Abstract
To enhance ambulation and facilitate hospital discharge of total artificial heart (TAH)–supported patients, we adapted a mobile ventricular assistance device (VAD) driver (Excor) for TAH use and report on the performance of Excor-driven TAH patients discharged home. Ten patients stabilized on a TAH, driven by the CSS (“Circulatory Support System”), were progressively switched over to the Excor in hospital over 14 days as a pilot, with daily hemodynamics and laboratory parameters measured. Twenty-two stable TAH patients were subsequently placed on the Excor, trained, and discharged home. Clinical and hemodynamic parameters were followed. All pilot study patients were clinically stable on the Excor, with no decrease in TAH output noted (6.3 + 0.3 L/min [day 1] vs. 5.8 + 0.2 L/min [day 14], p = 0.174), with a trend suggesting improvement of both hepatic and renal function. Twenty-two TAH patients were subsequently successfully discharged home on the portable driver and were supported out of hospital for up to 598 days (range, 2–598; mean = 179 ± 140 days), remaining ambulatory, New York Heart Association (NYHA) class I or II, and free of readmission for 88.5% of the time of support. TAH patients may be effectively and safely supported by a mobile drive system. As such, the utility of the TAH may be extended to support patients beyond the hospital, at home, with overall ambulatory freedom.
Keywords: total artificial heart, out-of-hospital, portable driver
The SynCardia Total Artificial Heart (TAH) (SynCardia Systems, Tucson, AZ) is a pneumatic, pulsatile, biventricular replacement system that is surgically implanted orthotopically following cardiac excision.1 The SynCardia TAH received Food and Drug Administration (FDA) approval for in-hospital bridge to transplantation in 2004. In the pivotal regulatory trial of the TAH, greater than 80% of patients were ambulatory within 2 weeks post-op.2 However, as initially configured, the SynCardia TAH system remains limited by the reliance on a large bulky pneumatic driver console (“Circulatory Support System” [CSS], aka “Big Blue Driver”), weighing more than 350 pounds, which the supported patient is incapable of maneuvering, relying on several assistants to move the console during ambulation (Figure 1A).
Figure 1.
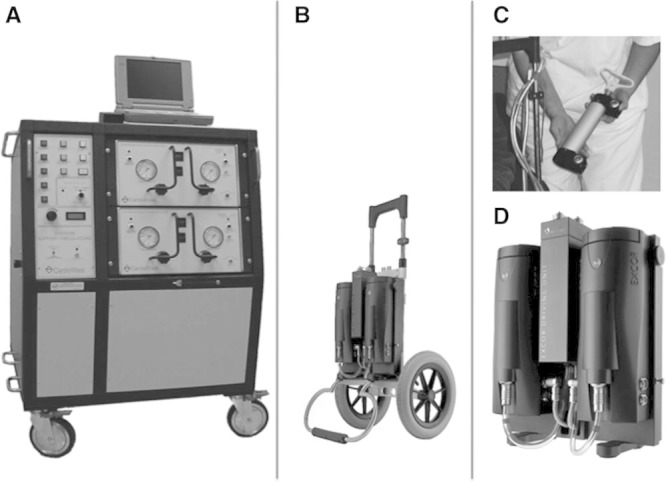
Modified small mobile pneumatic driver system to power the total artificial heart (TAH). A: Original TAH Circulatory Support System (CSS) console (Big Blue Driver). B: Modified Excor drive system mounted on dolly (photo is same scale as Big Blue for comparison). C: Hand pump capable of driving TAH in case of complete system failure. D: Close-up of the Excor with right and left pumping units and central valve system.
We explored the possibility of developing a system which would afford the TAH patient greater mobility as well as self-mobility, while being effective as a means of powering the TAH. Although work has been ongoing in designing and testing a specific portable driver for the TAH—the Freedom driver, as an initial step, to examine the clinical viability of this approach, we adapted a small pneumatic pulsatile driver, the Berlin Excor Mobile Drive System (Berlin Heart, Berlin, Germany), which had heretofore been developed to power pulsatile VADs, for use with the TAH. We hypothesized that a small pulsatile driver with adequate pneumatic output would effectively drive the TAH in clinically stable, recovered patients, affording them greater mobility, discharge from the hospital to the home environment, with overall ambulatory freedom. In this article, we report on an initial pilot progressive “switchover” study examining the efficacy of the modified drive system to support TAH patients followed by a larger study examining the efficacy and safety of the modified driver system in TAH patients, both in the hospital and upon discharge to home.
Methods
All human investigation was approved by the institutional review board and ethics committee of the Heart Center, North Rhine-Westphalia. All subjects provided written informed consent before study participation. Patient enrollment for this study was conducted between July 2003 and December 2005 with follow-up through April 2008.
The SynCardia Total Artificial Heart System
The SynCardia TAH (formerly CardioWest) (SynCardia Systems Inc., Tucson, AZ) is a biventricular, pulsatile, pneumatically driven blood pump consisting of two freely mobile ventricles that are implanted orthotopically.1,2 The maximal stroke volume of the TAH is 70 ml, with outputs exceeding 9.5 L/min achievable. In this study, the TAH was operated at rates of 90–130/min, with a vacuum of –4 to –8 mm Hg.
The Berlin Excor Mobile Pneumatic Drive System
The Excor drive system in clinical use today was modified to drive the SynCardia TAH system (Figure 1B). The Excor driver is a portable pneumatic drive system weighing 20 pounds, mobile on a handled dolly, originally designed to power the Excor VAD system.3 Its footprint is significantly smaller than the original CSS console. The drive system contains two driver modules, each functioning to pump a single VAD (Figure 1D). The powered system also is provided with a hand pump system, allowing alternative right- and left-sided pumping in case of complete powered system failure (Figure 1C). Five-foot pneumatic drivelines connect the console to the artificial heart.
To effectively drive the TAH, three major modifications were made to the Excor drive system. First, a change was made to the internal driver software, adding a safety pumping sequence in case of failure of one of the two drive modules. This safety backup sequence rapidly shifts pumping in the remaining functioning module to an alternating right side versus left side pumping mode, through coordinated opening and closing of a central valve mechanism. Second, the rate of pumping of the Excor was increased to accommodate the higher rates used in the TAH (up to 130 bpm) versus lower rates of VADs (70–80 bpm). Finally, changes were made to indicate full filling of the ventricles, to emulate the CSS, so as to allow adjustment of the rate of pumping to increase flow if clinically required. Vacuum was adjusted based on stroke volume and rate.
As a design specification, the maximum cardiac output that was sought with these modifications was 8 L/min, which was felt to be adequate for stabilized patients and patients well enough to be discharged.
In-Hospital Pilot Study—“Switchover” from the Approved CSS Console to the Modified Portable Driver
Volunteer TAH patients (n = 10), maintained on the CSS console, awaiting transplantation, were selected for the switchover trial with the following inclusion and exclusion criteria: Inclusion—clinical stability without evidence of end-organ failure, pump output on CSS < 8 L/min, and the ability to provide informed consent; and Exclusion—plasma free hemoglobin (Hgb) > 50 mg/dl, evidence of sepsis or driveline infection requiring systemic antibiotics, and multiple organ failure. Following selection for the study, baseline laboratories were drawn including hematocrit, plasma free Hgb, lactate dehydrogenase (LDH), blood urea nitrogen, creatinine, alanine transaminase (ALT), and total bilirubin. Baseline hemodynamics were obtained including mean arterial pressure, pump rate, and right and left pump output. Patients were then “switched over” to the Excor using escalating lengths of support as follows: day 1—2 hours, day 2—4 hours, day 3—8 hours, and day 4 and beyond—24 hours. The length of the trial was 14 days. At the completion of the switchover time, each day repeat laboratories and hemodynamics were obtained. If evidence of hemolysis or reduced hemodynamic support was apparent, patients were placed back on the CSS and were excluded from the remainder of the trial. Data for each parameter was recorded daily and results were then compared with those obtained while on the CSS. Device-related malfunctions and clinical adverse events were also carefully monitored and recorded.
Discharge Study: Demonstration of the Efficacy and Safety of Out-of-Hospital Utilization of the TAH with a Mobile Drive System
Volunteer TAH patients (n = 22, 8 of 22 from the pilot with 14 of 22 new patients) awaiting transplantation and stable on the CSS console were enrolled in the discharge study. All patients, and a caregiver companion, were trained on the use and maintenance of the Excor with the goal of being safe and prepared for discharge from the hospital to home. Training consisted of basic operation and maintenance of the Excor including battery change, care of the drivelines, console switch out, use of the hand pump as a bailout, and home anticoagulation monitoring. Patients were deemed suitable for discharge if they met the following criteria: hemodynamic stability, being full ambulatory, New York Heart Association (NYHA) class I or II, free of end-organ failure and wound infections, and having adequate personal support, including a family member or caregiver present with the patient 24 hours a day. Patients unable to have a constant companion were not enrolled in this study. Patients selected for discharge were then sent home and followed up as outpatients weekly for 4 weeks and then monthly. At each visit, patients were interviewed for state of health and overall well-being, presence of problematic symptoms, and underwent physical examination.
Statistical Analysis
Data are presented as mean ± SD. Statistical comparisons were made using the Student’s t-test or paired-samples t-test. Significance was defined at the p less than 0.05 level.
Results
In-Hospital Pilot Study—Demonstration of Safe “Switchover” from the CSS Console to the Modified Portable Driver
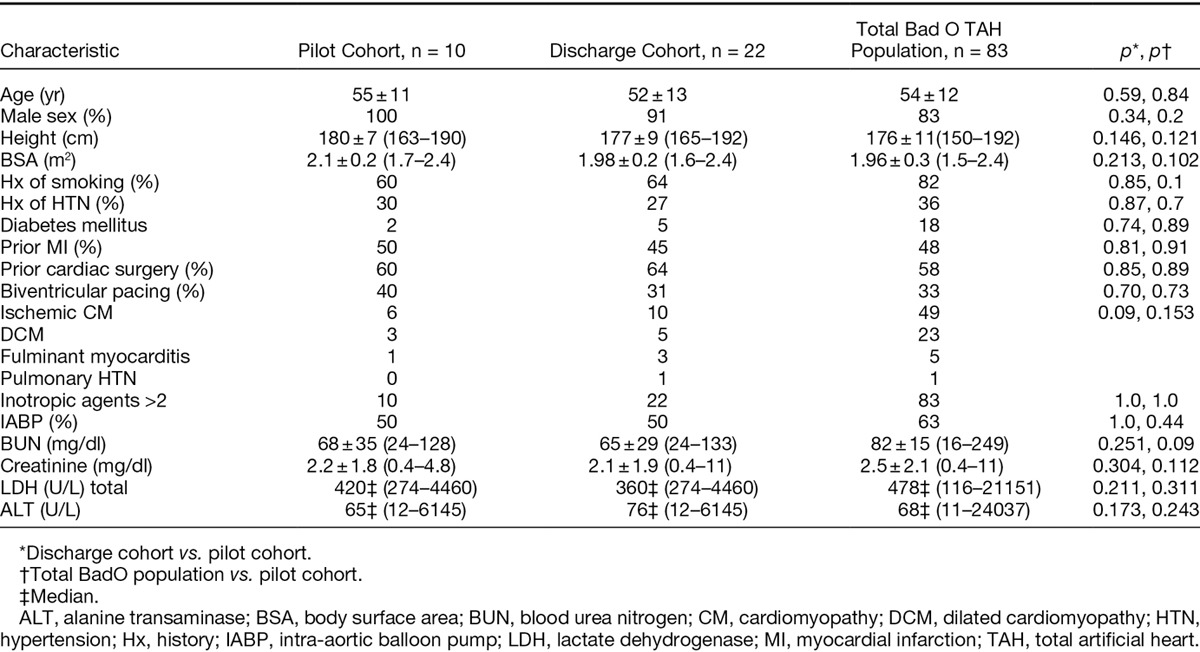
Ten patient volunteers with end-stage heart failure, salvaged and stabilized on the TAH driven by the CSS driver, were enrolled in the pilot study. On average, these patients had been maintained on the TAH for 69 + 58 days (range, 11–190 days) before study entrance. The baseline characteristics of these patients, pre-TAH implantation, are outlined (Table 1) and were similar to the characteristics of patients from the overall Bad Oeyenhausen TAH patient experience, up to and contemporaneous with this study.
Table 1.
Baseline Patient Characteristics Pre-TAH Implantation of the Pilot Study Cohort, the Discharge Study Cohort, and the Overall Bad Oeyenhausen TAH Population
Overall, the Excor provided adequate pneumatic drive power to support the TAH in all patients. Pressure and flow were well maintained on the Excor system during the pilot study (Table 2). Mean arterial pressure (mean, mm Hg) at baseline was 82 + 10 on the CSS versus 88 + 4 by day 14 while on the Excor, p = 0.195. Pump rate (bpm) did not vary, being 117 ± 11 at baseline versus 117 + 9 at day 14, p = 0.187. Left heart output (L/min) did not vary significantly over the 14 day period, being 6.5 ± 0.9 at baseline versus 6.1 + 0.6 at day 14, p = 0.502. Right heart output varied slightly, being 6.6 ± 0.9 at baseline and 5.8 ± 0.5 at day 14, p = 0.027. No significant decline in patient overall well-being or general health was reported or observed, over the course of support with the Excor during the pilot study.
Table 2.
Hemodynamic Performance of TAH Patients Supported by the Modified Excor Driver During the Pilot Study

Interestingly, differences in the pressure and suction needed for emptying and filling of the TAH were found to be significantly different for the Excor versus the CSS. The driving pressure (the pressure required for complete emptying of the ventricles) was lower with the Excor (mean at day 4, mm Hg, 63 ± 13 R side, 154 + 21 L side) versus the CSS (87 + 4 R side, 184 + 7 L side), p = 0.002 and 0.004, respectively. Conversely, the suction requirement (vacuum needed for filling of the ventricles) was higher for the Excor (mean at day 4, mm Hg, –15.5 + 3) versus the CSS (10 ± 1.4), p = 0.001.
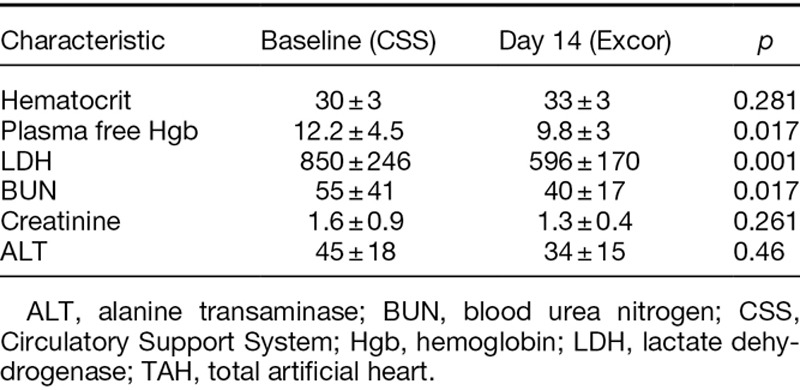
Renal and hepatic function, upon switching to the Excor, compared favorably to that observed while on the CSS driver (Table 3). There was no evidence of decline of function while on the Excor, rather a trend toward improvement was observed. Similarly switching to the Excor was not associated with an increase in hemolysis. Hemolysis appeared to be reduced on the Excor, as evidenced by a reduction in both plasma free hemoglobin (12.2 + 4.5 [CSS Baseline] vs. 9.8 + 3 [Excor day 14], p = 0.017) and LDH release (596 + 170 [CSS baseline] vs. 850 + 246 [Excor day 14], p = 0.047).
Table 3.
Laboratory Parameters of TAH Patients Supported by the Modified Excor Driver During the Pilot Study
Use of the Excor significantly increased the mobility and ambulatory freedom of the trial patients. By day 5, all of the enrolled patients were able to self-ambulate more than 1,000 feet/day, wheeling the driver in tow, with at least three excursions per day. Beyond 1 week, all 10 patients were routinely walking, towing the driver themselves, without frequency or time restrictions, within the run of the hospital grounds, although still accompanied by a trained companion for safety purposes. By day 14, all of the Excor patients were observed to take frequent walking excursions, with an average of 5 ± 2 trips/day. At this point, patients walked on average 3,600 ± 1,000 feet/day. In comparison, stable TAH patients maintained on the CSS had walking excursions only twice per day, with staff assistance needed to push the CSS, with an average distance walked of 900 + 180 feet/day, p less than 0.05.
There were minimal device-related malfunctions during the switchover pilot study. In one case, one of the two drive units of the Excor driver intermittently stopped pumping. The driver appropriately shifted to the emergency alternate right-left pumping sequence for the remaining functioning drive unit with no harm to the patient. In two other instances, frank drive unit failure occurred in one of the two drive units of the Excor due to a defect in the power transistor of the motor control unit. Similarly, no patient harm resulted, as the driver again shifted to the emergency backup alternate pumping sequence. In two cases, low pump output was detected. In both of these cases, this was associated with uncontrolled hypertension (BP systolic > 220) rather than true device malfunction, with the driver unable to overcome the excessive afterload. One of the two patients developed transient pulmonary edema due to reduced left-sided output. Through antihypertensive and diuretic administration, achieving BP control, the low output state resolved in both patients.
As to other adverse events, one of the 10 patients had a clinically adverse neurologic event despite adequate anticoagulation, suffering a persistent hemiparesis at day 10 on the Excor. Following this event, he was placed back on the CSS console and over a 2 month period had significant resolution of his paresis with minimal residua and was eventually successfully transplanted. One of the 10 patients developed weight gain and lower limb edema while on the Excor. This patient was large with a body surface area of 2.4 m2, which was a challenge for the drive system for adequate perfusion. Edema successfully resolved with concomitant continued diuretic use. No adverse sequelae occurred in this patient.
Discharge Study: Efficacy and Safety of Out-of-Hospital Utilization of the TAH with a Mobile Drive System
Twenty-two TAH patients volunteered for the home discharge study. Eight of the 22 were in the initial pilot switchover study, with an additional 14 being directly enrolled in this study. On average, these patients had been maintained on the TAH in hospital on the CSS driver for 40 ± 10 days following implantation. These patients were then shifted to the Excor and maintained in the hospital for 18 ± 7 days before discharge. During this period, they underwent training on use of the driver in simulated out of hospital circumstances. They were encouraged to steadily increase their degree of ambulation to build confidence with the drive system. Following discharge, patients were maintained on the Excor for 179 ± 140 days (range, 2–598 days). The baseline characteristics of the patients in the discharge study, pre-TAH implantation, are outlined and were similar to those of the total Bad Oeyenhausen patient experience (Table 1).
All 22 patients met the criteria for discharge (as outlined above) and were successfully discharged home, with none to an intermediate care facility. All patients were self-ambulatory upon discharge. Of the 22 patients, 15 (68%) were successfully transplanted with seven dying before transplantation. Of the 15 transplanted patients, the average length of support out of the hospital on the Excor averaged 175 + 116 days (range, 2–598 days).
All patients remained fully ambulatory while out of the hospital, save for events which necessitated readmission. In follow-up examination, patients overall reported feeling well while on the TAH driven by the Excor system. All patients reported feeling psychologically better out of hospital on the TAH than in the hospital; however, as a limitation of this study, no formal quality-of-life instrument was employed. No increase in fatigability or weakness was noted while out of the hospital on the Excor, with all patients remaining NYHA class I or II. Six of the 22 patients resumed driving an automobile, three patients resumed work, with one patient resuming her duties as a mother of a family of four with small children. Eight of the patients resumed sexual activity. All discharged patients were found to maintain a mean arterial pressure greater than 95 (range, 80–110 mm Hg), at every follow-up clinic visit. Hypertension rather than hypotension became an issue, with all patients requiring maintenance on angiotensin-converting enzyme inhibitors (enalapril, mean dose, 14 + 6 mg) to avoid hypertension while on the Excor.
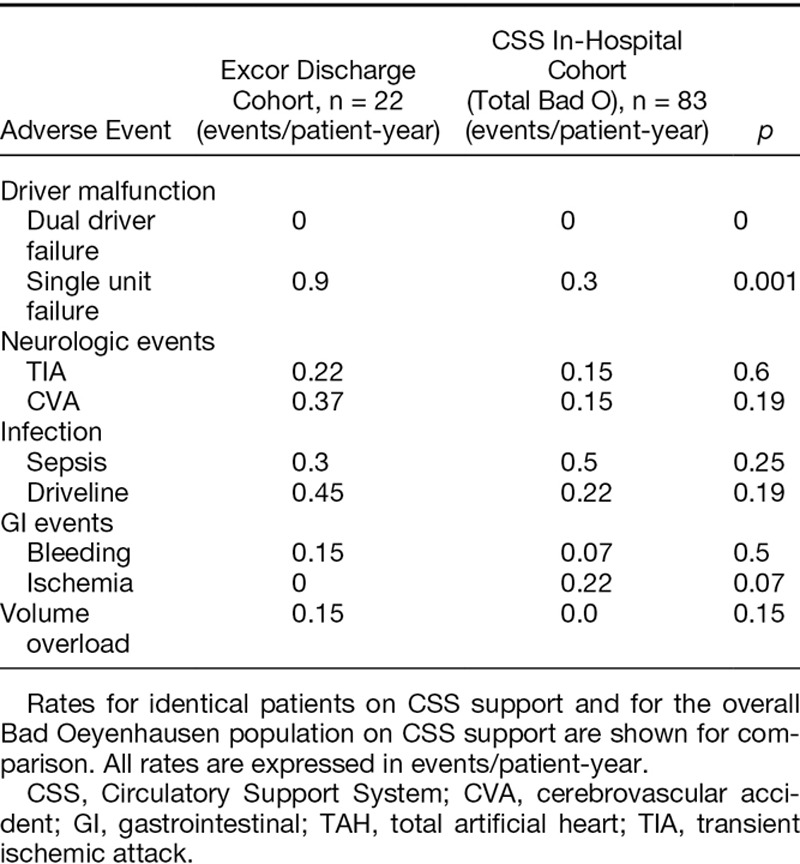
Adverse event rates for the 22 patients on the Excor were comparable with those observed while on the CSS, as well as those of the entire Bad Oeyenhausen in-hospital TAH experience on the CSS (Table 4).
Table 4.
Adverse Events Rates for TAH Patients in the Discharge Study While on Modified Excor Driver Support
The combined total days of out-of-hospital support for the 22 patients was 3,938 patient-days. From the perspective of total days of support (4,432), patients were maintained out of the hospital, free of readmission, for 88.5% of the time. Nineteen of the 22 patients required readmission during this study for a total of 32 readmission events. Of the nineteen patients, 11 of 19 (58%) had a single readmission, four of 19 (21%) required two admissions, three of 19 (16%) required three admissions, and one (5%) had four readmissions. The bulk of readmissions (55%) occurred within the initial 3 month time period postdischarge. The adverse events necessitating readmission were transient ischemic attack (TIA) in three patients, strokes in five patients, infection in six patients, gastrointestinal (GI) bleeds in two patients, technical driver issues in nine patients, volume overload (congestion and edema) in three patients, and social issues in four patients. The average length of stay for readmissions was 15.4 ± 25 days, ranging from 1 day to 101 days. The number of readmission days for the entire cohort studied compared to the total out-of-hospital days was 494 vs. 3,938, for a percent time of readmission while on Excor support of 12.5%. Patients readmitted because of Excor driver failure or required rehospitalization for longer than 72 hours were switched to a big blue driver.
The overall postdischarge survival rate at 1 year on the Excor was 76% (Figure 2). Seven of the 22 patients (32%) died while on the Excor, with all deaths occurring in the hospital following readmission, no patient dying at home. The causes of death were as follows: sepsis in five patients at 56, 201 271, 275, and 558 days postdischarge and stroke in two patients—one hemorrhagic and one embolic—at 154 and 66 days postdischarge, respectively.
Figure 2.
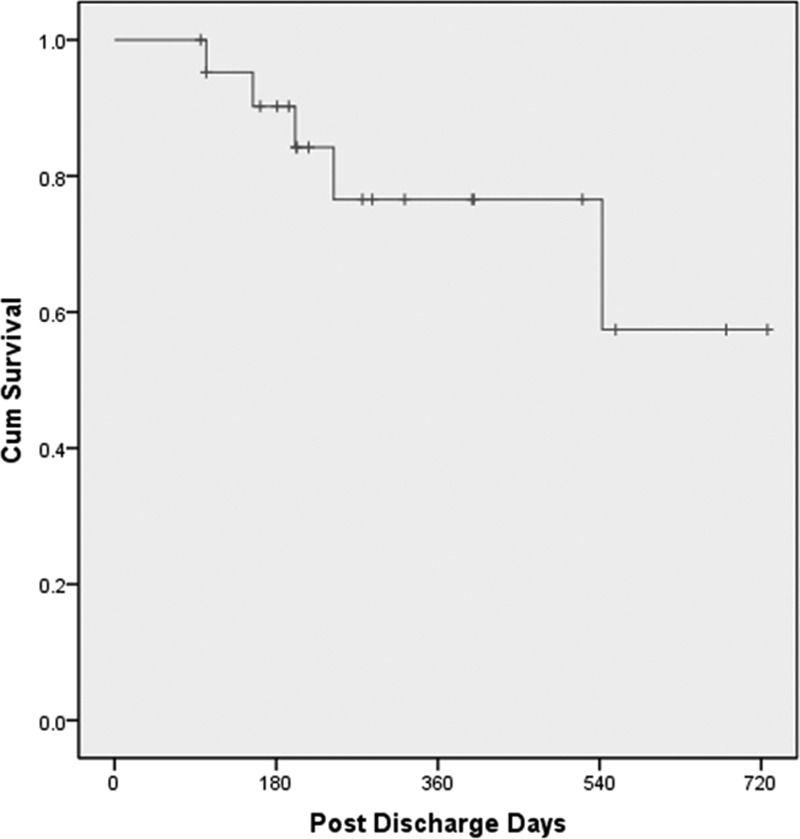
Overall survival of total artificial heart patients supported by the modified Excor driver from the time of hospital discharge.
Discussion
The principal finding of this study is that failing end-stage heart failure patients, salvaged via implantation of the TAH, recovering and clinically stable postimplantation, could be successfully transitioned, maintained, and discharged from the hospital to home on a mobile portable driver. This is the first report of the successful discharge of a cohort of patients on a full biventricular replacement, TAH system utilizing portable driver technology.
To date, there have been more than 1,060 implants of the SynCardia TAH worldwide, with more than 500 implants since FDA approval in 2004. On the TAH, patients typically improve rapidly, with more than 80% of patients able to ambulate within 14 days with greater than 60% ambulating more than 100 feet.2 Despite recovery and significant rates of ambulation, the quality of this mobility remains limited by the reliance on the original CSS console for pneumatic drive of the TAH. This original driver is extremely bulky, heavy at 380 pounds, not movable by the implanted patient, with patient walks requiring a team to assist the patient. By the end of 1 month post-TAH implantation, most patients have made a significant recovery with improved functional status and are active, routinely walking within the hospital, and engaged in full physical rehabilitation, though only with assistance in movement of the CSS driver. The option of a portable driver, small enough and light enough for a patient to move by themselves, dramatically expands the quality and quantity of ambulation and activity level achievable for the patient. Portability additionally extends the ambulatory freedom of these patients, allowing discharge to home, resulting in an improved overall quality of life.
A small low-weight portable driver system, specifically designed for the SynCardia TAH, has recently been developed—the Freedom driver.4 The regulatory approval trial for this driver has recently completed enrollment with good overall results with this system to date. The current study was conducted before the emergence and clinical testing of the new Freedom driver. This study served as an overall “clinical pilot” to the present Freedom trial, testing the viability and potential efficacy of home discharge and life out of the hospital on a TAH system with an ambulatory driver.
In the present pilot switchover study, the modified Excor system, in general, functioned well, differences in driving pressures and vacuum might be related to the shorter drivelines in Excor cohort (five foot versus seven foot). In several cases, over time the mean arterial pressure declined slightly compared to the baseline before switching while on the CSS. Over the 2 week trial period in the hospital, all patients did well overall on the portable driver, with no decline in general health detected, save for one patient who experienced a neurologic event. Objectively, no evidence of end-organ compromise was evident on the small driver, other than this cited patient. On the contrary, renal and hepatic function showed trends toward improvement. Hemolysis also appeared to be reduced compared to the CSS. All patients felt better with enhanced ambulatory freedom with greater daily excursions and a clear improvement in general mood and outlook.
In the discharge cohort similarly the majority of patients were well supported by the modified mobile driver over the duration of their outpatient experience. The 22 patients were supported for a total of 3,938 patient-days with more than 88% of time being spent out of the hospital. All discharged patients in general felt well upon follow-up visit questioning and many were able to resume normal daily life activities, including walking, driving, and sexual activity with several returning to part-time work. Only one of the 22 patients was readmitted for driver failure as evidenced by persistent peripheral edema. This contrasts dramatically to the preimplant status of these patients who were all initially bedridden, Class IV, and largely inotrope-dependent before TAH implantation. Subjectively, all patients described a further improvement in overall health and outlook upon being switched to a mobile driver and being discharged home.
In contrast to the CSS cohort, Excor patients experienced initially significant higher rates of driver malfunction (single unit failure), this has been resolved after changing the switching valve. The higher rates of the neurologic and the GI bleeding events were most probably related to the tight anticoagulation management protocols for in-hospital patients. The resuming daily life activities in Excor patients are most likely the reason for the higher rates of driveline infection.
This preliminary experience is the first description of the successful discharge of a series of patients maintained solely on a biventricular replacement, TAH. Over the lifespan of investigations with TAHs, an initial isolated experience was described by Semb in Sweden in which an earlier Jarvik design pneumatic TAH was implanted in a patient who was successfully discharged and maintained out of the hospital on a small portable driver.5 This patient remained self-ambulatory and survived for nearly 1 year. A home discharge experience was described for the AbioCor fully implantable TAH. This electrohydraulic device has had limited clinical experience being implanted in 15 patients, with only one patient being discharged surviving for 525 days, with the bulk of patients dying in-hospital, with adverse neurologic events observed in more than 80% of patients.6 The first successful discharge of a SynCardia TAH patient with the new Freedom driver was recently reported.4 As of the time of this writing, an additional 30 patients have been successfully transitioned from the CSS to the Freedom driver in the U.S. trial. It is anticipated that over the coming months the formal analysis of this experience will become available.
Similarly, limited experience exists in the broad mechanical circulatory support literature with regard to successful discharge of patients supported with biventricular assist devices. In a report of patients from the German Heart Center in Berlin, with more than 10 years of experience with discharge of patients on VADs, only five patients were successfully discharged with a biventricular assist device (BiVAD) system.3 No information on survival, bridge to transplantation, or adverse events was reported. In a report of home discharge experience with Thoractec VADs driven by the portable VAD driver, only one patient with BiVAD support was successfully sent home.7 Our present TAH experience significantly extends the potential of support and home discharge for patients with biventricular heart failure.
Readmission was necessary in most patients in this series, although the majority of patients had a single readmission event. Only one patient was readmitted for technical driver dysfunction. No TAH device malfunction occurred with the modified driver compared with a single TAH failure, reported in the prior in-hospital trial.
In summary, in this study, we demonstrate that TAH patients may be successfully supported with a mobile drive system, allowing increased ambulation, successful discharge, and maintenance out of the hospital while awaiting transplantation. With continued improvement in driver portability and durability, a viable TAH system capable of providing long-term support, with low levels of adverse events, returning patients to a reasonable functional class and a good quality of life is achievable.
Footnotes
Submitted for consideration May 2013; accepted for publication in revised form October 2013.
Disclosure: Dr. Copeland reports owning equity in SynCardia Systems. Mr. Smith and Dr. Slepian report owning equity in SynCardia Systems and being paid for part-time employment by the company. The other authors have no conflicts of interest to report.
Reprint Requests: Aly El Banayosy, MD, Penn State Hershey Medical Center, 500 University Drive, Hershey, PA 17033. Email: aelbanayosy@hmc.psu.edu.
References
- 1.Slepian MJ, Smith RG, Copeland JC. The SynCardia CardioWestTM total artificial heart. In: Baughman KL, Baumgartner WA, editors. Treatment of Advanced Heart Disease. New York, NY: Marcel Dekker; 2006. [Google Scholar]
- 2.Copeland JG, Smith RG, Arabia FA, et al. CardioWest Total Artificial Heart Investigators. Cardiac replacement with a total artificial heart as a bridge to transplantation. N Engl J Med. 2004;351:859–867. doi: 10.1056/NEJMoa040186. [DOI] [PubMed] [Google Scholar]
- 3.Drews TN, Loebe M, Jurmann MJ, Weng Y, Wendelmuth C, Hetzer R. Outpatients on mechanical circulatory support. Ann Thorac Surg. 2003;75:780–785. doi: 10.1016/s0003-4975(02)04648-9. [DOI] [PubMed] [Google Scholar]
- 4.Jaroszewski DE, Anderson EM, Pierce CN, Arabia FA. The SynCardia freedom driver: a portable driver for discharge home with the total artificial heart. J Heart Lung Transplant. 2011;30:844–845. doi: 10.1016/j.healun.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 5.Gil G. The artificial heart juggernaut. Hastings Cent Rep. 1989;19:24–31. [PubMed] [Google Scholar]
- 6.Frazier OH, Dowling RD, Gray LA, Jr, Shah NA, Pool T, Gregoric I. The total artificial heart: where we stand. Cardiology. 2004;101:117–121. doi: 10.1159/000075992. [DOI] [PubMed] [Google Scholar]
- 7.Slaughter MS, Sobieski MA, Martin M, Dia M, Silver MA. Home discharge experience with the Thoratec TLC-II portable driver. ASAIO J. 2007;53:132–135. doi: 10.1097/MAT.0b013e31802c189e. [DOI] [PubMed] [Google Scholar]


