Abstract
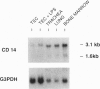
Mammals continually confront microbes at mucosal surfaces. A current model suggests that epithelial cells contribute to defense at these sites, in part through the production of broad-spectrum antibiotic peptides. Previous studies have shown that invertebrates can mount a host defense response characterized by the induction in epithelia] cells of a variety of antibiotic proteins and peptides when they are challenged with microorganisms, bacterial cell wall/membrane components, or traumatic injury [Boman, H.G. & Hultmark, D. (1987) Annu. Rev. Microbiol. 41, 103-126J. However, factors that govern the expression of similar defense molecules in mammalian epithelial cells are poorly understood. Here, a 13-fold induction of the endogenous gene encoding tracheal antimicrobial peptide was found to characterize a host response of tracheal epithelia] cells (TECs) exposed to bacterial lipopolysaccharide (LPS). Northern blot data indicated that TECs express CD14, a well-characterized LPS-binding protein known to mediate many LPS responses. A monoclonal antibody to CD14 blocked the observed tracheal antimicrobial peptide induction by LPS under serum-free conditions. Together the data support that CD14 of epithelial cell origin mediates the LPS induction of an antibiotic peptide gene in TECs, providing evidence for the active participation of epithelial cells in the host's local defense response to bacteria. Furthermore, the data allude to a conservation of this host response in evolution and suggest that a similar inducible pathway of host defense is prevalent at mucosal surfaces of mammals.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arditi M., Zhou J., Dorio R., Rong G. W., Goyert S. M., Kim K. S. Endotoxin-mediated endothelial cell injury and activation: role of soluble CD14. Infect Immun. 1993 Aug;61(8):3149–3156. doi: 10.1128/iai.61.8.3149-3156.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H. G., Hultmark D. Cell-free immunity in insects. Annu Rev Microbiol. 1987;41:103–126. doi: 10.1146/annurev.mi.41.100187.000535. [DOI] [PubMed] [Google Scholar]
- Boman H. G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. doi: 10.1146/annurev.iy.13.040195.000425. [DOI] [PubMed] [Google Scholar]
- Diamond G., Bevins C. L. Endotoxin upregulates expression of an antimicrobial peptide gene in mammalian airway epithelial cells. Chest. 1994 Mar;105(3 Suppl):51S–52S. doi: 10.1378/chest.105.3_supplement.51s. [DOI] [PubMed] [Google Scholar]
- Diamond G., Jones D. E., Bevins C. L. Airway epithelial cells are the site of expression of a mammalian antimicrobial peptide gene. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4596–4600. doi: 10.1073/pnas.90.10.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G., Zasloff M., Eck H., Brasseur M., Maloy W. L., Bevins C. L. Tracheal antimicrobial peptide, a cysteine-rich peptide from mammalian tracheal mucosa: peptide isolation and cloning of a cDNA. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3952–3956. doi: 10.1073/pnas.88.9.3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziarski R. Peptidoglycan and lipopolysaccharide bind to the same binding site on lymphocytes. J Biol Chem. 1991 Mar 15;266(8):4719–4725. [PubMed] [Google Scholar]
- Fearns C., Kravchenko V. V., Ulevitch R. J., Loskutoff D. J. Murine CD14 gene expression in vivo: extramyeloid synthesis and regulation by lipopolysaccharide. J Exp Med. 1995 Mar 1;181(3):857–866. doi: 10.1084/jem.181.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrero E., Hsieh C. L., Francke U., Goyert S. M. CD14 is a member of the family of leucine-rich proteins and is encoded by a gene syntenic with multiple receptor genes. J Immunol. 1990 Jul 1;145(1):331–336. [PubMed] [Google Scholar]
- Goldblum S. E., Brann T. W., Ding X., Pugin J., Tobias P. S. Lipopolysaccharide (LPS)-binding protein and soluble CD14 function as accessory molecules for LPS-induced changes in endothelial barrier function, in vitro. J Clin Invest. 1994 Feb;93(2):692–702. doi: 10.1172/JCI117022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann J. A., Hetru C., Reichhart J. M. The humoral antibacterial response of Drosophila. FEBS Lett. 1993 Jun 28;325(1-2):63–66. doi: 10.1016/0014-5793(93)81414-u. [DOI] [PubMed] [Google Scholar]
- Hultmark D. Insect immunology. Ancient relationships. Nature. 1994 Jan 13;367(6459):116–117. doi: 10.1038/367116a0. [DOI] [PubMed] [Google Scholar]
- Ip Y. T., Reach M., Engstrom Y., Kadalayil L., Cai H., González-Crespo S., Tatei K., Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993 Nov 19;75(4):753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr The immune system evolved to discriminate infectious nonself from noninfectious self. Immunol Today. 1992 Jan;13(1):11–16. doi: 10.1016/0167-5699(92)90198-G. [DOI] [PubMed] [Google Scholar]
- Jones D. E., Bevins C. L. Paneth cells of the human small intestine express an antimicrobial peptide gene. J Biol Chem. 1992 Nov 15;267(32):23216–23225. [PubMed] [Google Scholar]
- Kopp E. B., Ghosh S. NF-kappa B and rel proteins in innate immunity. Adv Immunol. 1995;58:1–27. doi: 10.1016/s0065-2776(08)60618-5. [DOI] [PubMed] [Google Scholar]
- Legrand E. K. An evolutionary perspective of endotoxin: a signal for a well-adapted defense system. Med Hypotheses. 1990 Sep;33(1):49–56. doi: 10.1016/0306-9877(90)90084-r. [DOI] [PubMed] [Google Scholar]
- Lehrer R. I., Lichtenstein A. K., Ganz T. Defensins: antimicrobial and cytotoxic peptides of mammalian cells. Annu Rev Immunol. 1993;11:105–128. doi: 10.1146/annurev.iy.11.040193.000541. [DOI] [PubMed] [Google Scholar]
- Mallow E. B., Harris A., Salzman N., Russell J. P., DeBerardinis R. J., Ruchelli E., Bevins C. L. Human enteric defensins. Gene structure and developmental expression. J Biol Chem. 1996 Feb 23;271(8):4038–4045. doi: 10.1074/jbc.271.8.4038. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Lei M. G., Kirikae T., Chen T. Y. Endotoxin receptors on mammalian cells. Immunobiology. 1993 Apr;187(3-5):212–226. doi: 10.1016/S0171-2985(11)80340-2. [DOI] [PubMed] [Google Scholar]
- Niederman M. S. Gram-negative colonization of the respiratory tract: pathogenesis and clinical consequences. Semin Respir Infect. 1990 Sep;5(3):173–184. [PubMed] [Google Scholar]
- Pugin J., Schürer-Maly C. C., Leturcq D., Moriarty A., Ulevitch R. J., Tobias P. S. Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2744–2748. doi: 10.1073/pnas.90.7.2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raetz C. R. Biochemistry of endotoxins. Annu Rev Biochem. 1990;59:129–170. doi: 10.1146/annurev.bi.59.070190.001021. [DOI] [PubMed] [Google Scholar]
- Read M. A., Cordle S. R., Veach R. A., Carlisle C. D., Hawiger J. Cell-free pool of CD14 mediates activation of transcription factor NF-kappa B by lipopolysaccharide in human endothelial cells. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):9887–9891. doi: 10.1073/pnas.90.21.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonwetter B. S., Stolzenberg E. D., Zasloff M. A. Epithelial antibiotics induced at sites of inflammation. Science. 1995 Mar 17;267(5204):1645–1648. doi: 10.1126/science.7886453. [DOI] [PubMed] [Google Scholar]
- Selsted M. E., Tang Y. Q., Morris W. L., McGuire P. A., Novotny M. J., Smith W., Henschen A. H., Cullor J. S. Purification, primary structures, and antibacterial activities of beta-defensins, a new family of antimicrobial peptides from bovine neutrophils. J Biol Chem. 1993 Mar 25;268(9):6641–6648. [PubMed] [Google Scholar]
- Simmons D. L., Tan S., Tenen D. G., Nicholson-Weller A., Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989 Jan;73(1):284–289. [PubMed] [Google Scholar]
- Tso J. Y., Sun X. H., Kao T. H., Reece K. S., Wu R. Isolation and characterization of rat and human glyceraldehyde-3-phosphate dehydrogenase cDNAs: genomic complexity and molecular evolution of the gene. Nucleic Acids Res. 1985 Apr 11;13(7):2485–2502. doi: 10.1093/nar/13.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R., Nolan E., Turner C. Expression of tracheal differentiated functions in serum-free hormone-supplemented medium. J Cell Physiol. 1985 Nov;125(2):167–181. doi: 10.1002/jcp.1041250202. [DOI] [PubMed] [Google Scholar]
- Wu R., Yankaskas J., Cheng E., Knowles M. R., Boucher R. Growth and differentiation of human nasal epithelial cells in culture. Serum-free, hormone-supplemented medium and proteoglycan synthesis. Am Rev Respir Dis. 1985 Aug;132(2):311–320. doi: 10.1164/arrd.1985.132.2.311. [DOI] [PubMed] [Google Scholar]
- Yang Z., Carter C. D., Miller M. S., Bochsler P. N. CD14 and tissue factor expression by bacterial lipopolysaccharide-stimulated bovine alveolar macrophages in vitro. Infect Immun. 1995 Jan;63(1):51–56. doi: 10.1128/iai.63.1.51-56.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Ulevitch R. J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993 Mar;14(3):121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]