Abstract
Hemodynamic performances comparisons between different types of left ventricular assist devices (LVADs) remain difficult in a clinical context. The aim of this study was to create an experimental model to assess and compare two types of LVAD under hemodynamic conditions that simulated physical effort and pulmonary hypertension. An experimental mock circulatory system was created to simulate the systemic and pulmonary circulations and consisted of pulsatile left and right cardiac simulators (cardiowest pump), air/water tanks to model compliances, and tubes to model the venous and arterial resistances. Two types of continuous-flow ventricular assist devices were connected to this pulsated model: an axial flow pump, Heartmate II (HTM II), and a centrifugal pump, VentrAssist (VTA). The hemodynamic conditions at rest and during exercise were replicated. Mean aortic pressures were not significantly different at rest and during effort but mean flow under maximum pump speed was higher with HTM II (13 L vs. 10 L, p = 0.02). Left atrial pressure was lower at rest and during effort for the HTM II (11 mm Hg vs. 3 mm Hg, p = 0.02 and 9 mm Hg vs. 2 mm Hg, p = 0.008) than with the VTA, but with greater risk of left-ventricle suck-down for the axial flow. Power consumption for a similar flow was lower with the VTA during rest (4.7 W vs. 6.9 W, p = 0.002) and during effort (4.3 W vs. 6.6 W, p = 0.008). In case of high pulmonary vascular resistance with preserved right ventricular function, lower right ventricular pressure was obtained with HTM II (21 mm Hg vs. 28 mm Hg, p = 0.03). Observed results are in favor of a better discharge of the left and right cavities with the HTM II compared to the VTA yet with a higher risk of left cavity collapse occurrence.
Keywords: LVAD, circulatory assistance devices, experimental surgery, heart failure, hemodynamics
Laminar flow left ventricular assist devices (LVADs) have seen substantial progress and these devices currently present excellent long-term durability1 for a significant flow (>5 L/min). These continuous-flow pumps can be classified in two subgroups depending on their mechanisms: centrifugal pump or axial pump. The Heartmate II (HTM II) (Thoratec Corporation, Pleasanton, CA) is a second-generation axial laminar flow assist device implanted for the first time in November 2003.2 The VTA (Ventracor, Chatswood, NSW, Australia) is a laminar flow assist device with a third-generation centrifugal pump. Its first implantation was performed in June 2003.3
These monoventricular pumps do not currently possess any autoregulation system: the pump thus does not increase its speed when the patient makes an additional effort. This absence of autoadaptation has been the basis of several studies these recent years to propose a regulation algorithm.4,5 Different parameters have been taken into consideration (shape of pressure curves, difference between the assistance’s inflow and outflow pressure, etc.). Although the first set of results seems encouraging, no regulation system is yet clinically available at this point in time.
Furthermore, the continuous-flow pumps diminish physiological pulsatility and do not allow a ventricle discharge as substantial as it would with the first pulsatile pumps.6,7 Their hemodynamic properties remain thus imperfectly known, notably in hemodynamic stress conditions such as effort. Another important point is that the right ventricle behavior remains still unpredictable sometimes after LVAD implantation. All these unanswered questions explain the recent creations of experimental models that artificially reproduce the human circulatory system (called MOCK circulation) to assess different assist devices in specific hemodynamic contexts.8–10 Although they cannot replace in vivo trials, they allow a first assessment of how these assist devices would react in different hemodynamic conditions.
The aim of this study was to create an experimental model to assess two types of LVADs (axial versus centrifugal) in different experimental hemodynamic conditions: during effort or in case of pulmonary hypertension.
Materials and Methods
Materials
The MOCK.
Pulmonary circulation and systemic circulation were simulated by using the MOCK which is normally used to set up the CardioWest external computer (SynCardia Systems, Inc., Tucson, AZ) (Figure 1). This MOCK consists of four tanks that respectively correspond to the aorta, the pulmonary artery, and the two atria. These four tanks contain different volumes so as to obtain the desired and adequate pressure. Residual air volume, present in each reservoir, makes changes in pressure control possible during the introduction of a supplementary volume in the compartment: the provision of volume resulting in diminished pressure in the original reservoir and increased pressure in the end reservoir (Boyle’s Law). They are connected among themselves and internally with different tubes so as to reproduce systemic and pulmonary circulations. These tanks are also connected externally via four polyvinyl chloride tubes to a biventricular pump (CardioWest) that simulates a pulsatile heart, driven by pneumatic energy.
Figure 1.
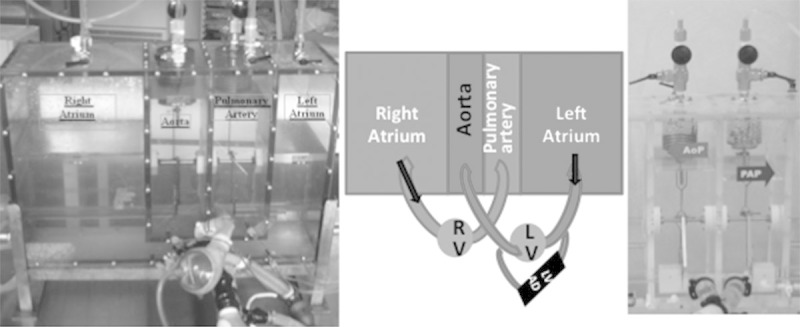
Photo (left side) and schema (middle) of the MOCK reproducing the human circulatory system connected to the CardioWest artificial ventricles. On the right, system composed of hollow springs filled with water and linked to external lateral compartments which recreates the systemic and pulmonary vascular resistances.
Between the tanks representing the aorta and the right atrium on the one hand and the pulmonary artery and the left atrium on the other is a system composed of hollow springs filled with water and linked to external lateral compartments which recreates the systemic and pulmonary vascular resistances. Raising or lowering these lateral compartments makes raising or decreasing pressure in these springs possible and thus enables variation in systemic and pulmonary vascular resistance (respective normal values for systemic and pulmonary vascular resistance at rest: 800–1,200 dynes/s/cm5 and 60–200 dynes/s/cm5, i.e., 10–15 Wood units and 1–3 Wood units) (Figure 1). The assist device to be tested was then connected in parallel between the pneumatic ventricle’s inflow and outflow, stimulating the left part of the heart (Figure 1). The pressure in each tank was continuously measured and displayed in real time on an adjacent screen.
The artificial ventricles.
In this MOCK, the heart is simulated by using artificial ventricles from the CardioWest total artificial heart (Figure 2), which is powered by pneumatic energy and controlled in this experimental model by an external pneumatic console (Dual Drive Console [DDC] from Thoratec). Each artificial ventricle consists of two compartments separated by a polyurethane membrane. One of these compartments is the ejection chamber of the ventricle, with a blood content, the other is a chamber with an air content where pressure is controlled by the pneumatic console. Mobilizing this polyurethane membrane thus enables the ejection chamber to completely empty, flow direction being controlled by two Medtronic Hall mechanical valves (Medtronic Inc., Minneapolis, MN) simulating the inflow and outflow valves (mitral/aortic and tricuspid/pulmonary) of each ventricle. The maximum output volume obtained is the one from the output chamber of these pneumatic ventricles, that is to say 70 ml.
Figure 2.

On the left, CardioWest artificial ventricles connected to the atrium, aorta, and pulmonary artery through four polyvinyl chloride tubes. On the right, Thoratec Dual Drive Console external console delivering and controlling pneumatic energy for the artificial ventricules.
Left ventricle circulatory assist device.
Circulatory assistance that was tested was then connected in parallel to the mock, between the left ventricle’s inflow and outflow, by means of a silicone tube, connected end-to-side to the mock. The inflow cannula (equivalent to the intraventricular cannula) was carried out down to the left ventricle’s inflow mitral valve so as to copy the physiological ventricular conditions of a ventricular support. To do so, the CardioWest mechanical valve, in “mitral” position, was removed and reinserted into the tube connecting the left ventricle to the left atrium. This reimplantation was carried out far from the reservoir reproducing the left atrial pressure (5–6 cm) so as to create a more physiological left atrial volume and more controllable by the person carrying out this experiment. The outflow cannula (equivalent to the reinjection cannula) of the circulatory assistance was carried out down to the ventricle’s aortic valve outflow on the tube connecting the artificial ventricle to the aortic reservoir (Figure 3). The tested LVAD in this model were laminar flow assist devices, whose functioning depends solely on upflow and downflow pressure and not on the end-diastolic volume present in the heart cavity. The assistance’s functioning thus obtained reasonably replicated the in vivo conditions.
Figure 3.
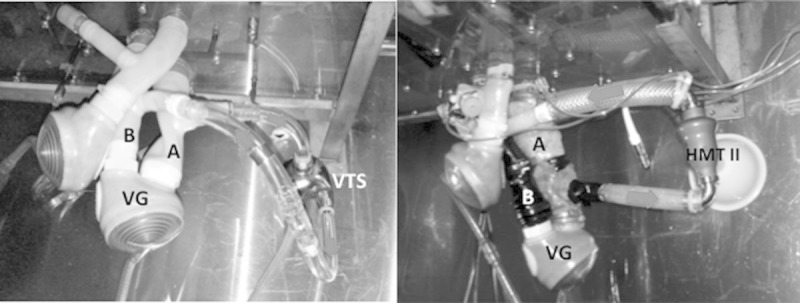
Connection of the VentrAssist (VTA) (at the left) and the HeartMate II (HTM II) (at the right) to the MOCK. (A: Tube connecting the left atrium to the left ventricle; B: Tube connecting the left ventricle to the aorta). Red arrows represent blood flow.
Methods
Experimental model validation.
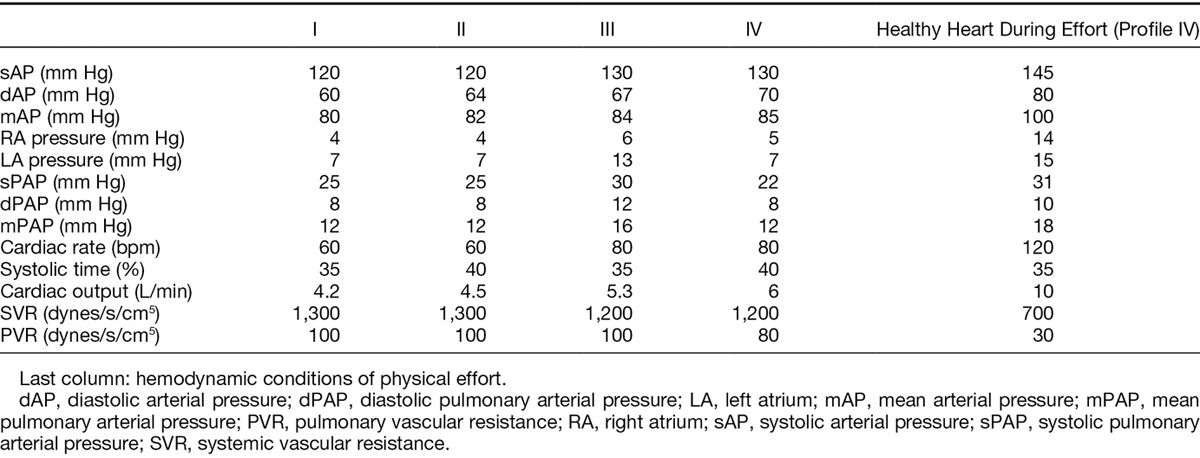
First, the hemodynamic conditions of a healthy heart at rest and then those of a pathological heart at rest were replicated in this MOCK model without LVAD support. The external pneumatic DDC was calibrated so as to get an end-systolic volume of 70 ml, a pulse of 80 bpm, for a systolic period representing 40% of the cardiac cycle. Insufflation pressures in the left and right ventricles were progressively increased to reach physiological values of aortic and pulmonary pressures (output 5 L/min, aortic pressure of 130/70 mm Hg, pulmonary artery pressure of 25/15 mm Hg). Several hemodynamic profiles of healthy hearts, different according to heart rate and systolic percentage time, were thus established and evaluated (Table 1).
Table 1.
Different Hemodynamic Profiles Replicating Healthy Hearts According to Heart Rate and Systole Percentage Time (States I, II, III, and IV)
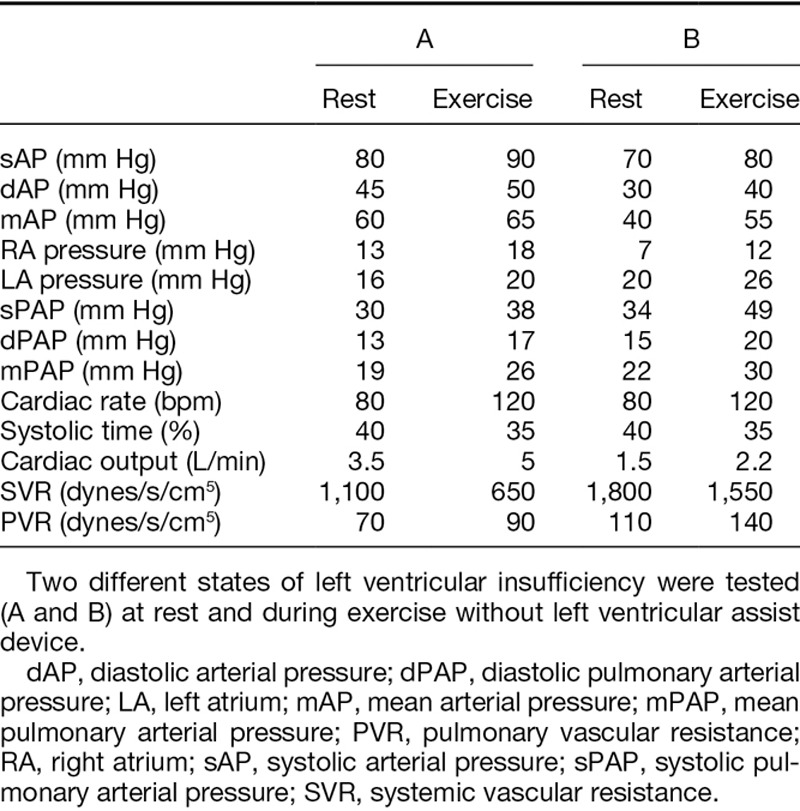
To reproduce the hemodynamic conditions of a heart failure, a progressive decrease of the insufflation pressure in the artificial left ventricle was realized. Two states (A and B) of left ventricular insufficiency (Table 2) were then replicated, distinguished by the severity of the ventricular insufficiency reproduced.
Table 2.
Reproduction of Hemodynamic Conditions of Heart Failure by Decreasing Insufflation Pressure in the Left Artificial Ventricle
To recreate the hemodynamic conditions of physical effort, the insufflation frequency was raised (up to 120 bpm), associated with the increase of the left ventricle’s preloading. At last, the pressures in the different tanks and the vascular resistances were then altered to replicate, at rest, isolated pulmonary hypertension on a healthy heart and then on a pathological heart.
In regard to the choice of fluid used inside the model, an aqueous solution containing 35% of glycerol was used, at a temperature of 25°C. The obtained viscosity of 4 cP was thus close to the blood viscosity (between 3 and 4 cP at a normal hematocrit level).
Test for LV pumps.
Two LVADs were tested on this model in those different hemodynamic states: the VTA (Ventracor), continuous-flow centrifugal pump, and the HTM II (Thoratec), continuous-flow axial pump (Figure 3). These monoventricular pumps were started once the obtained cardiac output was stable and satisfactory, initially at 1,800 revolutions/min for the VTA and 7,000 revolutions/min for the HTM II. The number of revolutions per minute was then progressively increased by stages of 100 or 200 cycles/min in a range of observable speeds in clinical practice (maximum 2,300 for the VTA and 12,000 for the HTM II), to assess the performance of each pump at different speeds.
Statistical tests.
Experiments have been reproduced three successive times. The pressures in the different tanks, the cardiac output, and the tested assist device functioning settings were noted every 5 minutes for 20 minutes, then the mean was calculated.
The endpoint was to compare the cardiac pressures with two different assist devices (left atrial pressure, right ventricular pressure, pulmonary artery pressure, and aortic pressure) and cardiac output. The Mann–Whitney test was used to compare two samples that were nonpaired with a non-Gaussian distribution. A p value inferior to 5% was considered significant (two-sided test).
Results
Experimental Model in the Absence of Monoventricular Circulatory Assistance Validation
The first results aimed to validate the mock’s capacity to reproduce the hemodynamic conditions of a natural circulatory system in the absence of circulatory assistance. The different hemodynamic profiles tested according to heart rate and systolic ejection time are represented in Table 1. Among those different hemodynamic states, profile IV associating a heartbeat of 80 bpm with 40% systolic ejection was selected for the rest of the experiments (Table 1). The hemodynamic profile of pressures in a healthy heart during effort is represented in Table 1. Maximum output obtained during effort was limited by the maximal systolic ejection volume of 70 ml in the pneumatic ventricles’ ejection chambers.
Results for the reproduction of a pathological heart with change on left ventricle function are represented in Table 2. At rest, simulating left ventricular failure lead to a drop in the mean aortic pressure (60 and 40 mm Hg according to the level of heart failure) associated with a slight increase in pulmonary pressure. Pressure in the left atrium was also higher (16 and 20 mm Hg for state A and B, respectively), reproducing a certain degree of pulmonary overload (Table 2).
During exercise simulation, systemic and pulmonary resistances were lowered, accompanied by an increase in output. Hemodynamic conditions observed during effort in case of left ventricular dysfunction are represented in Table 2 and show an increase in arterial and pulmonary pressures, associated with a slight increase in output. Finally, the hemodynamic conditions of pulmonary hypertension were replicated by increasing pulmonary resistance linked with healthy functioning of the right ventricle.
Assistances Test
Once the Mock validity confirmed, the VTA then the HTM II were successively connected to the experimental model. The conditions simulating a change in the left ventricle (statuses A and B) were replicated, at rest and then during effort. To compare obtained results through these different circulatory assistances, comparative tests were carried out according to the obtained global output and not according to pump rotor speed. Thus, the VTA was tested at rotation speed per minute ranging between 1,800 and 2,300 revolutions, and between 7,000 and 12,000 revolutions for the HTM II, so as to remain close to clinical practice.
At rest and during effort, the progressive increase in speed for the VTA and the HTM II was shown by a progressive increase in mean arterial pressure associated with an increase in output and a drop in flow pulsatility inside the mock (Table 3).
Table 3.
Reproduction of Hemodynamic Conditions of a Heart with Left Ventricular Dysfunction (State B) with a Left Ventricular Assist Device Support (VentrAssist or HeartMate II) at Rest and During Exercise Without Pulmonary Hypertension
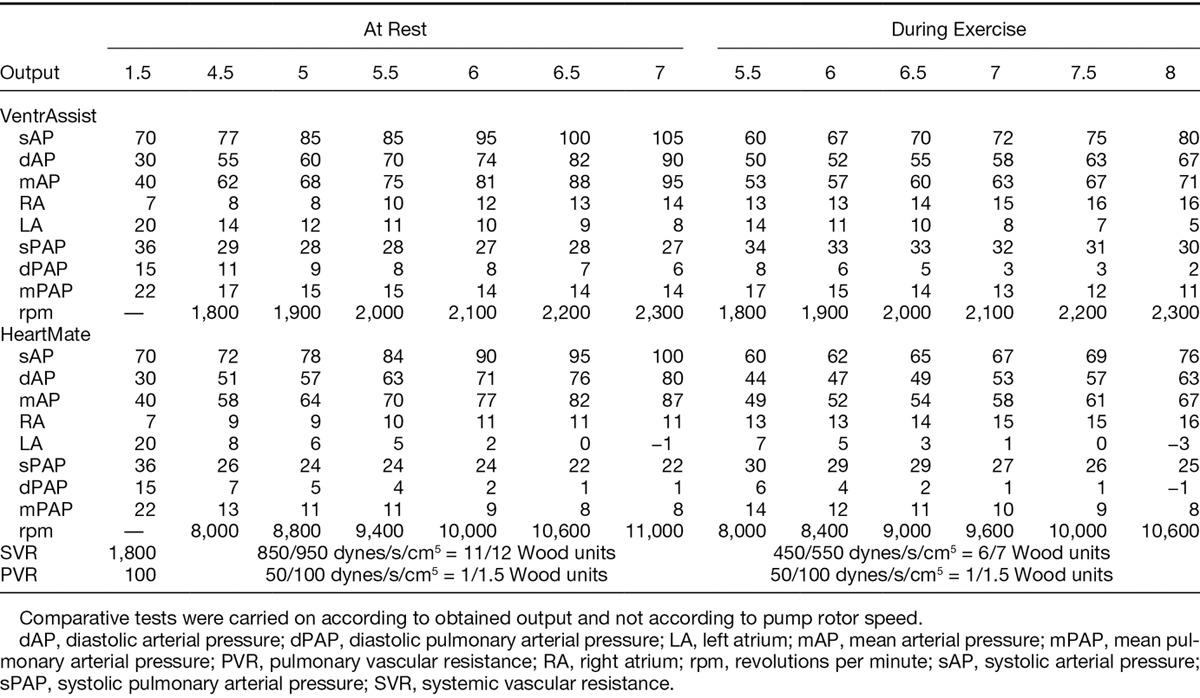
At the same time, a drop in left atrial pressure and an increase in right atrial pressure could be observed (Table 3). Finally, aortic valve opening did not take place from the moment the left ventricle discharge became too significant. Although hemodynamic profile evolution was similar for both statuses A and B for a pathological heart, mean aortic pressures increased more slowly with status B, recorded output was almost entirely due to the assistance (decline in aortic pressure differential), and the nonopening of the aortic valve appeared more precociously.
For similar systemic and pulmonary resistances, comparative assessment between the VTA and the HTM II found a more significant output increase in high rotation frequency for the HTM II (13 L/min against 10 L/min maximum for the VTA; p = 0.02), associated with a less significant increase in mean aortic pressure in lower frequencies. For high rotation frequencies, pressure in the left atrium was substantially lower at rest and during effort (3 mm Hg vs. 11 mm Hg; p = 0.02 and 2 mm Hg vs. 9 mm Hg; p = 0.008) (Figure 4) with the HTM II, to the detriment of a quicker occurrence of atrial depression. At corresponding output, energy consumption was more important for the HTM II at rest (4.7 W vs. 6.9 W, p = 0.002) and during effort (4.3 W vs. 6.6 W, p = 0.008) compared to the VTA. Mean aortic pressure evolution was not, however, statistically different between the two pumps. Finally, the aortic valve reacted identically with both pumps, its closing depending essentially on the left ventricle’s level of alteration, the output obtained by the assistance, and the left cavity discharge.
Figure 4.
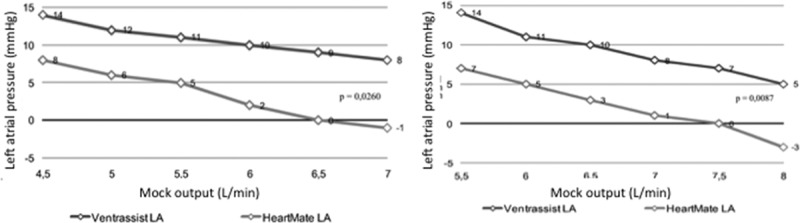
Left atrial pressure according to MOCK cardiac output and type of left ventricular assist device (HeartMate II [HTM II] vs. VentrAssist [VTA]) at rest (left side) and during exercise (at the right).
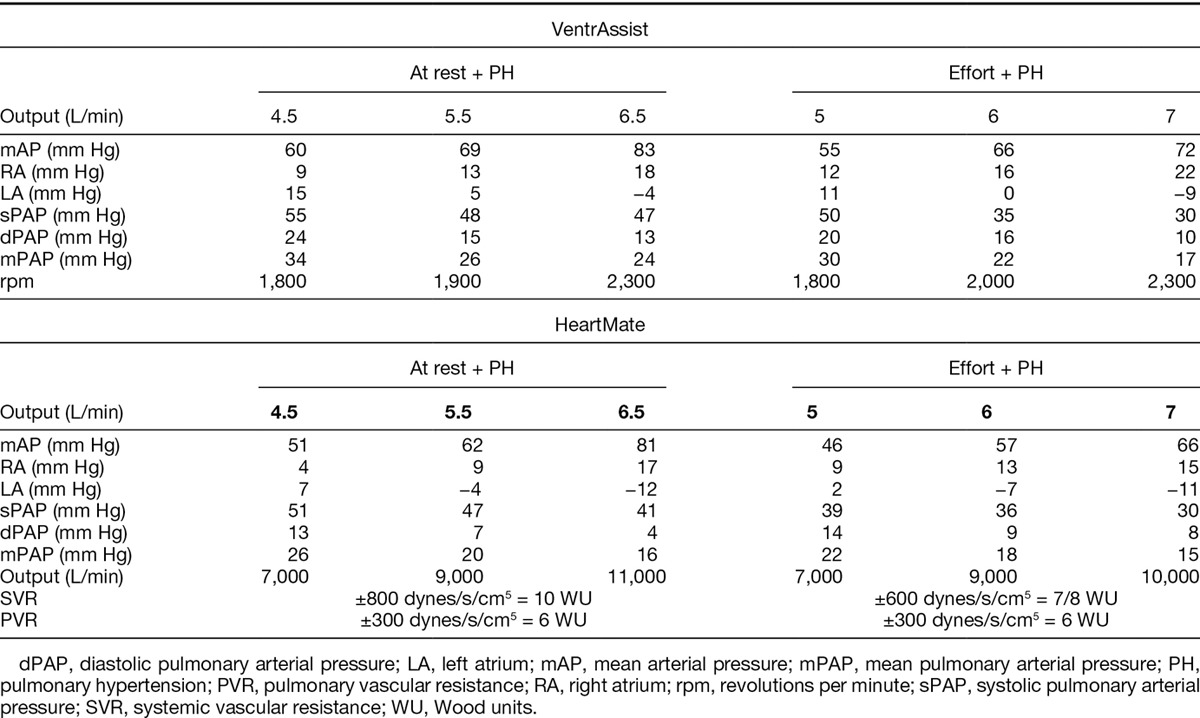
In case of fixed pulmonary hypertension associated with normal right ventricular functioning, similar evolution for pressures and outputs was equally observed (Table 4). A better output obtained with the HTM II is to be noted (the assistance enabling it to reach 11 L/min for the HTM II vs. 8–9 L/min for the VTA; p = 0.002), to the detriment of a quicker negation of left auricular pressure (Table 4). The HTM II would also enable a greater diminution of mean pulmonary (p = 0.031) and right auricular (21 mm Hg vs. 28 mm Hg, p = 0.03) pressures (Table 4). During effort, these differences were equally significant (especially for mock outputs ranging between 5 and 6 L/min).
Table 4.
Hemodynamic Conditions of a Heart with Left Ventricular Dysfunction with a Left Ventricular Assist Device Support in Case of Pulmonary Hypertension with Normal Right Ventricular Function at Rest and During Exercise
Discussion
A Circulatory Model
The aim of this work was to create an experimental model, able to compare two types of laminar flow pumps faced with similar hemodynamic conditions. This study’s first results demonstrated, first of all, the Mock’s capacity to entirely reproduce the heart’s functioning physiological conditions, in the presence of different hemodynamic conditions such as rest, effort, or pulmonary hypertension. Pneumatic ventricles, controlled by the external DDC console, enabled a reliable reproduction of existing pressures in different heart cavities, for heart beat corresponding to the hemodynamic conditions of an untrained and aged patient, at rest and during effort.
Assist Devices Comparison
One of the main differences between current continuous-flow pumps and pulsatile pumps is their filling mechanism. The pulsatile flow assist devices can be set up in a “fill to empty” mode and work in a synchronized manner with the ventricle to which they are connected so as to unload it completely. On the contrary, continuous-flow assist devices are preload-dependent, they need positive pressure in the atrium and see their performances improved when ventricle filling is satisfactory. In a discreet manner, the Starling’s Law is still in action: for a given pump speed, an increase in the patient’s activity will lead to a slight increase in cardiac output due to the native heart’s response, according to this law. In theory, this increase of the output would enable “assisted” patients to resume their daily activities.
Once left ventricular dysfunction was successfully reproduced, our model appeared to be of great interest in making it possible to compare several types of continuous-flow monoventricular assist devices in similar hemodynamic conditions. The results obtained in this study seem to consolidate the hypothesis of different hemodynamic properties between centrifugal and axial assist devices. The HTM II axial pump thus, in our study, presented better performances at rest than the VTA in terms of output and heart cavity discharge, yet with a greater risk of a drop in pressure in the left cavities. The VTA centrifugal pump seemed to ensure a slightly inferior output but with a lower risk of ventricular pressure drop. These results thus suggest the interest of automatic biofeedback for the HTM II depending on upflow pressures so as to avoid a “ventricular suction” event that could occur at rest.
Hemodynamic Adaptation in Case of Effort or Pulmonary Hypertension
In stressful hemodynamic conditions such as physical exercise, it is generally acknowledged that the increase in cardiac output observed in a cardiac assisted patient is due to a slight increase in the native heart’s response (Starling’s Law) with no change in the assist device’s rotation speed.11,12 In these stress conditions, the HTM II seems to, once again, ensure better output than the VTA again at the expense of faster negative pressures in left cavities. Autoregulation for this assist device, according to left ventricular pressure, could enable an increase in global cardiac output during effort.
In the presence of pulmonary hypertension with good right ventricular functioning, the help of the left ventricle brought by a monoventricular assist device would make the decrease of pulmonary pressure in patients, suffering from pulmonary hypertension and awaiting heart transplant, possible.13–15 These results are probably due to satisfactory discharge for the left ventricle and thus direct and indirect improvement of the right ventricular functioning. The right ventricle thus finds itself with better precharge (venous return increased through output improvement), a diminished postcharge (drop in left atrial pressure), an increased provision in oxygen (through the improvement of coronary output), and an improved geometrical configuration (no paradoxal septum during the systole). In our model reproducing a left ventricular failure associated with pulmonary hypertension, the HTM II made better left auricle discharge possible and a more efficient decrease in mean pulmonary, right ventricular, and right atrial pressures than the VTA with a increased risk, however, of ventricular suction event occurrence, this event appearing more quickly in the presence of high pulmonary vascular resistances.
Finally, concerning the yield of these two pumps for a similar output, the VTA had a significantly lower energy consumption than the HTM II at rest and during effort. These results are explained by the lower number of revolutions per minute for the VTA compared to the HTM II.
Clinical Impacts
Although these results remain to be confirmed by other in vivo studies, the clinical consequences of this work are potentially interesting because they show an advantage in favor of the HTM II, axial pump, compared to the VTA, centrifugal pump, to discharge the left cavities and enable more significant output. Thus, the choice of the type of centrifugal or axial pump could be adapted to the patient’s hemodynamic profile. The HTM II axial pump could therefore have a particular interest for patients of tall build, to diminish high pulmonary pressures in patients temporarily contraindicated for heart transplant, or even for young patients eager to resume physical and professional activity.
Limitations of the Study
The interpretation of all these results must, however, take certain limits of this experimental model into consideration. Starling’s Law cannot be applied in artificial pneumatic ventricles. This physiological condition cannot be ignored in a healthy heart, but is not as important in case of ventricular failure, where ventricles are almost incapable of increasing their ejection volume as a response to an increase in preload. Passive filling of the ventricle could, however, be reproduced in our model.
Another difficulty in reproducing a viable model is that in a clinical situation, a drop in pressure in the left cavities by the assist device results in a risk of left ventricle collapse, called “ventricular suction” or “suckdown,” leading to a drop in cardiac output. Indeed, the left ventricular unload brought by an increase in the assist device’s rotation speed can cause a collapse of the ventricular wall against the intraventricular inflow cannula. This event is not reproduced in this model because of the rigid and nonphysiological nature of the tubes and ventricles used in the Mock. Furthermore, the assist device is downstream from the tank that replicates the pressures of the left auricle, but for a volume 10 times greater to the one existing in the left auricle of a normal heart. This suction event is thus reproduced with difficulty because the left auricle is never empty. To try to recreate this ventricle suction, it was then decided that the distance separating the outflow of the left auricle and the pneumatic ventricle’s inflow valve should be increased (approximately 5–6 cm), thus recreating a shorter “atrium” with a more physiologic volume. Unfortunately, even then, the ventricular suction event was not entirely reproduced; the silicone tube was bending without totally collapsing. The use of more flexible material could most probably solve this problem.
In addition, the choice of pneumatic ventricles to reproduce the pulsatile flow inside the mock limits the maximal systolic ejection volume to their ejection chamber’s maximal volume, that is to say 70 ml for the CardioWest ventricle. This volume is low compared to a young patient with no heart insufficiency, especially during effort. This partly explains the low output and pressure obtained during the simulation of an exercise with no ventricular alteration. This volume is, however, more compatible with observable ejection volumes in a ventricular insufficiency case.
At last, like in all mechanical models, the physiological regulations seen in chronic heart failure like alterations of the β-adrenergic signal, altered expression of natriuretic peptides, and abnormal plasma renin activity could not be reproduced in this model. The evolution of these regulations after connection of the LVAD and their impact could not be either. The long-term evolution of the left ventricular function under LVAD and the potential part of recovery cannot be anticipated.
Conclusion
This experimental model enables the simulation of pressures and physiological outputs encountered in a case of left ventricular dysfunction, at rest and during effort, with or without pulmonary hypertension. Although this kind of test can never replace in vivo studies, it enables us to assess the hemodynamic profiles of LVADs, to assess a pump in given physiological conditions, or else to compare several assist devices among themselves. The experimental results obtained in this model, comparing the HTM II and the VTA, find an advantage in favor of axial pumps compared to centrifugal pumps to discharge left cavities and enable a greater output at the expense of greater “ventricular suction” event risk for the HTM II. Setting up autoregulation for the axial assist device according to anterior pressures could enable better blood output while preventing the risk of ventricular suction. In vivo studies that compare the hemodynamic performances of axial and centrifugal pumps must however be carried out to confirm these results.
Acknowledgment
The authors thank the SFCTCV who participated in this work through the attribution of a study grant. They thank Thoratec for lending their circulatory assist device HTM II and the DDC console, Syncardia for authorizing us to use the CardioWest mock as our experimental model base, and Ventracor who unfortunately could not pursue their circulatory assist device adventure. They also thank Prof. Tsui’s team from the Papworth hospital where this study was carried out.
Footnotes
Submitted for consideration February 2013; accepted for publication in revised form January 2014.
Disclosure: Steven Tsui is a member of Thoratec European Advisory Board and Heartware Scientific Advisory Board. Daniel Duveau is a member of Carmat Advisory Board. Thoratec has provided the Heartmate II pump and DDC console. Ventracor has provided the VentrAssist pump and Syncardia the MOCK circulation. Jean Noel Trochu is a member of Thoratec European Advisory Board. The remaining authors have no conflicts of interest to disclose.
This work received a grant from the French Society of Thoracic and Cardiovascular Surgery.
Reprint Requests: Thomas Sénage, Service de Chirurgie Thoracique et Cardiovasculaire, Hôpital Laënnec, CHU de Nantes, Bd Jacques Monod, 44093 Nantes Cedex 1, France. Email: thomas.senage@chu-nantes.fr.
References
- 1.Stonehouse A, Cook M, Woodard J. VentrAssist left ventricular assist systems long-term in vitro reliability. Artif Organs. 2009;33:860–864. doi: 10.1111/j.1525-1594.2009.00804.x. [DOI] [PubMed] [Google Scholar]
- 2.Frazier OH, Delgado RM, III, Kar B, Patel V, Gregoric ID, Myers TJ. First clinical use of the redesigned HeartMate II left ventricular assist system in the United States: A case report. Tex Heart Inst J. 2004;31:157–159. [PMC free article] [PubMed] [Google Scholar]
- 3.Esmore DS, Kaye D, Salamonsen R, et al. First clinical implant of the VentrAssist left ventricular assist system as destination therapy for end-stage heart failure. J Heart Lung Transplant. 2005;24:1150–1154. doi: 10.1016/j.healun.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Olegario PS, Yoshizawa M, Tanaka A, et al. Outflow control for avoiding atrial suction in a continuous flow total artificial heart. Artif Organs. 2003;27:92–98. doi: 10.1046/j.1525-1594.2003.07185.x. [DOI] [PubMed] [Google Scholar]
- 5.Bullister E, Reich S, Sluetz J. Physiologic control algorithms for rotary blood pumps using pressure sensor input. Artif Organs. 2002;26:931–938. doi: 10.1046/j.1525-1594.2002.07126.x. [DOI] [PubMed] [Google Scholar]
- 6.Klotz S, Deng MC, Stypmann J, et al. Left ventricular pressure and volume unloading during pulsatile versus nonpulsatile left ventricular assist device support. Ann Thorac Surg. 2004;77:143–149. doi: 10.1016/s0003-4975(03)01336-5. discussion 149. [DOI] [PubMed] [Google Scholar]
- 7.Haft J, Armstrong W, Dyke DB, et al. Hemodynamic and exercise performance with pulsatile and continuous-flow left ventricular assist devices. Circulation. 2007;116(11 Suppl):I8–I15. doi: 10.1161/CIRCULATIONAHA.106.677898. [DOI] [PubMed] [Google Scholar]
- 8.Timms D, Hayne M, McNeil K, Galbraith A. A complete mock circulation loop for the evaluation of left, right, and biventricular assist devices. Artif Organs. 2005;29:564–572. doi: 10.1111/j.1525-1594.2005.29094.x. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Allaire P, Wood H, Olsen D. Design and initial testing of a mock human circulatory loop for left ventricular assist device performance testing. Artif Organs. 2005;29:341–345. doi: 10.1111/j.1525-1594.2005.29058.x. [DOI] [PubMed] [Google Scholar]
- 10.Legendre D, Fonseca J, Andrade A, et al. Mock circulatory system for the evaluation of left ventricular assist devices, endoluminal prostheses, and vascular diseases. Artif Organs. 2008;32:461–467. doi: 10.1111/j.1525-1594.2008.00569.x. [DOI] [PubMed] [Google Scholar]
- 11.Foray A, Williams D, Reemtsma K, Oz M, Mancini D. Assessment of submaximal exercise capacity in patients with left ventricular assist devices. Circulation. 1996;94(9 Suppl):II222–II226. [PubMed] [Google Scholar]
- 12.de Jonge N, Kirkels H, Lahpor JR, et al. Exercise performance in patients with end-stage heart failure after implantation of a left ventricular assist device and after heart transplantation: An outlook for permanent assisting? J Am Coll Cardiol. 2001;37:1794–1799. doi: 10.1016/s0735-1097(01)01268-2. [DOI] [PubMed] [Google Scholar]
- 13.Salzberg SP, Lachat ML, von Harbou K, Zünd G, Turina MI. Normalization of high pulmonary vascular resistance with LVAD support in heart transplantation candidates. Eur J Cardiothorac Surg. 2005;27:222–225. doi: 10.1016/j.ejcts.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Mikus E, Stepanenko A, Krabatsch T, et al. Reversibility of fixed pulmonary hypertension in left ventricular assist device support recipients. Eur J Cardiothorac Surg. 2011;40:971–977. doi: 10.1016/j.ejcts.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 15.Maeder MT, Leet A, Ross A, Esmore D, Kaye DM. Changes in right ventricular function during continuous-flow left ventricular assist device support [corrected]. J Heart Lung Transplant. 2009;28:360–366. doi: 10.1016/j.healun.2009.01.007. [DOI] [PubMed] [Google Scholar]


