Abstract
Invasive species are a major threat to biodiversity when dominant within their newly established habitat. The globally distributed Argentine ant Linepithema humile has been reported to break the trade-off between interference and exploitative competition, achieve high population densities, and overpower nests of many endemic ant species. We have used the sensitivity of the Argentine ant to the synthetic trail pheromone (Z)-9-hexadecanal to investigate species interactions for the first time. We predicted that disrupting Argentine ant trail following behaviour would reduce their competitive ability and create an opportunity for three other resident species to increase their foraging success. Argentine ant success in the control was reduced with increasing pheromone concentration, as predicted, but interactions varied among competing resident species. These behavioural variations provide an explanation for observed differences in foraging success of the competing resident species and how much each of these individual competitors can increase their foraging if the competitive ability of the dominant invader is decreased. The mechanism for the observed increase in resource acquisition of resident species appears to be a decrease in aggressive behaviour displayed by the Argentine ant, which may create an opportunity for other resident species to forage more successfully. Our demonstration of species interactions with trail pheromone disruption is the first known case of reduced dominance under a pheromone treatment in ants.
Introduction
Invasive species are considered to be a major threat to biodiversity and can drive native species to extinction through competitive exclusion [1] or niche displacement [2], [3]. Invasive species can also have adverse effects on ecosystem functionality [4], and cause economic losses through decreased agricultural productivity and costs of control measures [4]–[6].
Several tramp ant species are among the 100 of the world's worst invasive species [7]. Invasive tramp ants can drastically change the structure and community dynamic of an ecosystem [8], [9]. They have also been reported many times to replace native ant species [10]–[12] without providing the same ecosystem services for plants and other community members [13], [14].
In ants, a well-defined ecological classification of three competition hierarchy levels has been established [15], [16], distinguishing among dominant, subdominant and subordinate species. Dominant species often place severe limitations on the foraging success of subordinate species at local food resources [17]–[23]. Behavioural traits [24] play an important role for the success of invasive species [25], [26] and may also be a factor for biotic resistance as the reactions of local community species influence the likelihood of native species to engage and interfere with the invader [27], [28].
Originally native to South America, the Argentine ant Linepithema humile has successfully become a global invader [9], [29]–[31]. It quickly monopolizes all resources in the local habitat [9], [32] and causes large reductions in abundances of native ants. The mechanisms to explain the Argentine ants' ability to displace native ant has been attributed to their capability of breaking of the trade-off usually involved between interference and exploitative capacities [1], [33], thus attaining ecological dominance [10], [34]–[36]. However there have also been reports of biotic resistance of native species against Argentine ants [1], [27], which use physical aggression or chemicals to successfully defend themselves and their territory.
Like many social insects, Argentine ants utilize a trail pheromone-based communication system to mark routes to valuable resources and exploit them efficiently. This trail-pheromones signal was demonstrated to deteriorate within approximately 30 minutes [37]. It has been hypothesized that one of the causes for the Argentine ants' success and their high impact on native species is their effective recruitment system [9]. This recruitment allows them to quickly divert workers from almost-depleted to newly discovered and presumably more worthwhile resources, thus gathering more food and enabling/supporting greater population abundance and thereby exploitatively outcompeting other resident species.
Previous laboratory and field tests have demonstrated that the synthetic trail pheromone (Z)-9-hexadecenal (Z9-16:Ald) disrupts Argentine ant trail-following behaviour by interfering with track angles to reduce foraging success [38]–[41]. High pheromone concentrations prevent Argentine ants from establishing stable trails and recruiting to resources in the area [39], although existing heavy traffic requires more pheromone for disruption [40]. Study designs to measure the impacts of invaders usually focus on before/after and control/impact comparisons, supported by experimental manipulations to maintain or decrease invasive populations and observe the effects on the local ecosystem and communities [42]–[45]. However, the Argentine ants and their sensitivity to synthetic trail pheromone provide an opportunity for a different approach to measure the impacts of an invasive species by altering their competitive abilities, rather than excluding them or comparing invaded with non-invaded areas.
Here, we attempt to reduce the competitive ability of the dominant Argentine ant using pheromones and assess the impact of these pheromones on the foraging success of competing community species. Firstly, we hypothesized that with increasing pheromone concentrations, Argentine ants would experience reduced resource acquisition, thus creating an opportunity for other resident species to increase their foraging success. Secondly, we hypothesized that increasing concentrations of synthetic pheromone would affect the foraging of co-occurring species differently, depending on the behavioural reactions between Argentine ants and the co-occurring species. Finally, as a mechanism for the increased foraging success of resident species in the presence of synthetic trail pheromone, we hypothesized that increasing pheromone concentrations could influence Argentine ant worker interactions with other species, with a variation in behavioural response between species.
Methods
Location
Experiments were conducted in an urban district of Lower Hutt, on the southern North Island of New Zealand, where Argentine ants have been well established since 2001. Three sites in the invasion zone (−41.222, 174.872; −41.221, 174.872 and −41.219, 174.879) were selected for competition experiments. No specific permissions were required to access the first two sampling sites, as they were on public ground. The last site was the garden of a private property, the owners gave oral permission to use their garden. None of the experiments involved any endangered or protected species. A preliminary survey had detected the highest abundance of individual workers for Argentine ants and the presence of other resident species at these locations. The three ant species encountered to approach food items together with Argentine ants were the native Monomorium antarcticum, the introduced Ochetellus glaber and the introduced Technomyrmex jocosus, with relative species composition varying between the three sites. None of the species are endangered or protected. Experiments were conducted on dry and sunny days between November 2011 and April 2012.
Resource Competition Experiments
To assess the foraging success of L. humile under the influence of the pheromone treatment, 50 small marzipan pieces (average weight 0.024 g±0.012 g) were scattered on paper bait cards with 10 cm radius and placed haphazardly in an area of 10×10 m at the three sites. Marzipan (Odense Marzipan, Andre Prost, Inc.) was selected as the food because its compounds (∼30% sugar, glycose syrup, almonds/almond oil) were found to be highly attractive for Argentine and a range of other ants in previous trials. Only one bait card was made available for foraging per plot at any point during the experiment. A fresh bait card was placed at a new location 5 minutes after the last experiment had concluded and was placed at least 3 meters away from the previous location to prevent habituation. Bait cards were treated with four droplets (25 µl each) of ethanol solutions of synthetic trail pheromone at concentrations of 100 µg/µl, 1 µg/µl, 0.01 µg/µl and a control with pure solvent. Six replicates were carried out in random order for each of the three treatments and the control on each of the three sites. One droplet was made on each side of the bait card to provide an equal distribution of the pheromone. Bait cards were observed until all 50 food items had been picked up and carried over the edge of the bait card by ants (average time 85.9 min±2.3 min). For each individual food item that was being carried away by an ant worker from the bait card, the species of the worker carrying it was noted. Workers of all ant species were observed to be physically able to carry food items away. Due to differences in species abundance only one other resident species was primarily competing with Argentine ants at each location.
Logistic regression was used to compare the number of food items taken by each species with pheromone concentrations. The dependent response variable was whether an individual food item had been taken or not by Argentine ants at the end of each trial using pheromone concentration and competing ant species as explanatory variables. A binary logistic regression fits the data to give predicted probabilities (and odds) of whether a food item is taken, given particular values of the explanatory variables. For details see [46].
Behavioural Scoring
To assess the difference in how encountered species would be treated by the Argentine ant and assess differences in their reaction in return, Argentine ant and workers of competing species were scored individually for both participating ants during encounters. At times between 1 to 5 minutes, a worker ant of M. antarcticum, O. glaber or T. jocosus entering the bait card was randomly chosen and its behaviour during its next interaction scored, as well as the behaviour displayed by the Argentine ant worker it was interacting with. Behaviour scores were taken: 0 = no interest or aggression; 1 = interest shown via antennation; 2 = ant retreats quickly; 3 = lunging, biting or leg-pulling, rising of gaster and exuding venom; 4 = prolonged (>5 s) incidences of aggression, individuals locked together and fighting [10]. Ants were considered “meeting” each other when they either touched each other physically, or when they were in close proximity (0.5 cm) to each other and both parties could be observed to display behaviours which could reasonably assumed to be addressed towards the other worker ant (for example stopping, antennating towards the other ant, turning around and quickly retreating). For half of the bait cards for each treatment and location, interactions of at least 10 individual ant workers were scored and no behaviour scores were taken after all food items had been removed by ants.
To investigate if Argentine ant behaviour in interactions would be influenced by the synthetic pheromone, the average of the behavioural scores was analysed with a Kruskal-Wallis test. Logistic regression was then used to test how interactions differ depending on species. The dependent response variable was whether an interaction was non-aggressive (scores 0, 1 and 2) versus aggressive behavioural (scores 3 and 4) with pheromone concentration and competing ant species as explanatory variables. Reactions of Argentine ant workers towards the competing species (M. antarcticum, O. glaber and T. jocosus) were tested as well as scores of the four species towards Argentine ant workers respectively. All data analysis was conducted using PASW Statistics v. 18.0. The data package associated with this manuscript has been made available at the Dryad data depository (Dryad accession number: doi: 10.5061/dryad.m64hs).
Results
Resource Competition Experiments
Our first hypothesis was that Argentine ants would experience reduced resource acquisition with increasing pheromone concentration, which disrupts their foraging. Argentine ant workers were observed to typically arrive first at the bait cards in all replicates, concurrent with the high exploitative abilities of these ants reported in previous publications [1], [10], [33]. The first scouts usually arrived within 2 to 18 minutes of the trial and substantially increased in number after that over the next 15 minutes. Although we did not quantify trail following behaviour or trail integrity, our observations indicate that Argentine ants always formed distinctive trails on and around the bait cards in controls, which were less distinctive in pheromone treatments of 1 µg/µl or higher. Furthermore in our observations during the experiment, Argentine ant workers appeared to take a slower approach on the bait card when pheromone was applied, often standing at the edge of the card for minutes and cleaning their antennae or walking in small circles before discovering food items. Workers of the three other species arrived much later, with the first worker ant picking up a food item between 14 to 31 minutes after the beginning of the experiment. By that time Argentine ants were nearly always already present in superior numbers and had started to retrieve food items, but this was reduced with increasing pheromone concentration.
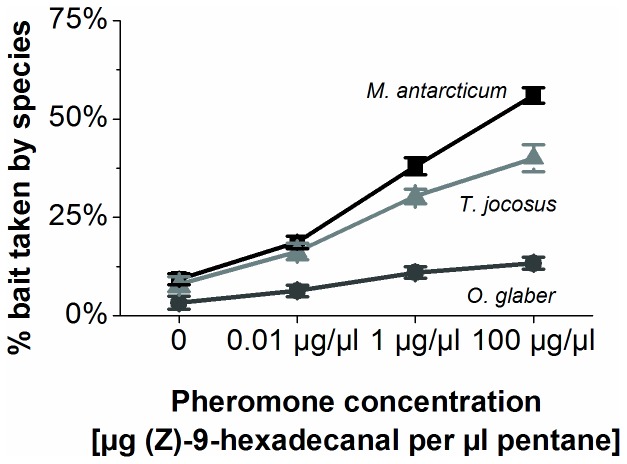
Logistic regression indicated that the trail pheromone had a substantial impact on the competition between Argentine ants and the three tested species M. antarcticum, O. glaber and T. jocosus. The synthetic trail pheromone affected the Argentine ants' ability to dominate the bait cards and retrieve food items, but the effect was considerably stronger at higher pheromone concentrations. The number of food items retrieved by each of the three species was significantly higher with each increase in pheromone concentration (control vs. any of the pheromone treatments P<0.001). There was also a significant difference for the number of food items taken between species for each concentration level (Figure 1, Table 1). The native ant M. antarcticum appeared to benefit the most from the pheromone treatment and increased the number of collected food items from an average of 4.6 in the controls to 28 at a treatment of 100 µg/µl (a 608% increase). The exotic ants T. jocosus increased their foraging success from an average of 4 to 20 (a 500% increase) and O. glaber increased from an average of 1.6 retrieved food items to 6.7 (a 416% increase).
Figure 1. Food items taken by ants.
Percentage of average number of food items taken in trials (n = 6 per treatment and site) by M. antarcticum, O. glaber and T. jocosus from Argentine ants depending on the treatment with different concentrations of synthetic trail pheromone (Z)-9-hexadecanal (measured in µg pheromone per µl solvent). Error bars represent standard error of average food items taken.
Table 1. Statistics for number of food items taken.
| ß | S.E. | Wald | df | P | Exp(ß) | |
| O. glaber vs. M. antarcticum | 0.39 | 0.10 | 15.77 | 1 | 0.001 ** | 1.47 |
| T. jocosus vs. M. antarcticum | 1.68 | 0.13 | 178.27 | 1 | 0.001 ** | 5.35 |
| 0.01 µg/µl vs. Control | −0.79 | 0.17 | 22.91 | 1 | 0.001 ** | 0.45 |
| 1 µg/µl vs. Control | −1.65 | 0.15 | 113.94 | 1 | 0.001 ** | 0.19 |
| 100 µg/µl vs. Control | −2.16 | 0.15 | 201.34 | 1 | 0.001 ** | 0.12 |
| Constant | 2.11 | 0.14 | 226.75 | 1 | 0.001 ** | 8.23 |
* P<0.05;
** P<0.01.
Logistic regression on the number of food items taken in trials by Argentine ants versus M. antarcticum, O. glaber and T. jocosus. First two rows show the difference between food items taken by Argentine ants from a species, with M. antarcticum as reference. The next three rows show the difference of food items taken by Argentine ants depending on pheromone concentration with the control treatment as a reference. ß is the log-odds units, S.E. the standard errors associated with the coefficients, Wald the Wald chi-square value, df the degrees of freedom, P the statistical significance and Exp(ß) the odds ratio.
Behavioural Scoring
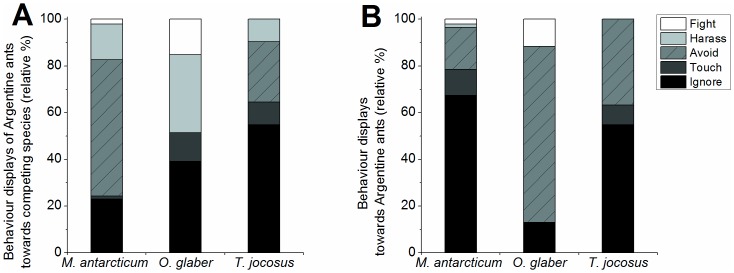
We had hypothesized that behavioural interactions between Argentine ants and the co-occurring species would be different for each species, affecting foraging of co-occurring species differently. The average behavioural score for Argentine ant behaviour towards their interaction partner was significantly different between the three species (Kruskal-Wallis P<0.01), as was the reaction of M. antarcticum, O. glaber and T. jocosus in return (Kruskal-Wallis P<0.01) (Figure 2). Workers of the native ant M. antarcticum were often avoided (aggression score 2) by Argentine ants, which retreated from M. antarcticum workers even when outnumbering it (Figure 2A). In return, M. antarcticum workers were often observed to ignore Argentine ants (aggression score 0), often walking on the bait card without any sign of hesitation, picking up a food item and leaving again even when harassed (biting, leg pulling) by Argentine ant workers on rare occasions (Figure 2B).
Figure 2. Relative percentage of behavioural scores during interactions.
(A) Argentine ants towards M. antarcticum, O. glaber and T. jocosus and (B) M. antarcticum, O. glaber and T. jocosus towards Argentine ants. Numbers surrounding each graph (0–4) represent the displayed behaviours during interactions, which was scored as 0 = no interest or aggression; 1 = interest shown via antennation; 2 = ant retreats quickly; 3 = lunging, biting or leg-pulling, rising of gaster and exuding venom; 4 = prolonged (>5 s) incidences of aggression, individuals locked together and fighting. Small numbers and dashed lines mark intervals of 0, 25, 50, 75 and 100 for the percentage of interactions during which these behaviours were being displayed.
Workers of the smaller exotic ant O. glaber were treated with considerably higher aggression by Argentine ants when encountered. The Argentine ants actively engaged and chased the smaller ants off the bait card. No occurrence of avoidance behaviour (aggression score 2) by Argentine ant workers towards O. glaber workers was ever observed (Table 2). In return the O. Glaber workers never displayed any touching (aggression score 1) or harassment (aggression score 3) (Table 3) and appeared to show great hesitation to go near the food items when they appeared to perceive Argentine ant workers in the vicinity, often stopping and turning immediately when coming as close as 0.5 cm to an Argentine ant worker.
Table 2. Total number of behavioural observations.
| Species | Treatment | Argentine ants | Competitors | ||
| Non Aggressive | Aggressive | Non Aggressive | Aggressive | ||
| M. antarcticum | 0 | 20 | 10 | 26 | 4 |
| 0.01 µg/µl | 28 | 5 | 33 | 0 | |
| 1 µg/µl | 36 | 5 | 40 | 1 | |
| 100 µg/µl | 35 | 5 | 40 | 0 | |
| O. glaber | 0 | 11 | 18 | 23 | 6 |
| 0.01 µg/µl | 14 | 22 | 32 | 4 | |
| 1 µg/µl | 23 | 15 | 34 | 4 | |
| 100 µg/µl | 23 | 12 | 33 | 2 | |
| T. jocosus | 0 | 25 | 6 | 31 | - |
| 0.01 µg/µl | 35 | 3 | 38 | - | |
| 1 µg/µl | 35 | 3 | 38 | - | |
| 100 µg/µl | 35 | 2 | 37 | - | |
Total number of observations of non-aggressive (behaviour scores 0, 1 and 2) and aggressive (behaviour scores 3 and 4) behaviour displayed by Argentine ants towards each competing species and observations for this species in return, as a function of pheromone concentration or no treatment.
Table 3. Statistics for aggression by Argentine ants.
| ß | S.E. | Wald | df | P | Exp(ß) | |
| O. glaber vs. M. antarcticum | 1.558 | 0.287 | 29.485 | 1 | 0.001 ** | 4.751 |
| T. jocosus vs. M. antarcticum | −0.718 | 0.363 | 3.918 | 1 | 0.048 * | 0.488 |
| 0.01 µg/µl vs. Control | −.0566 | 0.340 | 2.771 | 1 | 0.096 | 0.568 |
| 1 µg/µl vs. Control | −1.108 | 0.352 | 9.921 | 1 | 0.002 ** | 0.330 |
| 100 µg/µl vs. Control | −1.287 | 0.366 | 12.398 | 1 | 0.001 ** | 0.276 |
| Constant | −0.836 | 0.297 | 7.933 | 1 | 0.005 ** | 0.433 |
* P<0.05;
** P<0.01.
Logistic regression on displayed aggression by Argentine ants towards M. antarcticum, O. glaber and T. jocosus. The first two rows show the difference between aggressions of Argentine ants towards a species, with M. antarcticum as reference. The next three rows show aggression of Argentine ants depending on pheromone concentration with the control treatment as a reference. ß is the log-odds units, S.E. the standard errors associated with the coefficients, Wald the Wald chi-square value, df the degrees of freedom, P the statistical significance and Exp(ß) the odds ratio.
Workers of T. jocosus and the Argentine ant most frequently ignored and avoided each other, and while Argentine ant worker occasionally harassed T. jocosus, no occurrences of prolonged combat (aggression score 4) were observed between these species throughout the experiment. Additionally no instances of aggression from T. jocosus towards Argentine ants (behavioural score 3 or 4) were observed.
The results of the logistic regression (Table 3) analysing differences in non-aggressive versus aggressive behavioural reactions show significant differences between the aggression displayed by Argentine ants towards M. antarcticum and O. glaber (Wald = 29.485; df = 1; P<0.001), with O. glaber having a 4.75 times higher chance of being attacked than M. antarcticum. Between M. antarcticum and T. jocosus, the latter had an average of 0.49 chance of being attacked (Wald = 3.918; df = 1; P = 0.048). There was also a significant difference in the reactions of these species towards Argentine ants. Displayed aggression differed significantly between M. antarcticum and O. glaber towards Argentine ants (Wald = 6.148; df = 1; P = 0.013). The difference in aggression between M. antarcticum and T. jocosus towards Argentine ants could not be tested for with logistic regression, due to the absence of any aggression displayed by T. jocosus (no instances of scores 3 or 4 observed; (Table 2).
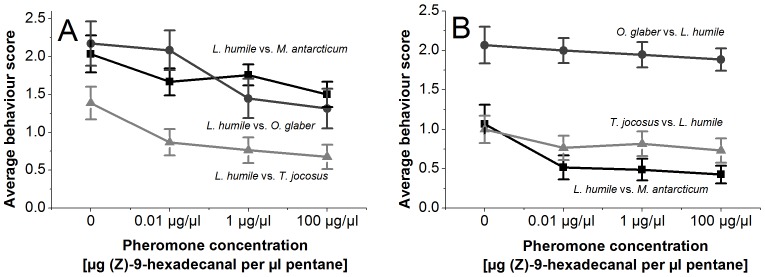
In our third hypothesis, we investigated if increasing pheromone concentrations could influence Argentine ant worker interactions with other species. The treatment with synthetic trail pheromone had a substantial impact on the behaviour displayed by Argentines ants towards M. antarcticum, O. glaber and T. jocosus (Figure 3A), lowering the average behavioural score (Kruskal-Wallis P<0.01). The behaviour displayed by O. glaber, T. jocosus and M. antarcticum appeared to be only slightly influenced by the application of pheromone, although all showed a small reduction (Figure 3B). The results of the logistic regression (Table 3) analysing differences in non-aggressive versus aggressive behavioural reactions reveal a highly significant reduction of displayed aggression by Argentine ants with pheromone concentrations of 1 µg/µl (Wald = 9.921; df = 1; P<0.001) and 100 µg/µl (Wald = 12.398; df = 1; P<0.001) compared to untreated controls. Furthermore, aggressive reactions of M. antarcticum, O. glaber and T. jocosus towards Argentine ants decreased with increasing pheromone treatments (Table 4).
Figure 3. Average aggression scores of ants.
Average aggression of (A) Argentine ants towards M. antarcticum, O. glaber and T. jocosus and (B) M. antarcticum, O. glaber and T. jocosus towards Argentine ants depending on the treatment with different concentrations of synthetic trail pheromone (Z)-9-hexadecanal (measured in µg pheromone per µl solvent). Aggression was scored as 0 = no interest or aggression; 1 = interest shown via antennation; 2 = ant retreats quickly; 3 = lunging, biting or leg-pulling, rising of gaster and exuding venom; 4 = prolonged (>5 s) incidences of aggression, individuals locked together and fighting. Error bars represent Standard Error of average aggression scores.
Table 4. Statistics for aggression by M. antarcticum, O. glaber and T. jocosus towards Argentine ants.
| ß | S.E. | Wald | df | P | Exp(ß) | |
| O. glaber vs. M. antarcticum | 1.33 | 0.54 | 6.148 | 1 | 0.013 * | 3.78 |
| T. jocosus vs. M. antarcticum | −17.85 | 3251.82 | 0.000 | 1 | 0.996 | 0.000 |
| 0.01 µg/µl vs. Control | −1.278 | 0.632 | 4.090 | 1 | 0.043 * | 0.279 |
| 1 µg/µl vs. Control | −1.13 | 0.59 | 3.712 | 1 | 0.054 | 0.32 |
| 100 µg/µl vs. Control | −2.032 | 0.804 | 6.385 | 1 | 0.012 * | 0.131 |
| Constant | −2.388 | 0.520 | 21.062 | 1 | 0.000 ** | 0.092 |
* P<0.05;
** P<0.01.
Logistic regression on displayed aggression by M. antarcticum, O. glaber and T. jocosus towards Argentine ants. The first two rows show the difference between aggressions by a species towards Argentine ants, with M. antarcticum as reference. The next three rows show aggression depending on pheromone concentration with the control treatment as a reference. ß is the log-odds units, S.E. the standard errors associated with the coefficients, Wald the Wald chi-square value, df the degrees of freedom, P the statistical significance and Exp(ß) the odds ratio.
Discussion
Our data support the hypothesis that confusing a dominant invasive species and reducing its competitive ability can reduce its resource acquisition and create an opportunity for other resident species to increase their foraging. While dominating untreated bait cards, Argentine ants arrived sporadically and in a more disorganized manner, when treated with synthetic pheromone. This is consistent with results in previous studies [38]–[41], which had reported that trail following behaviour of Argentine ants could be disrupted with synthetic pheromone. These studies provided a proof of concept for the pheromone disruption in Argentine ants by analysing trail integrity [38] and providing evidence for a negative impact on their foraging [39], [41]. However, it was previously unclear whether this interference with Argentine ants recruitment actually translates into an effect on the resident ant community, which could be used to support native species. In our experimental approach the trail disruption gave co-occurring species an opportunity to locate and remove baits much more successfully than without the pheromone.
Argentine ants reacted differently to each of the three species, showing a variety of patterns in frequency and type of non-aggressive and aggressive behaviours towards their competitors. Similar observations have been made in other ant species, which react differently towards certain competitors [47], [48]. All three tested competing species M. antarcticum, O. glaber and T. jocosus were able to utilize the advantage arising through the previously reported confusion caused by the pheromone application and significantly increase their foraging success. However, the increase in the number of retrieved food items varied among species, with the relative increase of M. antarcticum being the most substantial. Comparatively with increasing pheromone concentration, O. glaber showed less dramatic increase.
The differences in foraging success can be well explained by the differences in behavioural traits [25] and their manifestations in interactions between Argentine ants and the three species. For example, M. antarcticum is frequently avoided by Argentine ants, which make way for approaching M. antarcticum workers even when outnumbering them. Workers of M. antarcticum mostly seemed to ignore Argentine ants and single M. antarcticum workers were frequently observed to walk on the bait card and retrieve a food item without hesitation, apparently uninterested in present Argentine ant workers. These behaviour patterns are well within expected interaction ranges, as M. antarcticum workers are larger than Argentine ant workers and, unless dramatically outnumbered [49], have been shown to annihilate Argentine ant workers in physical interactions by biting them in half. However, M. antarcticum are also slow moving and slower to recruit than Argentine ants, especially at higher temperatures [50], which may explain why Argentine ants are still dominant at food sources. While O. glaber does benefit from the pheromone disruption, their foraging success increases less than the other species, since their workers are frequently harassed or attacked by Argentine ant workers. Workers of O. glaber were frequently observed to retreat when near Argentine ant workers, even when Argentine ants were walking in circles, apparently confused, and not directly interacting with O. glaber individuals. Workers of T. jocosus and Argentine ants display a similar pattern of ignoring and avoidance towards each other, with some harassment being exhibited by Argentine ant workers. The observed behavioural patterns are concurrent with aggression and avoidance patterns appropriate to dominance ranks of these species within the ant community [15], [16], with the Argentine ant taking a dominant role, while M. antarcticum and T. jocosus are subdominant and O. glaber subordinate.
As a mechanism for increased foraging success of the three competing resident species, we observed decreased aggression being exhibited by dominant Argentine ant workers when treated with pheromone, combined with their reduced ability to locate the food items. Our data indicate a significant reduction in aggression displayed by Argentine ant workers with increasing pheromone concentrations. While previous studies have investigated changes of aggression in social insects related to cuticular hydrocarbons [51], [52], queen pheromones [53] and alarm pheromones [54], the impact on aggression due to experimental pheromone changes in a wider area are unexplored at this point. In the presence of synthetic pheromone, Argentine ants seemed to spread out more and no longer followed distinct trails, which could increase the chance of encounters between species. However, Argentine ant workers also more frequently stand around or move in small circles. This behaviour appeared to allow workers of the other species to pass them in close proximity (∼0.5 cm) without provoking any aggressive reaction and thus retrieving food more easily. We propose two non-mutually exclusive hypotheses to explain this reduction in aggressiveness. First the trail disruption caused by higher concentrations of trail pheromone could simply decrease the likelihood of Argentine ants workers being able to perceive the workers of other species, even at very close distance, thus ignoring them much more often. Alternatively high pheromone concentrations might alter the reactions of Argentine ants, making individual workers less aggressive. Ants frequently defend established trails to resources, so it might be possible that, due to the disruption of the trail, the territoriality of Argentine ants is reduced, which therefore decreases their aggressive behaviour within the area of the pheromones' influence.
We have demonstrated that it is possible to significantly reduce the foraging success and displayed aggression of the Argentine ant, a globally dominant invader [9], [29]–[31]. Argentine ants have been reported many times to competitively exclude subdominant and subordinate species due to their ability to quickly locate food and defend it efficiently [1], [10], [33]. Our experiments showed that it is possible to reduce the competitiveness of the Argentine ants by interfering with their ability to find and defend food sources. Species from a lower dominance hierarchy were able to take advantage of this opportunity. This provides evidence that pheromone-based control measures could help to stabilize or even increase local populations of native species that were previously being suppressed. Insecticides have often led to losses of non-target species [55], [56] and have recently shown to even have the potential of giving an advantage to invasive species [57]. Over a longer time, reduced foraging ability of Argentine ants could lead to reduced populations of this invader, potentially even to a point where the invaders' population collapses and they are replaced by other local species. Future experimental studies should focus on whether or not long-term reduction of competitive abilities of a dominant species is possible and results in anticipated changes in the species' community which could be utilized in invasive species management.
Our demonstration of species interactions with trail pheromone disruption is the first known case of reduced dominance from pheromone-based treatment in ants, and this area offers new prospects for behavioural interference in invasive species management.
Acknowledgments
We thank the property owners who kindly allowed us permission to trap and bait ants on their property. We also thank Monica Gruber, Rafael Barbieri and anonymous reviewers for their constructive criticism and valuable comments.
Funding Statement
This research was funded by a Victoria University of Wellington doctoral scholarship. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Holway D (1999) Competetive Mechanisms Underlying the Displacement of native ants by the invasive Argentine ant. Ecology 80: 238–251 Available: 10.1890/0012-9658(1999)080[0238:CMUTDO]2.0.CO;2 [Google Scholar]
- 2. Kenward RE, Holm JL (1993) On the replacement of the red squirrel in Britain: a phytotoxic explanation. Proc Biol Sci 251: 187–194 Available: http://rspb.royalsocietypublishing.org/content/251/1332/187.abstract. Accessed 2014 January 25. [DOI] [PubMed] [Google Scholar]
- 3. Race M (1982) Competitive displacement and predation between introduced and native mud snails. Oecologia 54: 337–347 Available: http://link.springer.com/article/10.1007/BF00380002. Accessed 2013 April 17. [DOI] [PubMed] [Google Scholar]
- 4. Mack RN, Simberloff D, Mark Lonsdale W, Evans H, Clout M, et al. (2000) Biotic Invasions: Causes, Epidemiology, Global Consequences, and Control. Ecol Appl 10: 689–710 Available: http://dx.doi.org/10.1890/1051-0761(2000)010[0689:BICEGC]2.0.CO. Accessed 2013 July 31. [Google Scholar]
- 5. Pimentel D, Lach L, Zuniga R, Morrison D (2000) Enviromental and economic costs associated with nonindigenous species in the united states. Bioscience 50: 53–65. [Google Scholar]
- 6. Pimentel D, Zuniga R, Morrison D (2005) Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ 52: 273–288 Available: http://linkinghub.elsevier.com/retrieve/pii/S0921800904003027. Accessed 2013 July 30. [Google Scholar]
- 7. Alowe S, Browne M, Boudjelas S (2000) 100 of the world's worst invasive alien species. Aliens 12: 2–12. [Google Scholar]
- 8. O'Dowd DJ, Green PT, Lake PS (2003) Invasional “meltdown” on an oceanic island. Ecol Lett 6: 812–817 Available: http://doi.wiley.com/10.1046/j.1461-0248.2003.00512.x. Accessed 2012 July 19. [Google Scholar]
- 9. Holway D, Lach L, Suarez AV, Tsutsui ND, Case TJ, et al. (2002) the Causes and Consequences of Ant Invasions. Annu Rev Ecol Syst 33: 181–233 Available: http://arjournals.annualreviews.org/doi/abs/10.1146/annurev.ecolsys.33.010802.150444. Accessed 2011 June 14. [Google Scholar]
- 10. Rowles AD, O'Dowd DJ (2006) Interference competition by Argentine ants displaces native ants: implications for biotic resistance to invasion. Biol Invasions 9: 73–85 Available: http://springerlink.metapress.com/content/x8428536gk474831/fulltext.pdf. Accessed 2014 January 21. [Google Scholar]
- 11. Sanders NJ, Gotelli NJ, Heller NE, Gordon DM (2003) Community disassembly by an invasive species. Proc Natl Acad Sci U S A 100: 2474–2477 Available: http://www.pnas.org/cgi/reprint/100/5/2474. Accessed 2014 February 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barton KE, Gordon DM, Sanders NJ (2001) Long-term dynamics of the distribution of the invasive Argentine ant, Linepithema humile, and native ant taxa in northern California. Oecologia 127: 123–130 Available: http://web.utk.edu/~nsanders/Pubs/Oecologia2001.pdf or http://seedlingscience.org/Sanders_etal_2001.pdf. Accessed 2014 February 5. [DOI] [PubMed] [Google Scholar]
- 13. Blancafort X, Gómez C (2005) Consequences of the Argentine ant, Linepithema humile (Mayr), invasion on pollination of Euphorbia characias (L.) (Euphorbiaceae). Acta Oecologica 28: 49–55 Available: http://www.udg.edu/portals/92/Bio Animal/pdf/sdarticle.pdf. Accessed 2014 February 5. [Google Scholar]
- 14. Gómez C, Oliveras J (2003) Can the Argentine ant (Linepithema humile Mayr) replace native ants in myrmecochory? Acta Oecologica 24: 47–53 Available: http://www.udg.edu/portals/92/Bio Animal/pdf/Acta oecologica 2003 oliveras Gomez.pdf. Accessed 2013 August 7. [Google Scholar]
- 15. Vepsäläinen K, Pisarski B (1982) Assembly of island ant communities. Ann Zool Fennici 19: 327–335 Available: http://www.sekj.org/PDF/anzf19/anz19-327-335.pdf. Accessed 2013 April 17. [Google Scholar]
- 16. Savolainen R, Vepsäläinen K (1989) Niche differentiation of ant species within territories of the wood ant Formica polyctena. Oikos 56: 3–16. [Google Scholar]
- 17. Fellers JH (1987) Interference and exploitation in a guild of woodland ants. Ecology 68: 1466–1478. [Google Scholar]
- 18. Savolainen R (1991) Interference by wood ant influences size selection and retrieval rate of prey by Formica fusca. Behav Ecol Sociobiol 28: 1–7. [Google Scholar]
- 19. Andersen AN (1992) Regulation of “momentary” diversity by dominant species in exceptionally rich ant communities of the Australian seasonal tropics. Am Nat 140: 401–420. [DOI] [PubMed] [Google Scholar]
- 20. Andersen AN, Patel AD (1994) Meat ants as dominant members of Australian ant communities: an experimental test of their influence on the foraging success and forager abundance of other species. Oecologia 98: 15–24 Available: http://link.springer.com/article/10.1007/BF00326085. Accessed 2013 September 2. [DOI] [PubMed] [Google Scholar]
- 21. Morrison LW (1996) Community organization in a recently assembled fauna: the case of Polynesian ants. Oecologia 107: 243–256 Available: 1.5 MB http://www.springerlink.com/content/l2k42v311g8g0451/fulltext.pdf. Accessed 2014 February 5. [DOI] [PubMed] [Google Scholar]
- 22. Cerdá X, Retana J, Cros S (1998) Prey size reverses the outcome of interference interactions of scavenger ants. Oikos 82: 99–110. [Google Scholar]
- 23. GIBB H (2005) The effect of a dominant ant, Iridomyrmex purpureus, on resource use by ant assemblages depends on microhabitat and resource type. Austral Ecol 30: 856–867 Available: http://doi.wiley.com/10.1111/j.1442-9993.2005.01528.x. Accessed 2014 February 5. [Google Scholar]
- 24. Sih A, Bell A, Johnson JC (2004) Behavioral syndromes: an ecological and evolutionary overview. Trends Ecol Evol 19: 372–378 Available: http://www.ncbi.nlm.nih.gov/pubmed/16701288. Accessed 2013 July 31. [DOI] [PubMed] [Google Scholar]
- 25. Sih A, Cote J, Evans M, Fogarty S, Pruitt J (2012) Ecological implications of behavioural syndromes. Ecol Lett 15: 278–289 Available: http://www.ncbi.nlm.nih.gov/pubmed/22239107. Accessed 2012 July 12. [DOI] [PubMed] [Google Scholar]
- 26. Pintor L (2009) Behavioral correlations provide a mechanism for explaining high invader densities and increased impacts on native prey. Ecology 90: 581–587 Available: http://www.esajournals.org/doi/abs/10.1890/08-0552.1. Accessed 2012 August 11. [DOI] [PubMed] [Google Scholar]
- 27. Sorrells TR, Kuritzky LY, Kauhanen PG, Fitzgerald K, Sturgis SJ, et al. (2011) Chemical defense by the native winter ant (Prenolepis imparis) against the invasive Argentine ant (Linepithema humile). PLoS One 6: e18717 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3079705&tool=pmcentrez&rendertype=abstract. Accessed 2013 August 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Buczkowski G, Bennett GW (2007) Aggressive interactions between the introduced Argentine ant, Linepithema humile and the native odorous house ant, Tapinoma sessile. Biol Invasions 10: 1001–1011 Available: http://www.entm.purdue.edu/ants/pubs/16.pdf. Accessed 2014 February 5. [Google Scholar]
- 29. Roura-Pascual N, Suarez AV, McNyset K, Gómez C, Pons P, et al. (2006) Niche differentiation and fine-scale projections for Argentine ants based on remotely sensed data. Ecol Appl 16: 1832–1841 Available: http://www.esajournals.org/doi/abs/10.1890/1051-0761%282006%29016%5B1832%3ANDAFPF%5D2.0.CO%3B2. Accessed 2014 February 5. [DOI] [PubMed] [Google Scholar]
- 30. Suarez AV, Holway DA, Case TJ (2001) Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc Natl Acad Sci U S A 98: 1095–1100 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=14714&too=pmcentrez&rendertypel=abstract. Accessed 2014 January 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wild AL (2004) Taxonomy and Distribution of the Argentine Ant, Linepithema humile (Hymenoptera: Formicidae). Ann Entomol Soc Am 97: 1204–1215 Available: 1 MB http://antbase.org/ants/publications/20351/20351.pdf or http://www.myrmecos.net/wild/Taxonomy and Distribution of the Argentine Ant.pdf. Accessed 2013 August 7. [Google Scholar]
- 32. Harris RJ, Ward D, Sutherland MA (2002) A survey of the current distribution of agentine ants, Linepithema humile, in native habitats in New Zealand, and assessment of future risk of establishment. [Google Scholar]
- 33. Human KG, Gordon DM (1996) Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia 105: 405–412 Available: http://www.springerlink.com/index/10.1007/BF00328744. Accessed 2014 February 5. [DOI] [PubMed] [Google Scholar]
- 34. Tsutsui ND, Suarez AV, Holway DA, Case TJ (2000) Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci U S A 97: 5948–5953 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=18539&tool=pmcentrez&rendertype=abstract. Accessed 2014 January 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsutsui ND, Suarez AV (2003) The Colony Structure and Population Biology of Invasive Ants. Conserv Biol 17: 48–58 Available: http://doi.wiley.com/10.1046/j.1523-1739.2003.02018.x. Accessed 2014 January 10. [Google Scholar]
- 36. Rowles AD, O'Dowd DJ (2009) Impacts of the invasive Argentine ant on native ants and other invertebrates in coastal scrub in south-eastern Australia. Austral Ecol 34: 239–248. [Google Scholar]
- 37. Deneubourg JL, Aron S, Goss S, Pasteels JM (1990) The self-organizing exploratory pattern of the Argentine ant. J Insect Behav 3: 159–168 Available: http://www.ulb.ac.be/sciences/use/publications/JLD/76.pdf. Accessed 2012 November 1. [Google Scholar]
- 38. Suckling DM, Peck RW, Manning LM, Stringer LD, Cappadonna J, et al. (2008) Pheromone disruption of Argentine ant trail integrity. J Chem Ecol 34: 1602–1609 Available: Electronic supplementary material The online version of this article (doi:10.1007/s10886-008-9566-4) contains supplementary material, which is available to authorized users. Accessed 2014 February 5 [DOI] [PubMed] [Google Scholar]
- 39. Suckling DM, Peck RW, Stringer LD, Snook K, Banko PC (2010) Trail pheromone disruption of Argentine ant trail formation and foraging. J Chem Ecol 36: 122–128 Available: http://www.springerlink.com/content/2575083q84g86w3m/fulltext.pdf. Accessed 2014 February 5. [DOI] [PubMed] [Google Scholar]
- 40. Suckling DM, Stringer LD, Corn JE (2011) Argentine ant trail pheromone disruption is mediated by trail concentration. J Chem Ecol 37: 1143–1149 Available: http://www.ncbi.nlm.nih.gov/pubmed/21964852. Accessed 2013 August 7. [DOI] [PubMed] [Google Scholar]
- 41. Nishisue K, Sunamura E, Tanaka Y, Sakamoto H, Suzuki S, et al. (2010) Long-term field trial to control the invasive Argentine Ant (Hymenoptera: Formicidae) with synthetic trail pheromone. J Econ Entomol 103: 1784–1789 Available: http://www.bioone.org/doi/abs/10.1603/EC10008. Accessed 2011 September 8. [DOI] [PubMed] [Google Scholar]
- 42. Parker IM, Simberloff D, Lonsdale WM, Goodwell K, Wonham M, et al. (1999) Impact: toward a framework for understanding the ecological effects of invaders. Biol Invasions 1: 3–19 Available: http://www.springerlink.com/index/l4l50605m41l2782.pdf. Accessed 2013 August 7. [Google Scholar]
- 43. Zavaleta ES, Hobbs RJ, Mooney H (2001) Viewing invasive species removal in a whole-ecosystem context. Trends Ecol Evol 16: 454–459 Available: http://linkinghub.elsevier.com/retrieve/pii/S0169534701021942. Accessed 2014 February 5. [Google Scholar]
- 44. Hejda M, Pyšek P, Jarošík V (2009) Impact of invasive plants on the species richness, diversity and composition of invaded communities. J Ecol 97: 393–403 Available: http://doi.wiley.com/10.1111/j.1365-2745.2009.01480.x. Accessed 2012 October 27. [Google Scholar]
- 45. McNatty A, Abbott KL, Lester PJ (2009) Invasive ants compete with and modify the trophic ecology of hermit crabs on tropical islands. Oecologia 160: 187–194 Available: http://www.victoria.ac.nz/staff/phil_lester/PDFFiles/McNatty et al 2009 YCA & hermit crabs.pdf or http://www.springerlink.com/content/x873136j122g8711/fulltext.pdf. Accessed 2014 January 10. [DOI] [PubMed] [Google Scholar]
- 46. Stringer LD, Haywood J, Lester PJ (2007) The influence of temperature and fine-scale resource distribution on resource sharing and domination in an ant community. Ecol Entomol 32: 732–740 Available: http://doi.wiley.com/10.1111/j.1365-2311.2007.00924.x. Accessed 2013 October 10. [Google Scholar]
- 47. Wilson E (1975) Enemy specification in the alarm-recruitment system of an ant. Science (80-) 190 Available: http://www.sciencemag.org/content/190/4216/798.short. Accessed 2013 September 2. [DOI] [PubMed] [Google Scholar]
- 48. Holldobler B (1983) Territorial behavior in the green tree ant (Oecophylla smaragdina). Biotropica 15: 241–250 Available: http://www.jstor.org/stable/10.2307/2387648. Accessed 2012 May 6. [Google Scholar]
- 49. Sagata K, Lester PJ (2009) Behavioural plasticity associated with propagule size, resources, and the invasion success of the Argentine ant Linepithema humile. J Appl Ecol 46: 19–27 Available: http://doi.wiley.com/10.1111/j.1365-2664.2008.01523.x. Accessed 2014 January 10. [Google Scholar]
- 50. McGrannachan CM, Lester PJ (2013) Temperature and starvation effects on food exploitation by Argentine ants and native ants in New Zealand. J Appl Entomol 137: 550–559 Available: http://doi.wiley.com/10.1111/jen.12032. Accessed 2013 August 8. [Google Scholar]
- 51. Smith A, Hölldober B, Liebig J (2009) Cuticular hydrocarbons reliably identify cheaters and allow enforcement of altruism in a social insect. Curr Biol 19: 78–81 Available: http://www.ncbi.nlm.nih.gov/pubmed/19135369. Accessed 2013 August 12. [DOI] [PubMed] [Google Scholar]
- 52. Ruther J, Sieben S, Schricker B (2002) Nestmate recognition in social wasps: manipulation of hydrocarbon profiles induces aggression in the European hornet. Naturwissenschaften 89: 111–114 Available: http://link.springer.com/10.1007/s00114-001-0292-9. Accessed 2013 August 15. [DOI] [PubMed] [Google Scholar]
- 53. Alonso L, Vander Meer R (2002) Queen primer pheromone affects conspecific fire ant (Solenopsis invicta) aggression. Behav Ecol Sociobiol 51: 122–130 Available: http://link.springer.com/10.1007/s002650100417. Accessed 2013 September 2. [Google Scholar]
- 54. Ali MF, Morgan ED (1990) Chemical Communication in Insect Communities: a Guide To Insect Pheromones With Special Emphasis on Social Insects. Biol Rev 65: 227–247 Available: http://doi.wiley.com/10.1111/j.1469-185X.1990.tb01425.x. Accessed 2012 June 18. [Google Scholar]
- 55. Blacquière T, Smagghe G, van Gestel CM, Mommaerts V (2012) Neonicotinoids in bees: a review on concentrations, side-effects and risk assessment. Ecotoxicology 21: 973–992 Available: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3338325&tool=pmcentrez&rendertype=abstract. Accessed 2013 August 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Whitehorn PR, O'Connor S, Wackers FL, Goulson D (2012) Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336: 351–352 Available: http://www.ncbi.nlm.nih.gov/pubmed/22461500. Accessed 2013 August 8. [DOI] [PubMed] [Google Scholar]
- 57. Barbieri RF, Lester PJ, Miller AS, Ryan KG (2013) A neurotoxic pesticide changes the outcome of aggressive interactions between native and invasive ants. Proc R Soc B Biol Sci 280: 20132157–20132157 Available: http://rspb.royalsocietypublishing.org/cgi/doi/10.1098/rspb.2013.2157. Accessed 2014 January 10. [DOI] [PMC free article] [PubMed] [Google Scholar]