Abstract
Reports on sex-related outcomes in left ventricular assist device (LVAD) patients are conflicting. In addition, females have been underrepresented in most multicenter randomized controlled trials for mechanical circulatory support (MCS). The objective of our study was to analyze our experience implanting 130 continuous-flow LVADs and to determine the impact of sex on survival. We identified 130 patients who underwent implantation of a continuous-flow LVAD at our institution. Patients were stratified into two groups based on sex. Variables were compared using two-sided t-tests, χ2 tests, Cox proportional hazards models, and log-rank tests to determine whether there was a difference between the two groups and if sex was a significant independent predictor of outcome. Of the 130 patients, 35 were females and 95 were males. Female patients had worse pre-LVAD cardiac output and cardiac index and were more likely to be on MCS at the time of implantation. Male patients had worse renal function. Survival was analogous for both cohorts with 30 day, 6 month, 1 year, and 2 year survivals of 97%, 90.8%, 90.8%, and 84.3%, respectively, for female patients versus 94.7%, 87.9%, 78.4%, and 72.8%, respectively, for male patients. The incidence of other LVAD-related complications was also similar in both groups. Gender did not predict postoperative mortality on univariate analysis. Contrary to most published reports, female and male LVAD patients have similar postoperative and midterm survival, length of hospital stay, readmission rates, and postoperative complications. It appears that females have gained more benefit from newer generation devices compared to males.
Keywords: left ventricular assist device, LVAD, sex, gender, female, male, outcomes
Sex-related differences in heart failure have received very little attention. Currently, the published literature on gender-related outcome disparities in heart failure patients is conflicting. Some reports have demonstrated that females have longer hospital stay, more frequent hospitalizations, and higher mortality, as females with congestive heart failure are older, and are referred to a heart failure specialist at a later stage of their disease.1–5 Other studies have shown that females are more likely to have heart failure with preserved ejection fraction, are less likely to have ischemic cardiomyopathy, history of smoking, chronic obstructive pulmonary disease (COPD), peripheral vascular disease (PVD), renal insufficiency, and overall exhibit improved outcomes compared to males.6–10 In terms of sex-related left ventricular assist device (LVAD) outcomes, published data are scant. In addition, females have been underrepresented in most multicenter randomized controlled trials for mechanical circulatory support (MCS). The limited enrollment of women in LVAD studies was especially dominant in the pulsatile device era, as female patients were unable to accommodate the large-sized displacement pumps.11–13 Sex disparities have also been reported in heart transplantation. Although the underlying etiology remains unclear, women are less likely to receive a heart transplant (26% of transplanted hearts in the United States) and overall have worse 5 year posttransplant survival.14 Similarly, higher postoperative morbidity and inferior short- and long-term survival have been demonstrated for women undergoing non-LVAD cardiac surgery.15–19
Therefore, we hypothesized that an analysis of sex-related outcomes in patients receiving continuous-flow LVADs as a bridge to transplantation (BTT) or destination therapy (DT) would show inferior results for females. The objective of our study was to analyze our 6 year experience implanting 130 continuous-flow LVADs and to determine the impact of gender on survival.
Methods
This retrospective study was approved by our health system’s Institutional Review Board. We reviewed our institution’s LVAD dataset and analyzed patients who underwent continuous-flow LVAD implantation as a BTT or DT from March 2006 until June 2012. One hundred thirty patients were identified and stratified into subgroups based on sex. Once transplanted, patients were censored from the survival analysis.
Patient Data
Patient demographics and preoperative characteristics included age, race, body surface area (BSA), body mass index (BMI), previous sternotomy, preoperative liver function tests (LFTs), and associated comorbidities—hypertension (HTN), diabetes mellitus (DM), chronic renal insufficiency (CRI), dialysis, COPD, and PVD. Hemodynamic and echocardiographic data included pre- and post-LVAD (at 1 and 6 months) central venous pressure (CVP), pulmonary artery (PA) pressure, pulmonary capillary wedge pressure (PCWP), LV ejection fraction (LVEF), cardiac output (CO), cardiac index (CI), left ventricular end-diastolic diameter and right ventricular end-diastolic diameter, and mitral regurgitation and tricuspid regurgitation. Operative characteristics included type of device (HeartMate II or HeartWare), implantation for BTT or DT, cardiopulmonary bypass (CPB), and cross-clamp times. Primary outcome variables were survival at 30 days, 180 days, 1 year, and 2 years. Secondary outcome variables were complications, hemodynamic data, and causes of death. Survival, free of death with censoring for transplant and explantation for recovery, was reported. Complications included reoperation for bleeding, driveline infections, pneumonia, right ventricular failure, respiratory failure, tracheostomy, acute renal failure, ischemic stroke, hemorrhagic stroke, gastrointestinal bleeding, severe aortic insufficiency, and pump thrombosis. Chronic renal insufficiency was defined as glomerular filtration rate < 60 ml/min/m2. Right ventricular failure was defined as 1) need for inotropic support for more than 2 weeks or 2) need for right ventricular assist device support. Ventilator-dependant respiratory failure was defined as inability to wean from the ventilator for at least 1 week.
Statistical Analysis
Patients were stratified into gender groups. Continuous variables were assessed for normality and reported as mean, standard deviation, minimum, median, and maximum and were compared between groups using two-sided two-sample t-tests. Alternatively, a two-sided Wilcoxon rank-sum test was used if severe departures from normality were observed in the distributions. Categorical variables were reported as count and percent and were compared between the groups using χ2 tests. Alternatively, Fisher’s exact test was used if expected counts were not sufficiently large. Similar tests were used to compare postoperative complications. Preoperative and operative characteristics were evaluated using Cox proportional hazards models to test whether or not each individual characteristic was a significant predictor of postoperative survival. Hazard ratios (HRs) and 95% confidence intervals (CIs) for HRs were reported. Owing to our small sample size, we did not perform a multivariate analysis. Tests were performed using SAS 9.2. Tests were considered significant at p < 0.05.
Results
Demographic Characteristics of Female and Male Patients Who Underwent LVAD Implantation
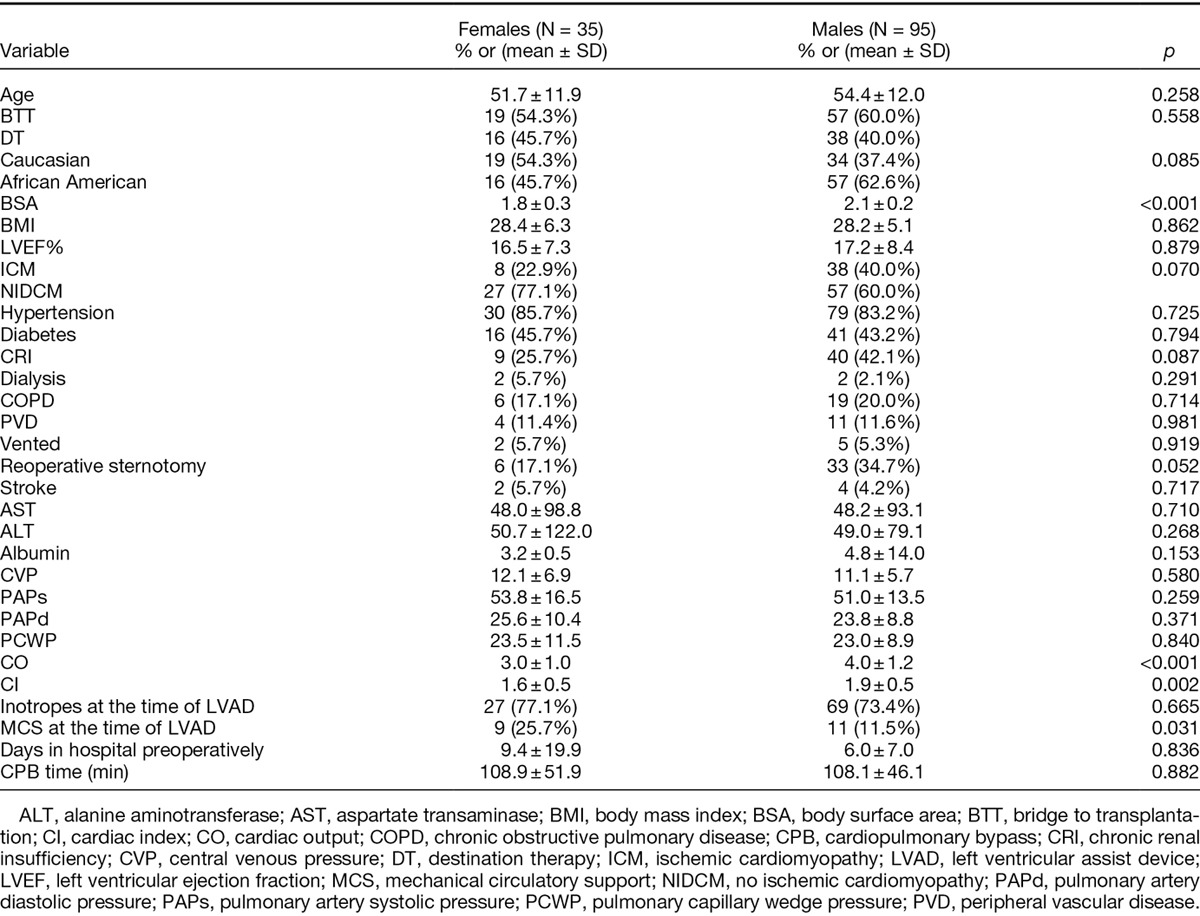
A total of 130 patients who underwent LVAD implantation as a BTT or DT were enrolled in this study. Of these 130 patients, 35 (29.7%) were female, while 95 (70.3%) were male. Demographic and preoperative characteristics for each subgroup are summarized in Table 1. There were no significant differences between females and males for age, race, LVEF, etiology of heart failure, the incidence of HTN, DM, CRI, COPD, PVD, history of stroke, previous sternotomy, preoperative ventilator support, LFTs, albumin, PA pressures, CVP, PCWP, preoperative inotropic support, and days of hospitalization preimplantation (p = not significant). Females had a lower preoperative CO (p < 0.001) and CI (p = 0.02) and were more likely to be on MCS at the time of LVAD implantation. Types of MCS included intra-arterial balloon pump (17/20, 85%), CentriMag (2/20, 10%), and Abiomed (1/20, 5%). Male patients had a significantly higher BSA (p = 0.0011) and BMI (p = 0.04) and worse renal function (p < 0.001).
Table 1.
Preoperative Characteristics of LVAD Recipients
Cardiopulmonary Bypass Time and Cross-Clamp Time
Both CPB times (108.9 min for females vs. 108.1 min for males, p = 0.882) and cross-clamp times (7.2 min for females vs. 6.3 min for males, p = 0.751) were equivalent for both subgroups.
Postimplant Survival for Females and Males
Survival was analogous for both genders, with 30 day, 6 month, 1 year, and 2 year survival of 97% (n = 1 death), 90.8% (n = 2 deaths), 90.8%, and 84.3% (n = 4 deaths), respectively, for female patients versus 94.7% (n = 5 deaths), 87.9% (n = 10 deaths), 78.4% (n = 16 deaths), and 72.8% (n = 20 deaths), respectively, for male patients (Figures 1–3).
Figure 1.
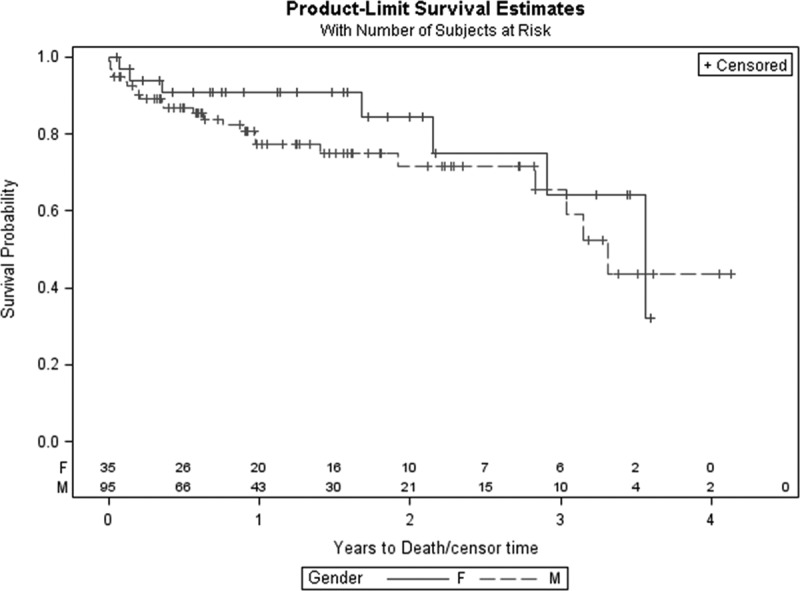
Kaplan–Meier survival curve for female and male left ventricular assist device patients.
Figure 2.
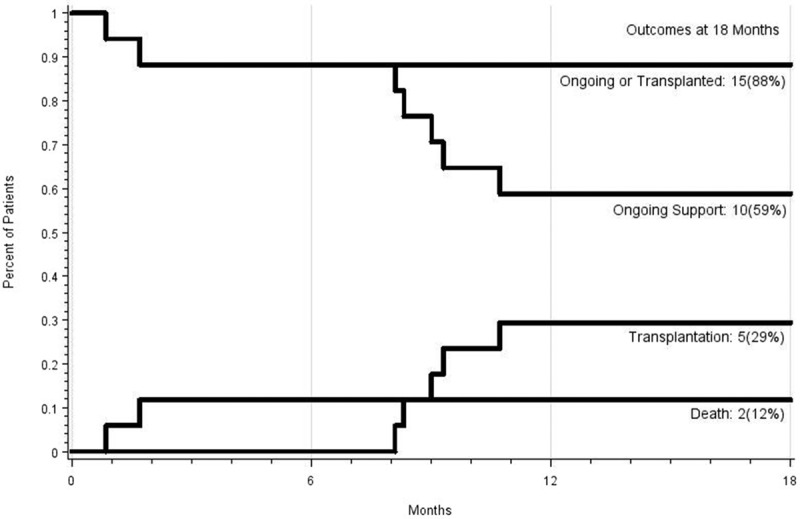
Competing outcomes curve for female bridge to transplantation patients: transplanted or ongoing, ongoing, transplanted, and death.
Figure 3.
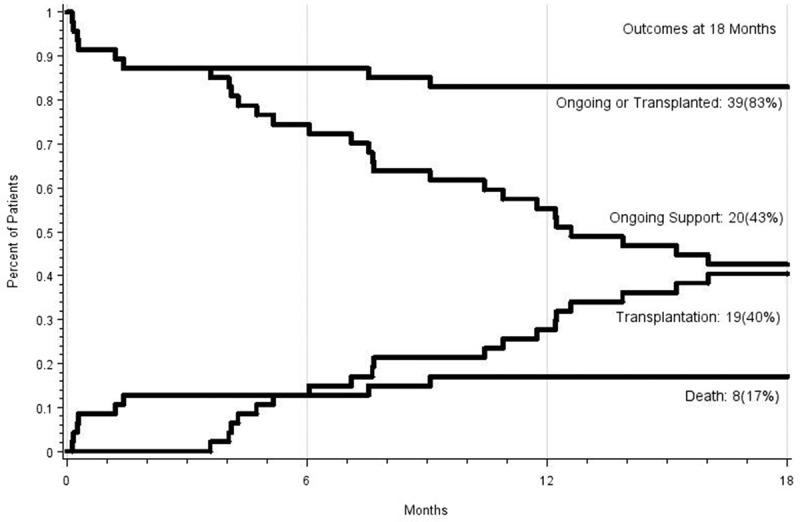
Competing outcomes curve for male bridge to transplantation patients: transplanted or ongoing, ongoing, transplanted, and death.
Transplantation Rates for Elective LVAD BTT Patients and Emergent LVAD BTT Patients
The indication for LVAD implantation was BTT in 54.2% (19/35) of females and 60% (57/95) of males (p = 0.558). Among patients implanted for the indication of BTT, 42.1% (8/19) female patients were transplanted versus 38.6% (22/57) male patients (p = 0.386).
Competing Outcomes Analysis at 18 Months
Figures 2 and 3 demonstrate the competing outcomes (ongoing LVAD therapy, transplantation, and death) for female and male patients up to 18 months.
Postoperative Complications, Hospital Length of Stay, and Readmission Rates
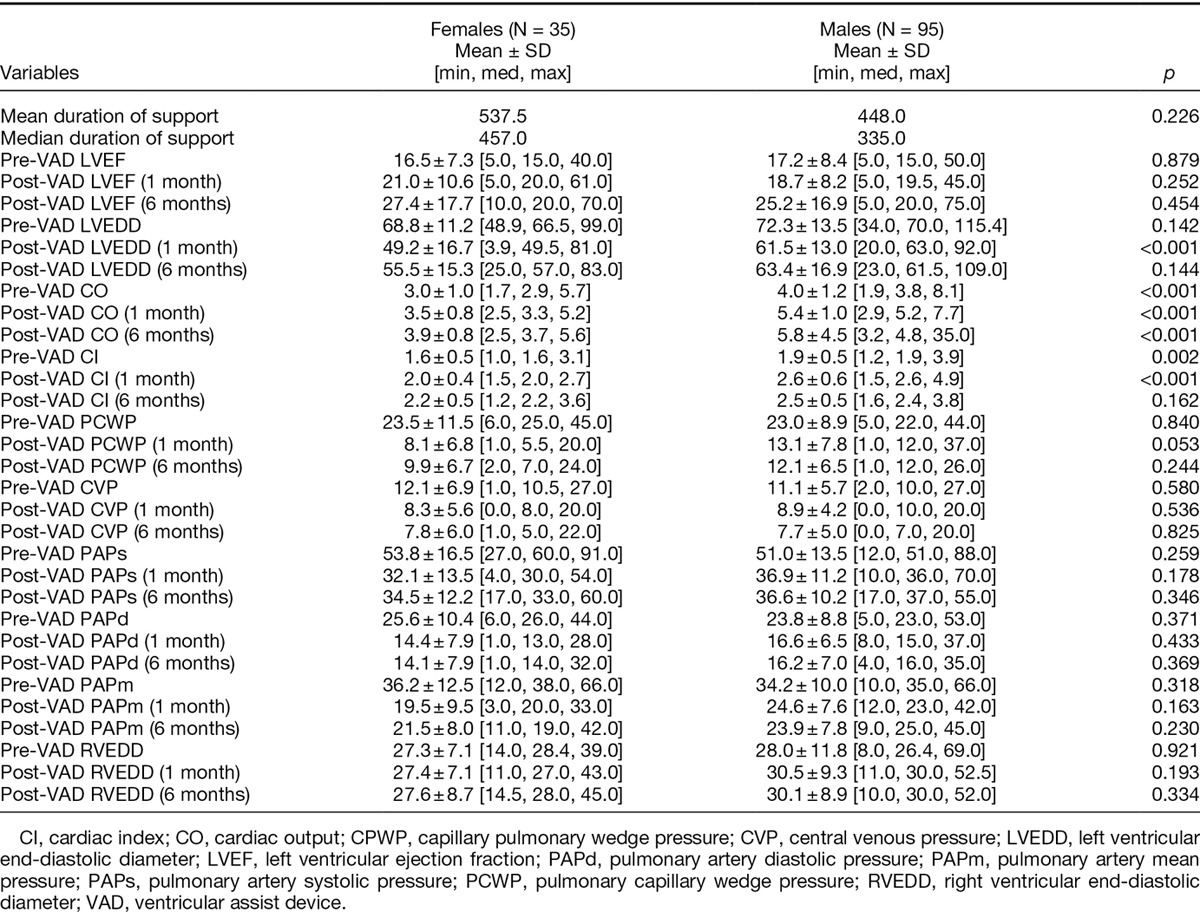
Postoperative complication rates were similar for female and male LVAD patients as shown in Table 2. There was no significant differences in length of hospital stay (23 days for females vs. 21.3 days for males, p = 0.55). Readmission rates within 30 days of discharge were also comparable for the two groups (31.4% for females vs. 23.2% for males, p = 0.337). Pre- and postoperative (at 1 and 6 months) hemodynamic data are demonstrated in Table 3.
Table 2.
Postoperative Complications
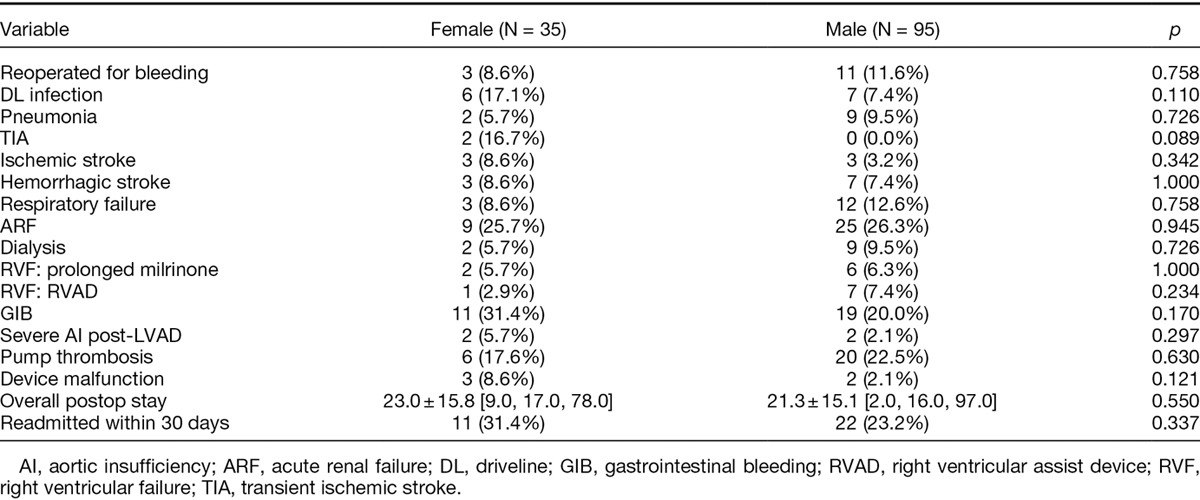
Table 3.
Preoperative and Postoperative (at 1 and 6 Months) Hemodynamic Data
Causes of Death
Causes of death for female patients included septic shock (50%, 2/4) and stroke (50%, 2/4). Causes of death in males were septic shock (30%, 6/20), right ventricular failure (20%, 4/20), stroke (20%, 4/20), multiple organ failure (15%, 3/20), refractory arrhythmia (5%, 1/20), bowel perforation (5%, 1/20), and disconnection from power source (5%, 1/20).
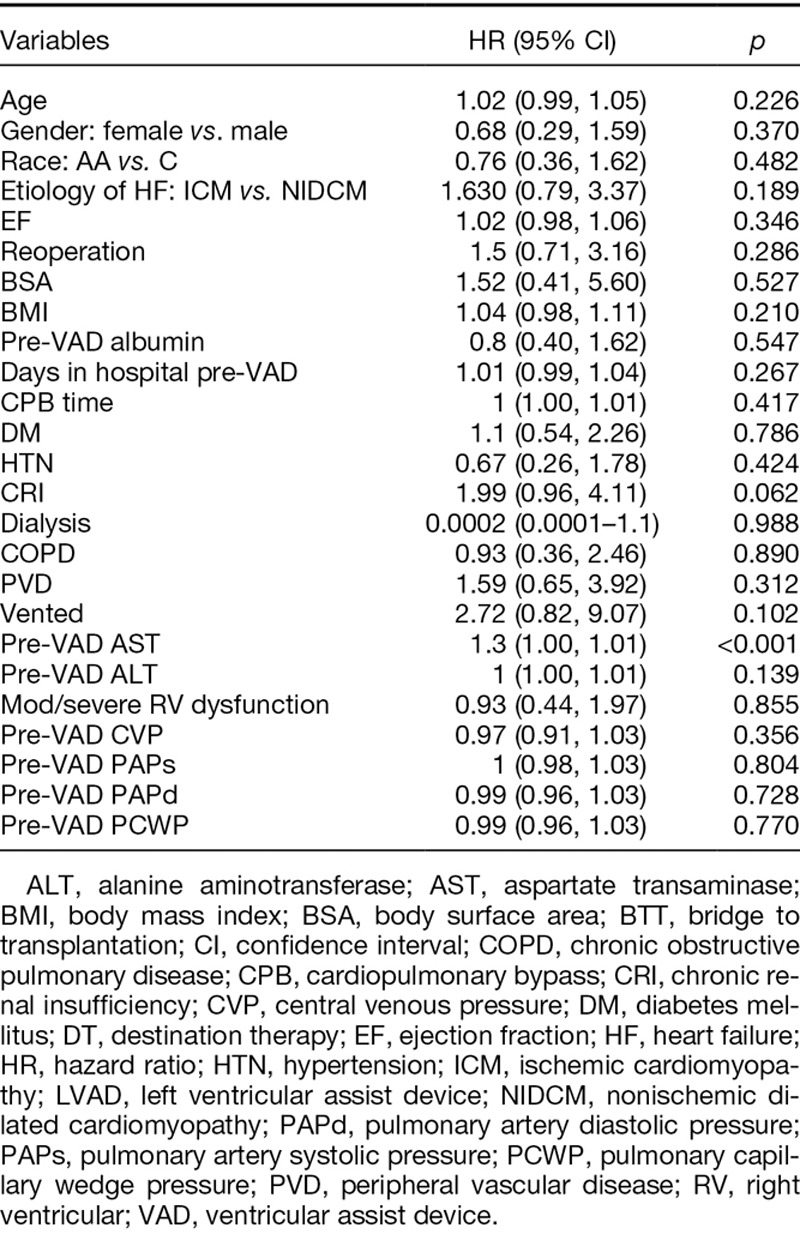
Univariate Analysis
When unadjusted, preoperative aspartate aminotransferase (HR, 1.3; 95% CI, 1–1.01; p < 0.001) was only a significant predictor of survival. Sex did not predict postoperative mortality (HR, 0.68; 95% CI, 0.29–1.59; p = 0.370) (Table 4).
Table 4.
Univariate Analysis: Predictors of Survival After LVAD Implantation
Discussion
This study was undertaken to ascertain the effect of sex on LVAD outcomes. Several reports have suggested that females exhibit higher mortality rates and increased incidence of postoperative hemorrhage and neurologic events after LVAD implantation.20–25 The main explanation for this is that when females are referred for MCS, they are usually sicker and have worse clinical features.26 Several underlying reasons have been identified for women presenting for LVAD or heart transplant evaluation at a later stage of their disease and include less social support, poor economic status, higher self-refusal rates, and more religious and pessimistic attitudes. The anticipated finding of our study was that both genders demonstrated similar postoperative complications and survival, with females actually exhibiting higher survival rates compared to men. This occurred despite the fact that more females were on MCS at LVAD implantation and had worse preoperative CO and CI. Our male population on the other hand were more likely to have CRI. This potentially could explain the improved survival in our female cohort. Otherwise patient comorbidities, preoperative hemodynamic measurements, and the etiology of heart failure were similar for both groups. Equivalent outcomes in our analysis can also be explained by our rigorous preoperative selection process, which confirms patient compliance and solidifies their access to postoperative care. Our multidisciplinary approach also contributes to improved outcomes. These findings are certainly encouraging and suggest that medical and social awareness needs to be increased in order to get female patients referred earlier for surgical treatment of advanced heart failure. Although males are the majority of our patient population (95 vs. 35), we have seen in recent years an increase in the number of females receiving LVAD. The smaller continuous-flow devices are now a viable option for females with lower BSA, who previously would not have been candidates, owing to anatomic body constraints.
The occurrence of post-LVAD complications, including stroke, was also comparable for females and males. This finding is contrary to published literature, which has demonstrated that females are at higher risk of neurologic events after LVAD implantation.27,28 However, none of these studies were able to identify sex-related differences in coagulability (international normalized ratio, partial thromboplastin time, platelet count, and von Willebrand factor). It is hypothesized that the higher risk of thromboembolism in females is from differences in pharmacokinetics of anticoagulation and antiplatelet drugs, which may also explain the higher risk of thromboembolism in female patients with atrial fibrillation.29 In addition, postoperative bleeding complications were similar for females and males despite consistent reports of increased rates of post-LVAD hemorrhage requiring re-exploration.20–25 It appears that the newer generation devices have equally neutralized the risk of bleeding in both genders.
After 18 months of LVAD therapy, more males were transplanted (40% vs. 29%, image 2 and 3), although transplantation rates appear to equilibrate at 2 years (42.1% of females vs. 38.6% of males). This possibly underlines the challenge of identifying suitable donors for females, owing to higher levels of panel-reactive antibodies.14
Few studies have analyzed the impact of sex on LVAD outcomes. Hsich et al.27 analyzed the INTERMACS database from 2006 to 2010 for 1,963 LVAD patients (401 women vs. 1,535 men) and reported no statistically significant sex differences in mortality and postoperative complications, except for the occurrence of stroke which was more frequent in females (HR, 1.44; 95% CI, 1.05–1.96; p = 0.02). This study included both pulsatile and continuous-flow devices. Bogaev et al.28 published a multi-institutional analysis of 465 patients (104 women and 361 men) who received an LVAD as BTT. In their study, there was no difference between females and males in survival or adverse events after 18 months of follow-up. However, women were more likely to develop stroke (0.1 vs. 0.04 events/year, p = 0.02) and less likely to develop infection-related complications (p = 0.006). Potapov et al.26 analyzed 1,667 VAD implantations from 1988 to 2010 at a single institution and reported a better 5 year survival in men aged 13–50 (53% vs. 42%, p = 0.02) and men aged >50 (49% vs. 25%, p = 0.026). Although this analysis was performed on a large number of patients with long-term follow-up, the cohort was heterogenous as patients received more than 15 types of devices. Morgan et al.25 also reported significantly higher 5 year survival in males compared to females (85.2% vs. 50.9%, p = 0.01) in a study of 191 males and 45 females who received LVAD between 1990 and 2002 at Columbia.
Worse outcomes in females have consistently been reported for non-LVAD cardiac surgery. Schwann et al.15 demonstrated an improved 12 year survival in males after coronary artery bypass grafting (CABG) from a cohort of 6,384 patients. Blankstein et al.16 showed that female gender was an independent predictor of operative mortality (4.24% vs. 2.23%, p = 0.001) in 15,440 patients who underwent CABG. Culler et al.18 demonstrated a higher risk-adjusted mortality rate over 2 years in patients undergoing CABG, especially in low-volume centers. Roedler et al.17 showed that females were an independent predictor (HR, 2.07; 95% CI, 1.28–3.35; p = 0.005) of in-hospital mortality after mechanical aortic valve replacement in an analysis of 629 patients. Finally, Doenst et al.19 showed that female gender was an independent predictor of stroke (relative risk, 1.52; 95% CI, 1.1–2.1) in 1,567 patients who had a combined CABG with aortic or mitral valve surgery.
Our study has several limitations. First, our sample size was small and it is possible that the statistical tests were insufficiently powered. Second, our study was not a prospective, randomized trial and is subject to limitations inherent to any retrospective study. Third, our study was a single-institution study and selection bias may have been present. Finally, our results were produced from short- and midterm follow-up.
In summary, our experience indicates that although females have worse preoperative CO/CI and are more likely to be on MCS at the time of LVAD implantation, they exhibit similar postoperative and midterm survival with males. Length of hospital stay, readmission rates, and postoperative complications are also analogous between the two groups. It appears that females have benefited more from newer generation devices, as recent literature demonstrates equivocal survival between genders, whereas older reports that studied pulsatile devices consistently showed worse short- and long-term outcomes in female LVAD patients. Stroke remains a more frequent occurrence in female patients after LVAD implantation, although this was not reproduced in our analysis.
Footnotes
Submitted for consideration August 2013; accepted for publication in revised form January 2014.
Disclosure: The authors have no conflicts of interest to report.
Reprint Requests: Athanasios Tsiouris, MD, Department of General Surgery, Henry Ford Hospital, Heart and Vascular Institute, 2799 West Grand Boulevard, K-14, Detroit, MI 48202. Email: atsiour1@hfhs.org.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–e209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christie JD, Edwards LB, Aurora P, et al. The Registry of the International Society for Heart and Lung Transplantation: Twenty-sixth Official Adult Lung and Heart-Lung Transplantation Report—2009. J Heart Lung Transplant. 2009;28:1031–1049. doi: 10.1016/j.healun.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Bourassa MG, Gurné O, Bangdiwala SI, et al. Natural history and patterns of current practice in heart failure. The Studies of Left Ventricular Dysfunction (SOLVD) Investigators. J Am Coll Cardiol. 1993;22(4 Suppl A):14A–19A. doi: 10.1016/0735-1097(93)90456-b. [DOI] [PubMed] [Google Scholar]
- 4.Doughty R, Yee T, Sharpe N, MacMahon S. Hospital admissions and deaths due to congestive heart failure in New Zealand, 1988–1991. N Z Med J. 1995;108:473–475. [PubMed] [Google Scholar]
- 5.Reitsma JB, Mosterd A, de Craen AJ, et al. Increase in hospital admission rates for heart failure in The Netherlands, 1980–1993. Heart. 1996;76:388–392. doi: 10.1136/hrt.76.5.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Meara E, Clayton T, McEntegart MB, et al. CHARM Investigators. Sex differences in clinical characteristics and prognosis in a broad spectrum of patients with heart failure: Results of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation. 2007;115:3111–3120. doi: 10.1161/CIRCULATIONAHA.106.673442. [DOI] [PubMed] [Google Scholar]
- 7.Galvao M, Kalman J, DeMarco T, et al. Gender differences in in-hospital management and outcomes in patients with decompensated heart failure: Analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Card Fail. 2006;12:100–107. doi: 10.1016/j.cardfail.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen MS, Harjola VP, Hochadel M, et al. Gender related differences in patients presenting with acute heart failure. Results from EuroHeart Failure Survey II. Eur J Heart Fail. 2008;10:140–148. doi: 10.1016/j.ejheart.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Ho KK, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 10.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 11.Rogers JG, Butler J, Lansman SL, et al. INTrEPID Investigators. Chronic mechanical circulatory support for inotrope-dependent heart failure patients who are not transplant candidates: Results of the INTrEPID Trial. J Am Coll Cardiol. 2007;50:741–747. doi: 10.1016/j.jacc.2007.03.063. [DOI] [PubMed] [Google Scholar]
- 12.Frazier OH, Rose EA, Oz MC, et al. HeartMate LVAS Investigators. Left Ventricular Assist System. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–1195. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 13.Rose EA, Gelijns AC, Moskowitz AJ, et al. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 14.Hsich EM, Piña IL. Heart failure in women: A need for prospective data. J Am Coll Cardiol. 2009;54:491–498. doi: 10.1016/j.jacc.2009.02.066. [DOI] [PubMed] [Google Scholar]
- 15.Schwann TA, Engoren M, Bonnell M, Clancy C, Habib RH. Comparison of late coronary artery bypass graft survival effects of radial artery versus saphenous vein grafting in male and female patients. Ann Thorac Surg. 2012;94:1485–1491. doi: 10.1016/j.athoracsur.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Blankstein R, Ward RP, Arnsdorf M, Jones B, Lou YB, Pine M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery: Contemporary analysis of 31 Midwestern hospitals. Circulation. 2005;112(9 suppl):I323–I327. doi: 10.1161/CIRCULATIONAHA.104.525139. [DOI] [PubMed] [Google Scholar]
- 17.Roedler S, Neuhauser J, Sodeck G, et al. Gender-related differences in patients undergoing mechanical aortic valve replacement with the CarboMedics valve. J Cardiovasc Surg (Torino) 2011;52:887–894. [PubMed] [Google Scholar]
- 18.Culler SD, Simon AW, Brown PP, Kugelmass AD, Reynolds MR, Rask KJ. Sex differences in hospital risk-adjusted mortality rates for Medicare beneficiaries undergoing CABG surgery. Arch Intern Med. 2008;168:2317–2322. doi: 10.1001/archinte.168.21.2317. [DOI] [PubMed] [Google Scholar]
- 19.Doenst T, Ivanov J, Borger MA, David TE, Brister SJ. Sex-specific long-term outcomes after combined valve and coronary artery surgery. Ann Thorac Surg. 2006;81:1632–1636. doi: 10.1016/j.athoracsur.2005.11.052. [DOI] [PubMed] [Google Scholar]
- 20.Kirklin JK, Naftel DC, Stevenson LW, et al. INTERMACS database for durable devices for circulatory support: First annual report. J Heart Lung Transplant. 2008;27:1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 21.Kormos RL, Kirklin JK, Naftel DC, et al. Early neurological adverse events (NAE) after pulsatile VAD implantation in 455 patients: Incidence, severity and outcome. J Heart Lung Transplant. 2009;28:S129. [Google Scholar]
- 22.Hernandez AF, Grab JD, Gammie JS, et al. A decade of short-term outcomes in post cardiac surgery ventricular assist device implantation: Data from the Society of Thoracic Surgeons’ National Cardiac Database. Circulation. 2007;116:606–612. doi: 10.1161/CIRCULATIONAHA.106.666289. [DOI] [PubMed] [Google Scholar]
- 23.Aaronson KD, Slaughter MS, Miller LW, et al. HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 24.Slaughter MS, Rogers JG, Milano CA, et al. HeartMate II Investigators. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 25.Morgan JA, Weinberg AD, Hollingsworth KW, Flannery MR, Oz MC, Naka Y. Effect of gender on bridging to transplantation and posttransplantation survival in patients with left ventricular assist devices. J Thorac Cardiovasc Surg. 2004;127:1193–1195. doi: 10.1016/s0022-5223(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 26.Potapov E, Schweiger M, Lehmkuhl E, et al. Gender differences during mechanical circulatory support. ASAIO J. 2012;58:320–325. doi: 10.1097/MAT.0b013e318251cdf9. [DOI] [PubMed] [Google Scholar]
- 27.Hsich EM, Naftel DC, Myers SL, et al. Should women receive left ventricular assist device support?: Findings from INTERMACS. Circ Heart Fail. 2012;5:234–240. doi: 10.1161/CIRCHEARTFAILURE.111.963272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bogaev RC, Pamboukian SV, Moore SA, et al. HeartMate II Clinical Investigators. Comparison of outcomes in women versus men using a continuous-flow left ventricular assist device as a bridge to transplantation. J Heart Lung Transplant. 2011;30:515–522. doi: 10.1016/j.healun.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]


