 activation was reduced by MEK1/2, PI3K and Src inhibition. Of note, Arf6 was activated by IFN-γ and inhibited by ERK1/2, PI3K or src inhibition, and siRNA-mediated knock-down of Arf6 significantly reduced IFN-γ-induced internalization of E.coli. These data suggest that ERK1/2-mediated Arf6 activation plays an important role in IFN-γ-induced host-pathogen interaction in the gut.
Arf6 promotes PMN recruitment during Salmonella typhimurium infection
During S. typhimurium infection, transmigration of polymorphonuclear leukocytes (PMN) across the intestinal epithelium depends on the pathogen type III effector protein SipA.147 SipA recruited Arf6 to the apical plasma membrane to stimulate PLD recruitment and activation. PLD in turn produced phosphatidic acid (PA) that was metabolized by a phosphohydrolase into the second messenger diacylglycerol (DAG) which recruited cytosolic PKC to the apical membrane. Activated PKC then mediated the production and release of chemoattractants to trigger transepithelial migration of PMN. Thus, Arf6 recruitment and activation during infection transduces a complex signaling cascade that triggers PMN recruitment. Although PMN recruitment is important for bacterial clearance, excessive PMN recruitment can induce significant epithelial damage.
Arf1 and 6 mediate endocytosis and intracellular trafficking of cholera toxin
In another study, it has been shown that enterocyte maturity is important for host-pathogen interactions in the intestinal mucosa.148 The authors compared the effect of cholera toxin (CT)-mediated Gαs activation in two epithelial cell lines, immature (H4) and mature (T84). Immature cells were more susceptible to CT-induced Gαs responses leading to increased cAMP production, a higher rate of CT endocytosis, chloride secretion and diarrhea. The authors demonstrated that Arf1 and Arf6 were required in H4 cells for CT-induced ADP-ribosylation of Gαs. Arf6 was necessary for regulating clathrin-mediated CT-endocytosis, while Arf1 regulated intracellular vesicle trafficking. These results demonstrate that the machinery required for CT endocytosis including Arfs are different in immature enterocytes compared with mature enterocytes.
The Arf1-GEF Golgi brefeldin-sensitive factor 1 controls AJC stability
Recently, an important role for the Arf1-GEF Golgi brefeldin-sensitive factor 1 (GBF1) has been demonstrated for AJC stability.149 Downregulation of GBF1 caused disassembly of both TJ and AJ leading to increased permeability as measured by TER. This effect was most likely mediated by disrupted Golgi function and vesicular transport of junction proteins from the Golgi to the apical plasma membrane. However, if these GBF1 effects are indeed a direct consequence of reduced Arf1 activation needs to be evaluated.
Arf6 as target during EPEC infection
EPEC infection is a major cause of diarrhea in the developing world associated with TJ disruption. The EPEC effector proteins EspG1 and EspG2 affected intestinal epithelial cell TJ via disruption of microtubule networks, thus preventing TJ restoration and contributing to EPEC-induced loss of barrier function.150 Another study also demonstrated EspG-induced stress fiber formation via activation of the GEF-H1/RhoA/ROCK1 axis.151 Crystal structure analyses revealed that EspG binds to GTP-loaded Arf6 to block GAP-mediated GTP hydrolysis.152 However, GTP hydrolysis and exchange on Arf6 has been shown to be required for proper membrane transport function suggesting that EspG can inhibit Golgi trafficking by blocking the guanine nucleotide cycle of Arf GTPases. Thus, it is tempting to speculate that EspG alters epithelial junction structure by interfering with Arf signaling to inhibit vesicular transport. If this indeed has consequences for epithelial barrier integrity and pathogen invasion remains to be investigated. In general, Arf GTPases seem to play a crucial role in host-pathogen interactions.
Arfrp1-deficiency causes malnutrition
Arfrp1 (ADP-ribosilation factor-related protein1) plays an important role in the regulation of membrane traffic.153 Recently, Arfrp1 has been implicated in the uptake and processing of dietary lipids in the small intestine.154 Using mice lacking intestinal Arfrp1 (Arfrp1vil−/−), the authors demonstrated that Arfrp1vil−/−-mice suffered from malnutrition. Studying the mechanism using Arfrp1-depleted Caco-2 cells, the authors found that Arfrp1, Rab2 and ApoA-1 were all localized at the trans-Golgi compartment and downregulation of either Rab2, ARL1 or Golgin-245 reduced the release of chylomicrons suggesting that an Arfrp1-Arl1-Golgin-Rab2 casacade is required for correct composition of chylomicrons in intestinal epithelial cells and distribution of nutrients. This mechanism is very important because several human diseases are characterized by defects in the synthesis and secretion of lipoproteins. However, whether this process is somehow related to epithelial barrier function is not known yet.
Arfrp1 and Arl1 are required for E-cadherin trafficking and AJ homeostasis
Another study using Arfrp1vil−/−-mice and an Arfrp1-depleted Hela cell line demonstrated that Arfrp1 was required for correct cell surface localization of the E-cadherin-catenin complex.155 In enterocytes of Arfrp1vil−/−-mice, E-cadherin was localized in intracellular compartments colocalizing with cis-Golgi structures, whereas in WT control enterocytes E-cadherin was normally localized at the lateral membrane suggesting that E-cadherin is retained in the Golgi without Arfrp1. Arl1 was also affected in Arfrp1vil−/−enterocytes by relocating from the Golgi membrane to the cytosol. However, downregulation of Arl1 alone did not lead to mistargeting of E-cadherin in Hela cells. Interestingly, E-cadherin and its binding partners α-catenin, β-catenin, γ-catenin and p120-catenin co-immunoprecipitated with Arfrp1 from MDCK lysates overexpressing myc-Arfrp1 suggesting direct interactions among these proteins. On the other hand, knockdown of Arfrp1 in the mouse fibroblast cell line Ltk-Ecad that stably expresses E-cadherin resulted in significantly reduced E-cadherin mediated cell-cell adhesion. These results suggest that Arfrp1 has an important role in the transport of E-cadherin from the Golgi to the plasma membrane and E-cadherin-mediated adhesion. Since Arf6 has also been shown to regulate dynamin-dependent endocytosis of E-cadherin in MDCK cells,156 it seems likely that Arf-controlled E-cadherin trafficking is a general mechanism in epithelial cells to regulate AJ homeostasis.
Conclusions
Obviously, signaling by small GTPases is of utmost importance to regulate cellular processes throughout the body. This is reflected by an overwhelming amount of studies on GTPase functions. Mechanisms of GTPase signaling have been extensively studied in various model organisms and cell types. While some of the known mechanisms have been confirmed to also occur in the intestines, others still await confirmation. However, accumulating evidence suggest that mechanisms identified in other organs and cell types also play a crucial role for the regulation of intestinal barrier integrity. These studies revealed that several GTPases of the Ras superfamily contribute to epithelial barrier function via common mechanisms such as regulation of actin dynamics, endosomal trafficking or activation of kinases among others (compare Tables 1 and 2). Only few mechanisms have so far been attributed to just one GTPase (Table 2). However, this may either be due to a true unique mechanism or solely because the common factors with other GTPases have not yet been identified.
The abundance of GTPases themselves and their regulators and effectors makes this field of investigation a very challenging but exciting task. Several open questions remain that need to be addressed in the future to reveal the usefulness of GTPase-mediated signaling pathways as targets for treatment of IBD and infectious intestinal diseases. First, we are only beginning to understand how GTPases are regulated in space and time to achieve different tasks depending on the status of the cell. Second, it will be important to better understand the mechanisms how signal and target specificity is regulated. For example, a given stimulus can activate several GTPases and one GTPase can be activated by various stimuli. The same is true for effector molecules. One effector can be activated by several GTPases while one GTPase can activate various effectors. However, in some cases only one GTPase is activated which then specifically targets one effector molecule. To better understand these principles will be instrumental for developing specific disease treatment strategies that interfere with GTPase signaling. Third, unraveling the network of GTPase interactions among themselves will also be helpful to understand the bridging of different pathways underlying GTPase-driven cellular responses. Fourth, several TJ and AJ components have been shown to interact with GEFs, GAPs or directly with GTPases to regulate their activation. However, the mechanisms by which GEFs and GAPs are finally modified remain elusive. Last but not least, even though knockout models exist for various GTPases and their regulators, a lot of mechanisms were examined in vitro and have not yet been confirmed in vivo to corroborate the physiological significance.
Addressing these questions will contribute to a better understanding of GTPase-mediated intestinal epithelial homeostasis and maybe to the development of novel treatment strategies for acute and chronic diseases that are characterized by epithelial barrier dysfunction.
Acknowledgments
We apologize to all colleagues whose relevant work has not been cited here due to space limitations. This work was supported by a grant to MS from the Mexican Council for Science and Technology (CONACyT, 179895).
Glossary
Abbreviations:
- AJ
adherens junction
- AJC
apical junctional complex
- Arf
ADP-ribosylation factor
- Cdc42
cell division cycle 42
- DSS
dextran sulfate sodium
- EGF
epidermal growth factor
- EPEC
enteropathogenic Escherichia coli
- ERK
extracellular-regulated kinase
- FPR
formyl peptide receptor-1
- GDP
guanylate-diphosphate
- GTP
guanylate-triphosphate
- GAP
GTPase-activating protein
- GDI
guanine nucleotide dissociation inhibitor
- GEF
guanine nucleotide exchange factor
- IBD
inflammatory bowel disease
- IEC
intestinal epithelial cells
- IL
interleukin
- JAM
junctional adhesion molecule
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MDCK
Madine Darby canine kidney
- mDia1
mammalian homolog of Drosophila diaphanous 1
- MEK
MAP/ERK kinase
- MLC
myosin light chain
- MLCP
myosin light chain phosphatase
- mTOR
mammalian target of rapamycin
- N-WASP
neural Wiskott-Aldrich syndrome protein
- PI3K
phosphatidyl-inositol-3-kinase
- Rac1
ras-related C3 botulinum toxin substrate 1
- Rap1
ras-related protein 1
- Ras
rat sarcoma viral oncogene homolog
- RhoA
ras homolog A
- ROCK1
Rho kinase 1
- TcdA/B
Clostridium difficile toxin A/B
- TJ
tight junction
- TNBS
2,4,6-trinitrobenzene sulfonic acid
- ZO
zonula occludens
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/tissuebarriers/article/26938
References
- 1.Koch S, Nusrat A. The life and death of epithelia during inflammation: lessons learned from the gut. Annu Rev Pathol. 2012;7:35–60. doi: 10.1146/annurev-pathol-011811-120905. [DOI] [PubMed] [Google Scholar]
- 2.Hering NA, Fromm M, Schulzke JD. Determinants of colonic barrier function in inflammatory bowel disease and potential therapeutics. J Physiol. 2012;590:1035–44. doi: 10.1113/jphysiol.2011.224568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–9. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brooke MA, Nitoiu D, Kelsell DP. Cell-cell connectivity: desmosomes and disease. J Pathol. 2012;226:158–71. doi: 10.1002/path.3027. [DOI] [PubMed] [Google Scholar]
- 5.Citi S, Pulimeno P, Paschoud S. Cingulin, paracingulin, and PLEKHA7: signaling and cytoskeletal adaptors at the apical junctional complex. Ann N Y Acad Sci. 2012;1257:125–32. doi: 10.1111/j.1749-6632.2012.06506.x. [DOI] [PubMed] [Google Scholar]
- 6.Ivanov AI. Structure and regulation of intestinal epithelial tight junctions: current concepts and unanswered questions. Adv Exp Med Biol. 2012;763:132–48. doi: 10.1007/978-1-4614-4711-5_6. [DOI] [PubMed] [Google Scholar]
- 7.Ivanov AI, Naydenov NG. Dynamics and regulation of epithelial adherens junctions: recent discoveries and controversies. Int Rev Cell Mol Biol. 2013;303:27–99. doi: 10.1016/B978-0-12-407697-6.00002-7. [DOI] [PubMed] [Google Scholar]
- 8.Marchiando AM, Graham WV, Turner JR. Epithelial barriers in homeostasis and disease. Annu Rev Pathol. 2010;5:119–44. doi: 10.1146/annurev.pathol.4.110807.092135. [DOI] [PubMed] [Google Scholar]
- 9.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics, and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:a000125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanov AI, Parkos CA, Nusrat A. Cytoskeletal regulation of epithelial barrier function during inflammation. Am J Pathol. 2010;177:512–24. doi: 10.2353/ajpath.2010.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodgers LS, Fanning AS. Regulation of epithelial permeability by the actin cytoskeleton. Cytoskeleton (Hoboken) 2011;68:653–60. doi: 10.1002/cm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–36. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Betanzos A, Javier-Reyna R, García-Rivera G, Bañuelos C, González-Mariscal L, Schnoor M, Orozco E. The EhCPADH112 complex of Entamoeba histolytica interacts with tight junction proteins occludin and claudin-1 to produce epithelial damage. PLoS One. 2013;8:e65100. doi: 10.1371/journal.pone.0065100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shifflett DE, Clayburgh DR, Koutsouris A, Turner JR, Hecht GA. Enteropathogenic E. coli disrupts tight junction barrier function and structure in vivo. Lab Invest. 2005;85:1308–24. doi: 10.1038/labinvest.3700330. [DOI] [PubMed] [Google Scholar]
- 15.Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- 16.Menke A, Giehl K. Regulation of adherens junctions by Rho GTPases and p120-catenin. Arch Biochem Biophys. 2012;524:48–55. doi: 10.1016/j.abb.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Vetter IR, Wittinghofer A. The guanine nucleotide-binding switch in three dimensions. Science. 2001;294:1299–304. doi: 10.1126/science.1062023. [DOI] [PubMed] [Google Scholar]
- 18.Cherfils J, Zeghouf M. Regulation of small GTPases by GEFs, GAPs, and GDIs. Physiol Rev. 2013;93:269–309. doi: 10.1152/physrev.00003.2012. [DOI] [PubMed] [Google Scholar]
- 19.Garcia-Mata R, Boulter E, Burridge K. The ‘invisible hand’: regulation of RHO GTPases by RHOGDIs. Nat Rev Mol Cell Biol. 2011;12:493–504. doi: 10.1038/nrm3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammar E, Tomas A, Bosco D, Halban PA. Role of the Rho-ROCK (Rho-associated kinase) signaling pathway in the regulation of pancreatic beta-cell function. Endocrinology. 2009;150:2072–9. doi: 10.1210/en.2008-1135. [DOI] [PubMed] [Google Scholar]
- 21.Bruewer M, Hopkins AM, Hobert ME, Nusrat A, Madara JL, Rho A. RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am J Physiol Cell Physiol. 2004;287:C327–35. doi: 10.1152/ajpcell.00087.2004. [DOI] [PubMed] [Google Scholar]
- 22.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–75. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young A, Lyons J, Miller AL, Phan VT, Alarcón IR, McCormick F. Ras signaling and therapies. Adv Cancer Res. 2009;102:1–17. doi: 10.1016/S0065-230X(09)02001-6. [DOI] [PubMed] [Google Scholar]
- 24.Braga VM, Yap AS. The challenges of abundance: epithelial junctions and small GTPase signalling. Curr Opin Cell Biol. 2005;17:466–74. doi: 10.1016/j.ceb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 25.Citi S, Spadaro D, Schneider Y, Stutz J, Pulimeno P. Regulation of small GTPases at epithelial cell-cell junctions. Mol Membr Biol. 2011;28:427–44. doi: 10.3109/09687688.2011.603101. [DOI] [PubMed] [Google Scholar]
- 26.Johnson DS, Chen YH. Ras family of small GTPases in immunity and inflammation. Curr Opin Pharmacol. 2012;12:458–63. doi: 10.1016/j.coph.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X, Bu X, Lu B, Avraham H, Flavell RA, Lim B. The hematopoiesis-specific GTP-binding protein RhoH is GTPase deficient and modulates activities of other Rho GTPases by an inhibitory function. Mol Cell Biol. 2002;22:1158–71. doi: 10.1128/MCB.22.4.1158-1171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ongusaha PP, Kim HG, Boswell SA, Ridley AJ, Der CJ, Dotto GP, Kim YB, Aaronson SA, Lee SW. RhoE is a pro-survival p53 target gene that inhibits ROCK I-mediated apoptosis in response to genotoxic stress. Curr Biol. 2006;16:2466–72. doi: 10.1016/j.cub.2006.10.056. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Reedquist KA, Tak PP. Signal transduction pathways in chronic inflammatory autoimmune disease: small GTPases. Open Rheumatol J. 2012;6:259–72. doi: 10.2174/1874312901206010259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel MCJF, Côté JF. Ras GTPases’ interaction with effector domains: Breaking the families’ barrier. Commun Integr Biol. 2013;6:e24298. doi: 10.4161/cib.24298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffe AB, Hall A. Rho GTPases: biochemistry and biology. Annu Rev Cell Dev Biol. 2005;21:247–69. doi: 10.1146/annurev.cellbio.21.020604.150721. [DOI] [PubMed] [Google Scholar]
- 32.Hutagalung AH, Novick PJ. Role of Rab GTPases in membrane traffic and cell physiology. Physiol Rev. 2011;91:119–49. doi: 10.1152/physrev.00059.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yudin D, Fainzilber M. Ran on tracks--cytoplasmic roles for a nuclear regulator. J Cell Sci. 2009;122:587–93. doi: 10.1242/jcs.015289. [DOI] [PubMed] [Google Scholar]
- 34.Inoki K, Li Y, Xu T, Guan KL. Rheb GTPase is a direct target of TSC2 GAP activity and regulates mTOR signaling. Genes Dev. 2003;17:1829–34. doi: 10.1101/gad.1110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fransson A, Ruusala A, Aspenström P. Atypical Rho GTPases have roles in mitochondrial homeostasis and apoptosis. J Biol Chem. 2003;278:6495–502. doi: 10.1074/jbc.M208609200. [DOI] [PubMed] [Google Scholar]
- 36.Boureux A, Vignal E, Faure S, Fort P. Evolution of the Rho family of ras-like GTPases in eukaryotes. Mol Biol Evol. 2007;24:203–16. doi: 10.1093/molbev/msl145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delprato A. Topological and functional properties of the small GTPases protein interaction network. PLoS One. 2012;7:e44882. doi: 10.1371/journal.pone.0044882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakano K, Takaishi K, Kodama A, Mammoto A, Shiozaki H, Monden M, Takai Y. Distinct actions and cooperative roles of ROCK and mDia in Rho small G protein-induced reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Mol Biol Cell. 1999;10:2481–91. doi: 10.1091/mbc.10.8.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menke A, Giehl K. Regulation of adherens junctions by Rho GTPases and p120-catenin. Arch Biochem Biophys. 2012;524:48–55. doi: 10.1016/j.abb.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 40.Terry S, Nie M, Matter K, Balda MS. Rho signaling and tight junction functions. Physiology (Bethesda) 2010;25:16–26. doi: 10.1152/physiol.00034.2009. [DOI] [PubMed] [Google Scholar]
- 41.Popoff MR, Geny B. Multifaceted role of Rho, Rac, Cdc42 and Ras in intercellular junctions, lessons from toxins. Biochim Biophys Acta. 2009;1788:797–812. doi: 10.1016/j.bbamem.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 42.Pertz O. Spatio-temporal Rho GTPase signaling - where are we now? J Cell Sci. 2010;123:1841–50. doi: 10.1242/jcs.064345. [DOI] [PubMed] [Google Scholar]
- 43.Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–27. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noren NK, Niessen CM, Gumbiner BM, Burridge K. Cadherin engagement regulates Rho family GTPases. J Biol Chem. 2001;276:33305–8. doi: 10.1074/jbc.C100306200. [DOI] [PubMed] [Google Scholar]
- 45.Aijaz S, D’Atri F, Citi S, Balda MS, Matter K. Binding of GEF-H1 to the tight junction-associated adaptor cingulin results in inhibition of Rho signaling and G1/S phase transition. Dev Cell. 2005;8:777–86. doi: 10.1016/j.devcel.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 46.Samarin SN, Ivanov AI, Flatau G, Parkos CA, Nusrat A. Rho/Rho-associated kinase-II signaling mediates disassembly of epithelial apical junctions. Mol Biol Cell. 2007;18:3429–39. doi: 10.1091/mbc.E07-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Terry SJ, Zihni C, Elbediwy A, Vitiello E, Leefa Chong San IV, Balda MS, Matter K. Spatially restricted activation of RhoA signalling at epithelial junctions by p114RhoGEF drives junction formation and morphogenesis. Nat Cell Biol. 2011;13:159–66. doi: 10.1038/ncb2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Itoh M, Tsukita S, Yamazaki Y, Sugimoto H. Rho GTP exchange factor ARHGEF11 regulates the integrity of epithelial junctions by connecting ZO-1 and RhoA-myosin II signaling. Proc Natl Acad Sci U S A. 2012;109:9905–10. doi: 10.1073/pnas.1115063109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Itoh M. ARHGEF11, a regulator of junction-associated actomyosin in epithelial cells. Tissue Barriers. 2013;1:e24221. doi: 10.4161/tisb.24221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. 2007;87:807–17. doi: 10.1038/labinvest.3700595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brazil JC, Louis NA, Parkos CA. The role of polymorphonuclear leukocyte trafficking in the perpetuation of inflammation during inflammatory bowel disease. Inflamm Bowel Dis. 2013;19:1556–65. doi: 10.1097/MIB.0b013e318281f54e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaturvedi LS, Marsh HM, Basson MD. Role of RhoA and its effectors ROCK and mDia1 in the modulation of deformation-induced FAK, ERK, p38, and MLC motogenic signals in human Caco-2 intestinal epithelial cells. Am J Physiol Cell Physiol. 2011;301:C1224–38. doi: 10.1152/ajpcell.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.MacManus CF, Campbell EL, Keely S, Burgess A, Kominsky DJ, Colgan SP. Anti-inflammatory actions of adrenomedullin through fine tuning of HIF stabilization. FASEB J. 2011;25:1856–64. doi: 10.1096/fj.10-170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ashizuka S, Inagaki-Ohara K, Kuwasako K, Kato J, Inatsu H, Kitamura K. Adrenomedullin treatment reduces intestinal inflammation and maintains epithelial barrier function in mice administered dextran sulphate sodium. Microbiol Immunol. 2009;53:573–81. doi: 10.1111/j.1348-0421.2009.00159.x. [DOI] [PubMed] [Google Scholar]
- 55.Zimmerman NP, Kumar SN, Turner JR, Dwinell MB. Cyclic AMP dysregulates intestinal epithelial cell restitution through PKA and RhoA. Inflamm Bowel Dis. 2012;18:1081–91. doi: 10.1002/ibd.21898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nusrat A, Giry M, Turner JR, Colgan SP, Parkos CA, Carnes D, Lemichez E, Boquet P, Madara JL. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci U S A. 1995;92:10629–33. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Samarin S, Nusrat A. Regulation of epithelial apical junctional complex by Rho family GTPases. Front Biosci (Landmark Ed) 2009;14:1129–42. doi: 10.2741/3298. [DOI] [PubMed] [Google Scholar]
- 58.Schlegel N, Meir M, Spindler V, Germer CT, Waschke J. Differential role of Rho GTPases in intestinal epithelial barrier regulation in vitro. J Cell Physiol. 2011;226:1196–203. doi: 10.1002/jcp.22446. [DOI] [PubMed] [Google Scholar]
- 59.Bruewer M, Luegering A, Kucharzik T, Parkos CA, Madara JL, Hopkins AM, Nusrat A. Proinflammatory cytokines disrupt epithelial barrier function by apoptosis-independent mechanisms. J Immunol. 2003;171:6164–72. doi: 10.4049/jimmunol.171.11.6164. [DOI] [PubMed] [Google Scholar]
- 60.Utech M, Ivanov AI, Samarin SN, Bruewer M, Turner JR, Mrsny RJ, Parkos CA, Nusrat A. Mechanism of IFN-gamma-induced endocytosis of tight junction proteins: myosin II-dependent vacuolarization of the apical plasma membrane. Mol Biol Cell. 2005;16:5040–52. doi: 10.1091/mbc.E05-03-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mihaescu A, Santén S, Jeppsson B, Thorlacius H. Rho kinase signalling mediates radiation-induced inflammation and intestinal barrier dysfunction. Br J Surg. 2011;98:124–31. doi: 10.1002/bjs.7279. [DOI] [PubMed] [Google Scholar]
- 62.Chandhoke SK, Mooseker MS. A role for myosin IXb, a motor-RhoGAP chimera, in epithelial wound healing and tight junction regulation. Mol Biol Cell. 2012;23:2468–80. doi: 10.1091/mbc.E11-09-0803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Bodegraven AA, Curley CR, Hunt KA, Monsuur AJ, Linskens RK, Onnie CM, Crusius JB, Annese V, Latiano A, Silverberg MS, et al. Genetic variation in myosin IXB is associated with ulcerative colitis. Gastroenterology. 2006;131:1768–74. doi: 10.1053/j.gastro.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Núñez C, Oliver J, Mendoza JL, Gómez-García M, Piñero A, Taxonera C, Díaz-Rubio M, López-Nevot MA, de la Concha EG, Nieto A, et al. MYO9B polymorphisms in patients with inflammatory bowel disease. Gut. 2007;56:1321–2. doi: 10.1136/gut.2007.121905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abouhamed M, Grobe K, San IV, Thelen S, Honnert U, Balda MS, Matter K, Bähler M. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol Biol Cell. 2009;20:5074–85. doi: 10.1091/mbc.E09-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pijls KE, Jonkers DM, Elamin EE, Masclee AA, Koek GH. Intestinal epithelial barrier function in liver cirrhosis: an extensive review of the literature. Liver Int. 2013;33:1457–69. doi: 10.1111/liv.12271. [DOI] [PubMed] [Google Scholar]
- 67.Elamin E, Jonkers D, Juuti-Uusitalo K, van Ijzendoorn S, Troost F, Duimel H, Broers J, Verheyen F, Dekker J, Masclee A. Effects of ethanol and acetaldehyde on tight junction integrity: in vitro study in a three dimensional intestinal epithelial cell culture model. PLoS One. 2012;7:e35008. doi: 10.1371/journal.pone.0035008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keshavarzian A, Farhadi A, Forsyth CB, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ. Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J Hepatol. 2009;50:538–47. doi: 10.1016/j.jhep.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tong J, Wang Y, Chang B, Zhang D, Wang B. Evidence for the Involvement of RhoA Signaling in the Ethanol-Induced Increase in Intestinal Epithelial Barrier Permeability. Int J Mol Sci. 2013;14:3946–60. doi: 10.3390/ijms14023946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schnoor M, Louis NA. Inflammatory Mediators Contributing to Intestinal Epithelial Cell Apoptosis and Barrier Disruption in IBD. J Clin Cell Immunol. 2011;S3:003. [Google Scholar]
- 71.Just I, Gerhard R. Large clostridial cytotoxins. Rev Physiol Biochem Pharmacol. 2004;152:23–47. doi: 10.1007/s10254-004-0033-5. [DOI] [PubMed] [Google Scholar]
- 72.Gerhard R, Nottrott S, Schoentaube J, Tatge H, Olling A, Just I. Glucosylation of Rho GTPases by Clostridium difficile toxin A triggers apoptosis in intestinal epithelial cells. J Med Microbiol. 2008;57:765–70. doi: 10.1099/jmm.0.47769-0. [DOI] [PubMed] [Google Scholar]
- 73.Santos AA, Braga-Neto MB, Oliveira MR, Freire RS, Barros EB, Santiago TM, Rebelo LM, Mermelstein C, Warren CA, Guerrant RL, et al. Glutamine and alanyl-glutamine increase RhoA expression and reduce Clostridium difficile toxin-a-induced intestinal epithelial cell damage. Biomed Res Int. 2013;2013:152052. doi: 10.1155/2013/152052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gerhard R, Schmidt G, Hofmann F, Aktories K. Activation of Rho GTPases by Escherichia coli cytotoxic necrotizing factor 1 increases intestinal permeability in Caco-2 cells. Infect Immun. 1998;66:5125–31. doi: 10.1128/iai.66.11.5125-5131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hopkins AM, Walsh SV, Verkade P, Boquet P, Nusrat A. Constitutive activation of Rho proteins by CNF-1 influences tight junction structure and epithelial barrier function. J Cell Sci. 2003;116:725–42. doi: 10.1242/jcs.00300. [DOI] [PubMed] [Google Scholar]
- 76.Sangari FJ, Goodman J, Bermudez LE. Mycobacterium avium enters intestinal epithelial cells through the apical membrane, but not by the basolateral surface, activates small GTPase Rho and, once within epithelial cells, expresses an invasive phenotype. Cell Microbiol. 2000;2:561–8. doi: 10.1046/j.1462-5822.2000.00080.x. [DOI] [PubMed] [Google Scholar]
- 77.Hänisch J, Kölm R, Wozniczka M, Bumann D, Rottner K, Stradal TE. Activation of a RhoA/myosin II-dependent but Arp2/3 complex-independent pathway facilitates Salmonella invasion. Cell Host Microbe. 2011;9:273–85. doi: 10.1016/j.chom.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 78.Keestra AM, Winter MG, Auburger JJ, Frässle SP, Xavier MN, Winter SE, Kim A, Poon V, Ravesloot MM, Waldenmaier JF, et al. Manipulation of small Rho GTPases is a pathogen-induced process detected by NOD1. Nature. 2013;496:233–7. doi: 10.1038/nature12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng Y, Bi F. miR-21 targets the tumor suppressor RhoB and regulates proliferation, invasion and apoptosis in colorectal cancer cells. FEBS Lett. 2011;585:2998–3005. doi: 10.1016/j.febslet.2011.08.014. [DOI] [PubMed] [Google Scholar]
- 80.Zhou J, Zhu Y, Zhang G, Liu N, Sun L, Liu M, Qiu M, Luo D, Tang Q, Liao Z, et al. A distinct role of RhoB in gastric cancer suppression. Int J Cancer. 2011;128:1057–68. doi: 10.1002/ijc.25445. [DOI] [PubMed] [Google Scholar]
- 81.Shi C, Liang Y, Yang J, Xia Y, Chen H, Han H, Yang Y, Wu W, Gao R, Qin H. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS One. 2013;8:e66814. doi: 10.1371/journal.pone.0066814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang Y, Ma Y, Shi C, Chen H, Zhang H, Chen N, Zhang P, Wang F, Yang J, Yang J, et al. Overexpression of miR-21 in patients with ulcerative colitis impairs intestinal epithelial barrier function through targeting the Rho GTPase RhoB. Biochem Biophys Res Commun. 2013;434:746–52. doi: 10.1016/j.bbrc.2013.03.122. [DOI] [PubMed] [Google Scholar]
- 83.Iizuka M, Konno S. Wound healing of intestinal epithelial cells. World J Gastroenterol. 2011;17:2161–71. doi: 10.3748/wjg.v17.i17.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Babbin BA, Jesaitis AJ, Ivanov AI, Kelly D, Laukoetter M, Nava P, Parkos CA, Nusrat A. Formyl peptide receptor-1 activation enhances intestinal epithelial cell restitution through phosphatidylinositol 3-kinase-dependent activation of Rac1 and Cdc42. J Immunol. 2007;179:8112–21. doi: 10.4049/jimmunol.179.12.8112. [DOI] [PubMed] [Google Scholar]
- 85.Dise RS, Frey MR, Whitehead RH, Polk DB. Epidermal growth factor stimulates Rac activation through Src and phosphatidylinositol 3-kinase to promote colonic epithelial cell migration. Am J Physiol Gastrointest Liver Physiol. 2008;294:G276–85. doi: 10.1152/ajpgi.00340.2007. [DOI] [PubMed] [Google Scholar]
- 86.Ray RM, Vaidya RJ, Johnson LR. MEK/ERK regulates adherens junctions and migration through Rac1. Cell Motil Cytoskeleton. 2007;64:143–56. doi: 10.1002/cm.20172. [DOI] [PubMed] [Google Scholar]
- 87.Ray RM, Guo H, Patel M, Jin S, Bhattacharya S, Johnson LR. Role of myosin regulatory light chain and Rac1 in the migration of polyamine-depleted intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2007;292:G983–95. doi: 10.1152/ajpgi.00356.2006. [DOI] [PubMed] [Google Scholar]
- 88.Espejo R, Rengifo-Cam W, Schaller MD, Evers BM, Sastry SK. PTP-PEST controls motility, adherens junction assembly, and Rho GTPase activity in colon cancer cells. Am J Physiol Cell Physiol. 2010;299:C454–63. doi: 10.1152/ajpcell.00148.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lemichez E, Aktories K. Hijacking of Rho GTPases during bacterial infection. Exp Cell Res. 2013;319:2329–36. doi: 10.1016/j.yexcr.2013.04.021. [DOI] [PubMed] [Google Scholar]
- 90.Schoentaube J, Olling A, Tatge H, Just I, Gerhard R. Serine-71 phosphorylation of Rac1/Cdc42 diminishes the pathogenic effect of Clostridium difficile toxin A. Cell Microbiol. 2009;11:1816–26. doi: 10.1111/j.1462-5822.2009.01373.x. [DOI] [PubMed] [Google Scholar]
- 91.Jin S, Ray RM, Johnson LR. Rac1 mediates intestinal epithelial cell apoptosis via JNK. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1137–47. doi: 10.1152/ajpgi.00031.2006. [DOI] [PubMed] [Google Scholar]
- 92.Kim KA, Kim JY, Lee YA, Min A, Bahk YY, Shin MH. Entamoeba histolytica induces cell death of HT29 colonic epithelial cells via NOX1-derived ROS. Korean J Parasitol. 2013;51:61–8. doi: 10.3347/kjp.2013.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Muise AM, Walters T, Xu W, Shen-Tu G, Guo CH, Fattouh R, Lam GY, Wolters VM, Bennitz J, van Limbergen J, et al. Single nucleotide polymorphisms that increase expression of the guanosine triphosphatase RAC1 are associated with ulcerative colitis. Gastroenterology. 2011;141:633–41. doi: 10.1053/j.gastro.2011.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Muise AM, Xu W, Guo CH, Walters TD, Wolters VM, Fattouh R, Lam GY, Hu P, Murchie R, Sherlock M, et al. NEOPICS NADPH oxidase complex and IBD candidate gene studies: identification of a rare variant in NCF2 that results in reduced binding to RAC2. Gut. 2012;61:1028–35. doi: 10.1136/gutjnl-2011-300078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, et al. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–96. doi: 10.1016/S1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 96.Kurkchubasche AG, Panepinto JA, Tracy TF, Jr., Thurman GW, Ambruso DR. Clinical features of a human Rac2 mutation: a complex neutrophil dysfunction disease. J Pediatr. 2001;139:141–7. doi: 10.1067/mpd.2001.114718. [DOI] [PubMed] [Google Scholar]
- 97.Fattouh R, Guo CH, Lam GY, Gareau MG, Ngan BY, Glogauer M, Muise AM, Brumell JH. Rac2-deficiency leads to exacerbated and protracted colitis in response to Citrobacter rodentium infection. PLoS One. 2013;8:e61629. doi: 10.1371/journal.pone.0061629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Surviladze Z, Waller A, Strouse JJ, Bologa C, Ursu O, Salas V, Parkinson JF, Phillips GK, Romero E, Wandinger-Ness A, et al. A Potent and Selective Inhibitor of Cdc42 GTPase. Probe Reports from the NIH Molecular Libraries Program. Bethesda (MD), 2010. [PubMed] [Google Scholar]
- 99.Jaffe AB, Kaji N, Durgan J, Hall A. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol. 2008;183:625–33. doi: 10.1083/jcb.200807121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Durgan J, Kaji N, Jin D, Hall A. Par6B and atypical PKC regulate mitotic spindle orientation during epithelial morphogenesis. J Biol Chem. 2011;286:12461–74. doi: 10.1074/jbc.M110.174235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kovacs EM, Verma S, Thomas SG, Yap AS. Tuba and N-WASP function cooperatively to position the central lumen during epithelial cyst morphogenesis. Cell Adh Migr. 2011;5:344–50. doi: 10.4161/cam.5.4.16717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Otani T, Ichii T, Aono S, Takeichi M. Cdc42 GEF Tuba regulates the junctional configuration of simple epithelial cells. J Cell Biol. 2006;175:135–46. doi: 10.1083/jcb.200605012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Melendez J, Liu M, Sampson L, Akunuru S, Han X, Vallance J, Witte D, Shroyer N, Zheng Y. Cdc42 coordinates proliferation, polarity, migration, and differentiation of small intestinal epithelial cells in mice. Gastroenterology. 2013;145:808–19. doi: 10.1053/j.gastro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sakamori R, Das S, Yu S, Feng S, Stypulkowski E, Guan Y, Douard V, Tang W, Ferraris RP, Harada A, et al. Cdc42 and Rab8a are critical for intestinal stem cell division, survival, and differentiation in mice. J Clin Invest. 2012;122:1052–65. doi: 10.1172/JCI60282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elbediwy A, Zihni C, Terry SJ, Clark P, Matter K, Balda MS. Epithelial junction formation requires confinement of Cdc42 activity by a novel SH3BP1 complex. J Cell Biol. 2012;198:677–93. doi: 10.1083/jcb.201202094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Walsh-Reitz MM, Huang EF, Musch MW, Chang EB, Martin TE, Kartha S, Toback FG. AMP-18 protects barrier function of colonic epithelial cells: role of tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2005;289:G163–71. doi: 10.1152/ajpgi.00013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chen P, Kartha S, Bissonnette M, Hart J, Toback FG. AMP-18 facilitates assembly and stabilization of tight junctions to protect the colonic mucosal barrier. Inflamm Bowel Dis. 2012;18:1749–59. doi: 10.1002/ibd.22886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Müller AJ, Hoffmann C, Galle M, Van Den Broeke A, Heikenwalder M, Falter L, Misselwitz B, Kremer M, Beyaert R, Hardt WD. The S. Typhimurium effector SopE induces caspase-1 activation in stromal cells to initiate gut inflammation. Cell Host Microbe. 2009;6:125–36. doi: 10.1016/j.chom.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 109.Krause-Gruszczynska M, Rohde M, Hartig R, Genth H, Schmidt G, Keo T, König W, Miller WG, Konkel ME, Backert S. Role of the small Rho GTPases Rac1 and Cdc42 in host cell invasion of Campylobacter jejuni. Cell Microbiol. 2007;9:2431–44. doi: 10.1111/j.1462-5822.2007.00971.x. [DOI] [PubMed] [Google Scholar]
- 110.Krause-Gruszczynska M, Boehm M, Rohde M, Tegtmeyer N, Takahashi S, Buday L, Oyarzabal OA, Backert S. The signaling pathway of Campylobacter jejuni-induced Cdc42 activation: Role of fibronectin, integrin beta1, tyrosine kinases and guanine exchange factor Vav2. Cell Commun Signal. 2011;9:32. doi: 10.1186/1478-811X-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harvey JJ. An Unidentified Virus Which Causes the Rapid Production of Tumours in Mice. Nature. 1964;204:1104–5. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- 112.Ellis RW, Defeo D, Shih TY, Gonda MA, Young HA, Tsuchida N, Lowy DR, Scolnick EM. The p21 src genes of Harvey and Kirsten sarcoma viruses originate from divergent members of a family of normal vertebrate genes. Nature. 1981;292:506–11. doi: 10.1038/292506a0. [DOI] [PubMed] [Google Scholar]
- 113.Trahey M, McCormick F. A cytoplasmic protein stimulates normal N-ras p21 GTPase, but does not affect oncogenic mutants. Science. 1987;238:542–5. doi: 10.1126/science.2821624. [DOI] [PubMed] [Google Scholar]
- 114.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mor A, Philips MR. Compartmentalized Ras/MAPK signaling. Annu Rev Immunol. 2006;24:771–800. doi: 10.1146/annurev.immunol.24.021605.090723. [DOI] [PubMed] [Google Scholar]
- 116.Buday L, Downward J. Many faces of Ras activation. Biochim Biophys Acta. 2008;1786:178–87. doi: 10.1016/j.bbcan.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 117.Pannekoek WJ, Kooistra MR, Zwartkruis FJ, Bos JL. Cell-cell junction formation: the role of Rap1 and Rap1 guanine nucleotide exchange factors. Biochim Biophys Acta. 2009;1788:790–6. doi: 10.1016/j.bbamem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 118.Bos JL, Fearon ER, Hamilton SR, Verlaan-de Vries M, van Boom JH, van der Eb AJ, Vogelstein B. Prevalence of ras gene mutations in human colorectal cancers. Nature. 1987;327:293–7. doi: 10.1038/327293a0. [DOI] [PubMed] [Google Scholar]
- 119.Yan F, John SK, Wilson G, Jones DS, Washington MK, Polk DB. Kinase suppressor of Ras-1 protects intestinal epithelium from cytokine-mediated apoptosis during inflammation. J Clin Invest. 2004;114:1272–80. doi: 10.1172/JCI21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Oron T, Elad-Sfadia G, Haklai R, Aizman E, Brazowski E, Kloog Y, Reif S. Prevention of induced colitis in mice by the ras antagonist farnesylthiosalicylic acid. Dig Dis Sci. 2012;57:320–6. doi: 10.1007/s10620-011-1880-y. [DOI] [PubMed] [Google Scholar]
- 121.Wang X, Lu Y, Wu L, Zhao C, Song C, Yu S, Zhao B, Zhao T, Liu H, Dou C, et al. Moxibustion Inhibits the ERK Signaling Pathway and Intestinal Fibrosis in Rats with Crohn’s Disease. Evid Based Complement Alternat Med. 2013;2013:198282. doi: 10.1155/2013/198282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wittchen ES, Aghajanian A, Burridge K. Isoform-specific differences between Rap1A and Rap1B GTPases in the formation of endothelial cell junctions. Small GTPases. 2011;2:65–76. doi: 10.4161/sgtp.2.2.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hogan C, Serpente N, Cogram P, Hosking CR, Bialucha CU, Feller SM, Braga VM, Birchmeier W, Fujita Y. Rap1 regulates the formation of E-cadherin-based cell-cell contacts. Mol Cell Biol. 2004;24:6690–700. doi: 10.1128/MCB.24.15.6690-6700.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hoshino T, Sakisaka T, Baba T, Yamada T, Kimura T, Takai Y. Regulation of E-cadherin endocytosis by nectin through afadin, Rap1, and p120ctn. J Biol Chem. 2005;280:24095–103. doi: 10.1074/jbc.M414447200. [DOI] [PubMed] [Google Scholar]
- 125.Sato T, Fujita N, Yamada A, Ooshio T, Okamoto R, Irie K, Takai Y. Regulation of the assembly and adhesion activity of E-cadherin by nectin and afadin for the formation of adherens junctions in Madin-Darby canine kidney cells. J Biol Chem. 2006;281:5288–99. doi: 10.1074/jbc.M510070200. [DOI] [PubMed] [Google Scholar]
- 126.Severson EA, Lee WY, Capaldo CT, Nusrat A, Parkos CA. Junctional adhesion molecule A interacts with Afadin and PDZ-GEF2 to activate Rap1A, regulate beta1 integrin levels, and enhance cell migration. Mol Biol Cell. 2009;20:1916–25. doi: 10.1091/mbc.E08-10-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Monteiro AC, Sumagin R, Rankin CR, Leoni G, Mina MJ, Reiter DM, Stehle T, Dermody TS, Schaefer SA, Hall RA, et al. JAM-A associates with ZO-2, afadin, and PDZ-GEF1 to activate Rap2c and regulate epithelial barrier function. Mol Biol Cell. 2013;24:2849–60. doi: 10.1091/mbc.E13-06-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tanaka-Okamoto M, Hori K, Ishizaki H, Itoh Y, Onishi S, Yonemura S, Takai Y, Miyoshi J. Involvement of afadin in barrier function and homeostasis of mouse intestinal epithelia. J Cell Sci. 2011;124:2231–40. doi: 10.1242/jcs.081000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Riedl JA, Brandt DT, Batlle E, Price LS, Clevers H, Bos JL. Down-regulation of Rap1 activity is involved in ephrinB1-induced cell contraction. Biochem J. 2005;389:465–9. doi: 10.1042/BJ20050048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ohba Y, Mochizuki N, Matsuo K, Yamashita S, Nakaya M, Hashimoto Y, Hamaguchi M, Kurata T, Nagashima K, Matsuda M. Rap2 as a slowly responding molecular switch in the Rap1 signaling cascade. Mol Cell Biol. 2000;20:6074–83. doi: 10.1128/MCB.20.16.6074-6083.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gloerich M, ten Klooster JP, Vliem MJ, Koorman T, Zwartkruis FJ, Clevers H, Bos JL. Rap2A links intestinal cell polarity to brush border formation. Nat Cell Biol. 2012;14:793–801. doi: 10.1038/ncb2537. [DOI] [PubMed] [Google Scholar]
- 132.Pujuguet P, Del Maestro L, Gautreau A, Louvard D, Arpin M. Ezrin regulates E-cadherin-dependent adherens junction assembly through Rac1 activation. Mol Biol Cell. 2003;14:2181–91. doi: 10.1091/mbc.E02-07-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tsygankova OM, Ma C, Tang W, Korch C, Feldman MD, Lv Y, Brose MS, Meinkoth JL. Downregulation of Rap1GAP in human tumor cells alters cell/matrix and cell/cell adhesion. Mol Cell Biol. 2010;30:3262–74. doi: 10.1128/MCB.01345-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pfeffer SR. Rab GTPase regulation of membrane identity. Curr Opin Cell Biol. 2013;25:414–9. doi: 10.1016/j.ceb.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–88. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coyne CB, Shen L, Turner JR, Bergelson JM. Coxsackievirus entry across epithelial tight junctions requires occludin and the small GTPases Rab34 and Rab5. Cell Host Microbe. 2007;2:181–92. doi: 10.1016/j.chom.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bruewer M, Utech M, Ivanov AI, Hopkins AM, Parkos CA, Nusrat A. Interferon-gamma induces internalization of epithelial tight junction proteins via a macropinocytosis-like process. FASEB J. 2005;19:923–33. doi: 10.1096/fj.04-3260com. [DOI] [PubMed] [Google Scholar]
- 138.Talmon G, Holzapfel M, DiMaio DJ, Muirhead D. Rab11 is a useful tool for the diagnosis of microvillous inclusion disease. Int J Surg Pathol. 2012;20:252–6. doi: 10.1177/1066896911430959. [DOI] [PubMed] [Google Scholar]
- 139.Ohira M, Oshitani N, Hosomi S, Watanabe K, Yamagami H, Tominaga K, Watanabe T, Fujiwara Y, Maeda K, Hirakawa K, et al. Dislocation of Rab13 and vasodilator-stimulated phosphoprotein in inactive colon epithelium in patients with Crohn’s disease. Int J Mol Med. 2009;24:829–35. doi: 10.3892/ijmm_00000300. [DOI] [PubMed] [Google Scholar]
- 140.Zahraoui A, Joberty G, Arpin M, Fontaine JJ, Hellio R, Tavitian A, Louvard D. A small rab GTPase is distributed in cytoplasmic vesicles in non polarized cells but colocalizes with the tight junction marker ZO-1 in polarized epithelial cells. J Cell Biol. 1994;124:101–15. doi: 10.1083/jcb.124.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yamamura R, Nishimura N, Nakatsuji H, Arase S, Sasaki T. The interaction of JRAB/MICAL-L2 with Rab8 and Rab13 coordinates the assembly of tight junctions and adherens junctions. Mol Biol Cell. 2008;19:971–83. doi: 10.1091/mbc.E07-06-0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Köhler K, Louvard D, Zahraoui A. Rab13 regulates PKA signaling during tight junction assembly. J Cell Biol. 2004;165:175–80. doi: 10.1083/jcb.200312118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7:347–58. doi: 10.1038/nrm1910. [DOI] [PubMed] [Google Scholar]
- 144.Funakoshi Y, Hasegawa H, Kanaho Y. Regulation of PIP5K activity by Arf6 and its physiological significance. J Cell Physiol. 2011;226:888–95. doi: 10.1002/jcp.22482. [DOI] [PubMed] [Google Scholar]
- 145.Paleotti O, Macia E, Luton F, Klein S, Partisani M, Chardin P, Kirchhausen T, Franco M. The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J Biol Chem. 2005;280:21661–6. doi: 10.1074/jbc.M503099200. [DOI] [PubMed] [Google Scholar]
- 146.Smyth D, McKay CM, Gulbransen BD, Phan VC, Wang A, McKay DM. Interferon-gamma signals via an ERK1/2-ARF6 pathway to promote bacterial internalization by gut epithelia. Cell Microbiol. 2012;14:1257–70. doi: 10.1111/j.1462-5822.2012.01796.x. [DOI] [PubMed] [Google Scholar]
- 147.Criss AK, Silva M, Casanova JE, McCormick BA. Regulation of Salmonella-induced neutrophil transmigration by epithelial ADP-ribosylation factor 6. J Biol Chem. 2001;276:48431–9. doi: 10.1074/jbc.M106969200. [DOI] [PubMed] [Google Scholar]
- 148.Lu L, Khan A, Walker WA. ADP-ribosylation factors regulate the development of CT signaling in immature human enterocytes. Am J Physiol Gastrointest Liver Physiol. 2009;296:G1221–9. doi: 10.1152/ajpgi.90686.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Naydenov NG, Brown B, Harris G, Dohn MR, Morales VM, Baranwal S, Reynolds AB, Ivanov AI. A membrane fusion protein αSNAP is a novel regulator of epithelial apical junctions. PLoS One. 2012;7:e34320. doi: 10.1371/journal.pone.0034320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tomson FL, Viswanathan VK, Kanack KJ, Kanteti RP, Straub KV, Menet M, Kaper JB, Hecht G. Enteropathogenic Escherichia coli EspG disrupts microtubules and in conjunction with Orf3 enhances perturbation of the tight junction barrier. Mol Microbiol. 2005;56:447–64. doi: 10.1111/j.1365-2958.2005.04571.x. [DOI] [PubMed] [Google Scholar]
- 151.Matsuzawa T, Kuwae A, Yoshida S, Sasakawa C, Abe A. Enteropathogenic Escherichia coli activates the RhoA signaling pathway via the stimulation of GEF-H1. EMBO J. 2004;23:3570–82. doi: 10.1038/sj.emboj.7600359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Selyunin AS, Sutton SE, Weigele BA, Reddick LE, Orchard RC, Bresson SM, Tomchick DR, Alto NM. The assembly of a GTPase-kinase signalling complex by a bacterial catalytic scaffold. Nature. 2011;469:107–11. doi: 10.1038/nature09593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kahn RA, Cherfils J, Elias M, Lovering RC, Munro S, Schurmann A. Nomenclature for the human Arf family of GTP-binding proteins: ARF, ARL, and SAR proteins. J Cell Biol. 2006;172:645–50. doi: 10.1083/jcb.200512057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jaschke A, Chung B, Hesse D, Kluge R, Zahn C, Moser M, Petzke KJ, Brigelius-Flohé R, Puchkov D, Koepsell H, et al. The GTPase ARFRP1 controls the lipidation of chylomicrons in the Golgi of the intestinal epithelium. Hum Mol Genet. 2012;21:3128–42. doi: 10.1093/hmg/dds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zahn C, Jaschke A, Weiske J, Hommel A, Hesse D, Augustin R, Lu L, Hong W, Florian S, Scheepers A, et al. ADP-ribosylation factor-like GTPase ARFRP1 is required for trans-Golgi to plasma membrane trafficking of E-cadherin. J Biol Chem. 2008;283:27179–88. doi: 10.1074/jbc.M802108200. [DOI] [PubMed] [Google Scholar]
- 156.Palacios F, Schweitzer JK, Boshans RL, D’Souza-Schorey C. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat Cell Biol. 2002;4:929–36. doi: 10.1038/ncb881. [DOI] [PubMed] [Google Scholar]
- 157.Babbin BA, Parkos CA, Mandell KJ, Winfree LM, Laur O, Ivanov AI, Nusrat A. Annexin 2 regulates intestinal epithelial cell spreading and wound closure through Rho-related signaling. Am J Pathol. 2007;170:951–66. doi: 10.2353/ajpath.2007.060647. [DOI] [PMC free article] [PubMed] [Google Scholar]