Abstract
In order to find a way to induce rooting on cuttings of Hemarthria compressa cv. Ya’an under controlled conditions, a project was carried out to study the effect of naphthalene acetic acid (NAA) on rooting in stem cuttings and related physiological changes during the rooting process of Hemarthria compressa. The cuttings were treated with five concentrations of NAA (0, 100, 200 300, 400 mg/l) at three soaking durations (10, 20, 30 minutes), and cuttings without treatment were considered as control. Samples were planted immediately into pots after treatment. IAA-oxidase (IAAO) activity, peroxidase (POD) activity and polyphenol oxidase (PPO) activity were determined after planting. Results showed that NAA had positive effect on rooting at the concentration of 200 mg/l compared to other concentrations at 30 days after planting (DAP). Among the three soaking durations, 20 minutes (min) of 200 mg/l NAA resulted in higher percentages of rooting, larger numbers of adventitious roots and heavier root dry weight per cutting. The lowest IAAO activity was obtained when soaked at 200 mg/l NAA for 20 min soaking duration. This was consistent with the best rooting ability, indicating that the lower IAAO activity, the higher POD activity and PPO activity could be used as an indicator of better rooting ability for whip grass cuttings and might serve as a good marker for rooting ability in cuttings.
Introduction
Hemarthria compressa (Poaceae), known as a warm-season perennial whip grass, mainly cultivated in southern China, south-central Florida and tropical southern Asia, is one of the most important crops in the world [1]. Recently, it is being cultivated in other parts of the world with suitable mild and humid climates. Hemarthria compressa (Whip grass) is used in animal feed and is effectively conserving of water logging and improving nutrient composition in soils [2]. Conventionally, whip grass is propagated by stem cuttings. However, the survival rate of mature stem cuttings was only 80% [3]. Semi-mature cuttings were used for large scale of propagation due to great demand, but the survival rate was limited by serious losses during rooting and hardening procedures [4].
The proper formation of adventitious roots at the base of stem cuttings is an important developmental phenomenon in the growth and survival of cuttings which involves the initiation of several new meristematic areas in different tissues of stem cuttings [5]. Many basic studies on adventitious root formation have been performed under in vitro [6]–[8] and in vivo [9] conditions to distinguish and delineate the successive phases of adventitious root formation and regulation, and three distinct phases of adventitious root formation in plants were found [10]–[12], i.e. induction, initiation and expression. However, different plants appeared to have different periods for the three phases in adventitious root formation. Nag et al. [9] reported that the period of induction, initiation and expression phase were 0–24 h, 24–72 h, after 72 h for mung bean, respectively. On the contrary, induction (0–12 days), initiation (12–14 days) and expression (14–18 days) were obtained during the adventitious rooting process of Camellia sinensis [13]. There is no such approach reported with grass cuttings. Based on our preliminary observation, root initiation developed at 14 days in most of the whip grass cuttings, the period of induction phase was 0–13 DAP, which was similar with Camellia sinensis.
In addition, adventitious root formation is generally promoted by auxin, and auxin signaling and transport has been shown to control plant root length, number of adventitious roots, root hair and root growth direction [14]. Nag et al. [9] reported that auxin was an essential factor for induction rather than initiation of roots in plants, which verified the hypothesis that the adventitious root formation initially occurred in two phases: an auxin-sensitive phase and an auxin-insensitive phase [15]. As a synthetic auxin, NAA is commonly used at relatively low dose to elicit auxin-type responses in cell growth, cell division, fruit setting, rooting, etc [16], [17]. The adventitious root production was increased rapidly at lower NAA concentration, while the number of roots was decreased at higher concentration [17]–[19], similar to our previous study in whip grass [20].
The activities of enzyme in the rooting zone of cuttings provided an easy, fast and reliable means of assessing cellular differentiation into roots [21]. Sato et al. [22] reported that a particular POD catalyzed the process of cell wall lignification during rooting in Zinnia cuttings. PPO catalyzes the oxidation of polyphenols and the hydroxylation of monophenols and lignification of plant cells in trees [23], [24]. Furthermore, an auxin-induced change in POD and IAAO occurred during the rooting processes [9]. But, the responses of enzyme activities to auxin in forage grass were unclear. Therefore, we examined the effects of NAA concentrations and soaking durations on enzyme activities in the rooting zone during the induction phase of adventitious root formation and rooting response in whip grass stem cuttings. These research results may be used as an indicator of rooting ability and lay a theoretical foundation for the improvement of whip grass rooting.
Materials and Methods
Plant materials and treatments
The cuttings of H. compressa (“Ya’an” cultivar) were selected from the Farm of Sichuan Agricultural University, which have been released by the China Forage Registration Committee, and have been widely used in the Yangtze River and played an important role in animal husbandry. Experimental stem cuttings of 15–16 cm length, 3.0 mm diameter and with 3 nodes were obtained from vigorously growing whip grass at the elongation stage on 9 May of 2011 and 2012. Leaves were removed from the cuttings, and the cuttings were immersed in a solution of fungicide 0.5% Bavistin [Methyl N- (1Hbenzoimidazol-2-yl) carbamate] for 15 min and thoroughly rinsed with distilled water. The basal ends (5 cm) of each cutting were then soaked in different concentrations (0, 100, 200, 300, and 400 mg/l) of NAA for different durations (10, 20, 30 min) and cuttings without treatment were considered as control. The treatments were based on the results from preliminary trials in the laboratory. There were three replicates of 20 cuttings in each treatment, which were planted immediately in pots (30 cm diameter, 45 cm deep) filled with a sterilized mixture of sand and loamy soil (1:2, v:v). The pots were then incubated in a controlled growth chamber for 30 days (25±2°C, 16 h photoperiod with cool, white fluorescent lamps and 65% relative humidity). Watering was applied every two days.
Scoring of data in rooting experiment
Ten cuttings were taken randomly from each treatment at 30 DAP for morphological analysis in three replicates. Rooting percentage, numbers of adventitious roots (longer than 2 cm) per cutting and root dry weight per cutting were recorded.
Physiological investigation
There were three replicates of ten cuttings in each treatment for physiological analysis. The physiological investigations on the basal part (about 0.5∼1.0 cm of the rooting zone) were carried out, cut up and mixed at 10 DAP (induction phase).
Extraction for enzyme assay
Fresh tissue (0.5 g) was weighed from the above mixed samples in each treatment with three replicates, frozen in liquid nitrogen and stored at –80°C. The frozen sample was ground in a mortar on ice with 10 ml of 100 mM pre-cold phosphate buffer (pH 6.0) containing 1 % (w/v) dithiothreitol for enzyme extract. The homogenate was centrifuged at 10,000 rpm for 20 min at 4°C and then the pellet was discarded and the supernatant was used for enzyme assay.
IAAO assay
The reaction mixture was made of the 0.5 ml enzyme extract, 1.5 ml of 100 mM potassium-phosphate buffer (pH 6.0), 1.0 ml of 1 mM MnCl2, 1.0 ml of 1 mM 2, 4-dichlorophenol and 1.0 ml of 1 mM IAA. Assay was conducted at 30±0.5°C for 30 min. Then, 0.08 ml of 0.5 M FeCl3 and 3.92 ml of 35% perchloric acid were added to the above enzyme mixture (2 ml). IAA degradation was determined by measuring the absorbance at 530 nm at 35±0.5°C for 30 min in the dark [25]. IAAO activity is represented by the amount of IAA degraded (µg) starting from 1 mg initial protein in 1 h. Measurements were performed in three replicates.
POD assay
The activity of POD was determined according to the method of Li et al. [26] based on the oxidation of guaiacol using H2O2. The enzyme extract (0.05 ml) was added to 3 ml reaction solution to start reaction and the absorbance was monitored at 470 nm for 2 min. POD activity was quantified by the amount of tetraguaiacol formed. The reaction solution was made by mixing 50 ml 100 mM cold phosphate buffer (pH 6.0), 28 µl guaiacol, then, heated and stirred until guaiacol was completely dissolved, finally, 19 µl 30% H2O2 was added after it was cooled and mixed evenly.
PPO assay
The activity of PPO was determined according to the method of Li et al. [26] with modification. The 1 ml reaction mixture contained 250 µl of the enzyme extract and 0.1 M phosphate buffer (pH 6.0). Each sample was aerated for 2 min in a small test tube followed by the addition of 0.2 M catechol as the substrate. PPO activity was expressed as changes in absorbance at 420 nm min−1 g−1 FW.
Statistical Analyses
Means of three replications were calculated. All data were analyzed using the two-way analysis of variance and the least significance difference (LSD) test at p = 0.05 [27] for comparisons between NAA dosages and soaking durations using the SAS program version 9.1 (SAS Institute, Cary, NC).
Results
Effect of NAA-treatment on rooting
Rooting characteristics were influenced by different NAA dosages and soaking durations for explants excised in the year 2011 and 2012 (Figure 1, Table 1). Interestingly, the treatments with lower dosages NAA (100 mg/l and 200 mg/l) increased rooting percentage, number of adventitious roots per cutting and root dry weight per cutting. Maximum rooting ability was observed in the 200 mg/l NAA treatment, whereas higher dosage NAA (400 mg/l) suppressed these parameters. However, rooting ability was not affected by soaking duration in the non-NAA condition. The rooting percentage, number of adventitious roots per cutting and root dry weight per cutting were significantly increased with the increase in soaking duration of 100 mg/l NAA at 30 DAP. When treated with 300 mg/l NAA, the maximum of the root parameters studied at 30 DAP, was observed in cuttings treated during 10 min soaking. However, the cuttings treated with 200 mg/l NAA ×20 min soaking duration showed higher percentages of rooting as well as larger numbers of adventitious roots and heavier root dry weight per cutting.
Figure 1. Rooting response in whip grass cuttings under treatment of NAA dosage and soaking duration in 2011.
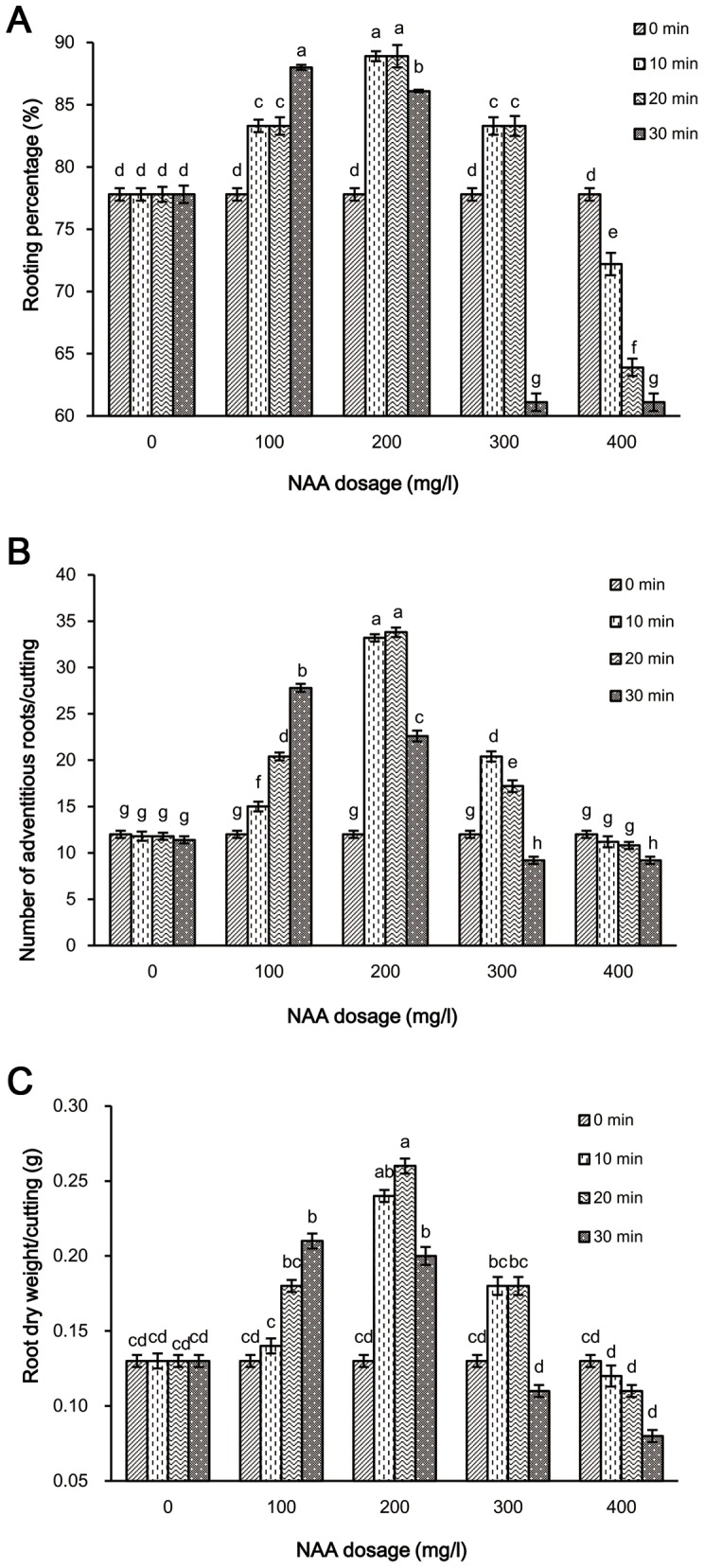
Comparative graph of rooting percentage (A), number of adventitious roots (longer than 2 cm) per cutting (B) and root dry weight per cutting (C). 0 min soaking duration of different NAA dosages (0 mg/l, 100 mg/l, 200 mg/l, 300 mg/l and 400 mg/l) was without treatment and considered as control. Different small letters mean significant differences among treatments of NAA dosage and soaking duration at 30 DAP at α = 0.05. Values represent a mean of 3 replicates with SD. LSD Test.
Table 1. Rooting response in cuttings of whip grass under treatment of NAA dosage and soaking duration in 2012.
| NAA dosage (mg/l) | ||||||
| Characteristics | Soaking duration (min) | 0 | 100 | 200 | 300 | 400 |
| Rooting percentage (%) | 0 | 80.9±0.50e | 80.9±0.50e | 80.9±0.50e | 80.9±0.50e | 80.9±0.50e |
| 10 | 80.9±0.51e | 88.9±0.53d | 97.2±0.42a | 86.7±0.76d | 78.1±0.94fg | |
| 20 | 80.8±0.63ef | 91.7±0.74c | 97.2±0.92a | 86.7±0.80d | 78.1±0.71fgh | |
| 30 | 80.7±0.72ef | 94.4±0.25b | 94.4±0.12b | 75.3±0.71gh | 72.6±0.72h | |
| Number of adventitious roots/cutting | 0 | 12.2±0.39g | 12.2±0.39g | 12.2±0.39g | 12.2±0.39g | 12.2±0.39g |
| 10 | 12.2±0.50g | 15.2±0.52f | 34.8±0.40a | 21.2±0.55d | 10.2±0.60hi | |
| 20 | 11.6±0.38gh | 20.8±0.43d | 35.4±0.51a | 17.6±0.63e | 9.0±0.38ij | |
| 30 | 11.2±0.39gh | 28.6±0.44b | 23.4±0.59c | 8.2±0.38jk | 7.0±0.39k | |
| Root dry weight/cutting (g) | 0 | 0.24±0.01cde | 0.24±0.01cde | 0.24±0.01cde | 0.24±0.01cde | 0.24±0.01cde |
| 10 | 0.21±0.02cde | 0.26±0.02cd | 0.34±0.01ab | 0.3±0.01b | 0.16±0.01ef | |
| 20 | 0.19±0.02de | 0.29±0.02bc | 0.4±0.02a | 0.28±0.01bc | 0.15±0.01ef | |
| 30 | 0.17±0.01e | 0.32±0.01b | 0.3±0.01b | 0.14±0.01ef | 0.07±0.01g | |
Data were recorded at 30 DAP. Value represented a mean of three replicates with SD of each treatment, which consisted of 10 cuttings. 0 min soaking duration of different NAA dosages (0 mg/l, 100 mg/l, 200 mg/l, 300 mg/l and 400 mg/l) was without treatment and considered as control. Within the same characteristics, means followed by the same letters are not significantly different at P<0.05 according to LSD tests.
Effect of NAA-treatment on IAAO activity
The IAAO activity was affected by NAA dosages for different soaking durations of cuttings excised from plants in 2011 and 2012 (Figure 2A, Table 2). The IAAO activity was significantly decreased in the cuttings treated with 100 mg/l and 200 mg/l NAA compared to the control, and the maximum reduction in IAAO activity was observed in 200 mg/l NAA treatment. However, IAAO activity was significantly increased in cuttings treated with 400 mg/l NAA. IAAO activity at 10 DAP was significantly decreased with the increase in soaking duration of 100 mg/l NAA. Among the different soaking durations of 300 mg/l NAA, the minimum value of IAAO activity at 10 DAP was obtained in cuttings treated during 10 min soaking. However, the most remarkable inhibition occurred at 200 mg/l NAA ×20 min soaking duration, and the IAAO activity values were 8.51% and 4.03% lower compared to the control in 2011 and 2012, respectively.
Figure 2. Effect of NAA dosage and soaking duration on some enzyme activity in whip grass cuttings in 2011.
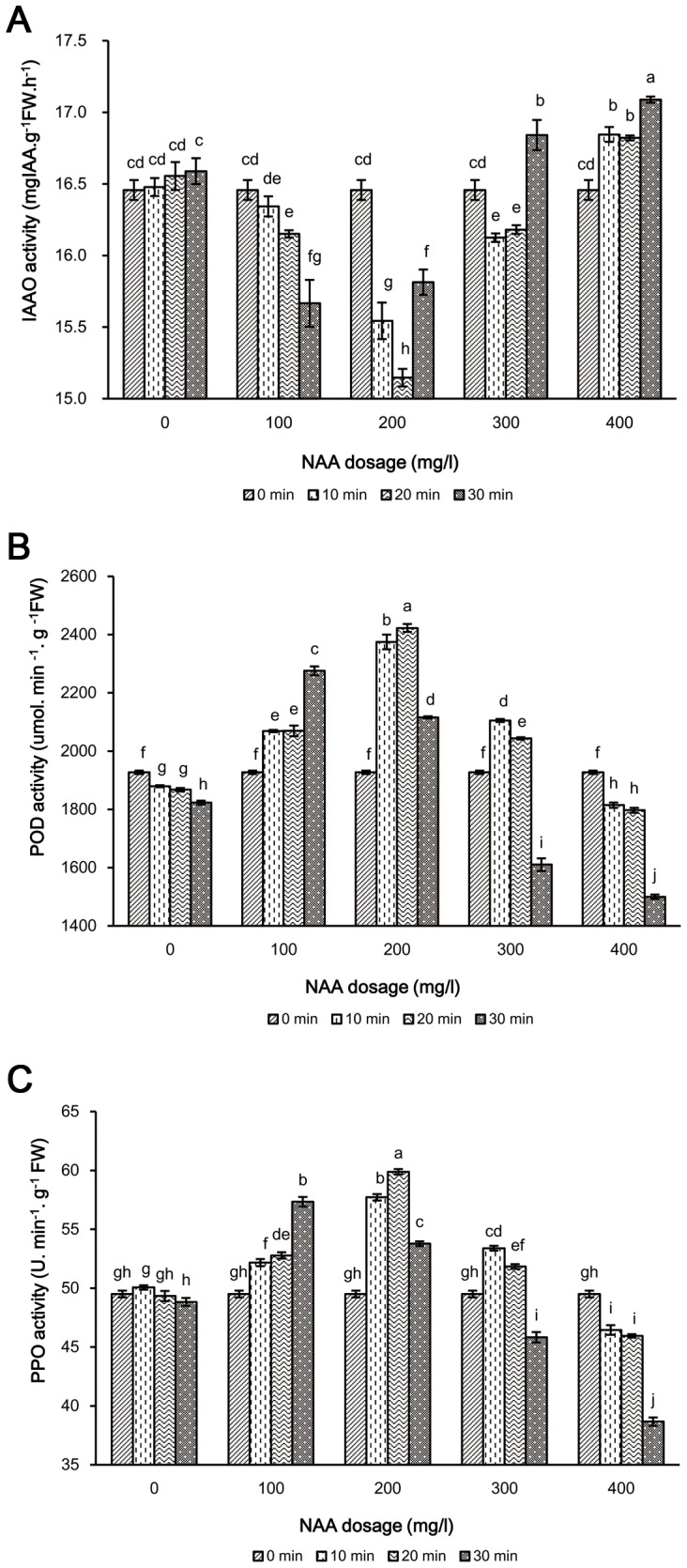
Comparative graph of IAAO activity (A), POD activity (B) and PPO activity (C). 0 min soaking duration of different NAA dosages (0 mg/l, 100 mg/l, 200 mg/l, 300 mg/l and 400 mg/l) was without treatment and considered as control. Different small letters mean significant differences among treatments of NAA dosage and soaking duration at 10 DAP (induction phase) at α = 0.05. Values represented a mean of 3 replicates with SD. LSD Test.
Table 2. Effect of NAA dosage and soaking duration on some enzyme activity in whip grass cuttings in 2012.
| NAA dosage (mg/l) | ||||||
| Characteristics | Soaking duration (min) | 0 | 100 | 200 | 300 | 400 |
| IAAO (mg IAAg−1FW.h−1) | 0 | 16.02±0.01f | 16.02±0.01f | 16.02±0.01f | 16.02±0.01f | 16.02±0.01f |
| 10 | 16.07±0.01f | 15.87±0.07g | 15.50±0.04j | 15.75±0.07gh | 16.26±0.03d | |
| 20 | 16.12±0.01ef | 15.78±0.06g | 15.40±0.02k | 15.85±0.09g | 16.38±0.08c | |
| 30 | 16.23±0.03de | 15.58±0.06ij | 15.64±0.08hi | 16.52±0.07b | 16.74±0.04a | |
| POD (μmol.min-1.g-1FW) | 0 | 2410.69±1.63h | 2410.69±1.63h | 2410.69±1.63h | 2410.69±1.63h | 2410.69±1.63h |
| 10 | 2395.83±5.52h | 2443.75±21.94g | 3237.50±6.88b | 2710.42±7.29e | 2141.67±6.14j | |
| 20 | 2233.33±6.04i | 2627.08±9.10f | 3668.75±14.79a | 2636.81±3.02f | 2134.03±14.08j | |
| 30 | 2152.08±11.58j | 3172.92±9.07c | 2745.83±12.33d | 2010.42±14.08k | 1960.42±17.07l | |
| PPO (U.min-1.g-1FW) | 0 | 59.17±0.27g | 59.17±0.27g | 59.17±0.27g | 59.17±0.27g | 59.17±0.27g |
| 10 | 57.94±0.09h | 60.44±0.38ef | 68.0±0.24b | 61.39±0.89de | 55.06±0.27i | |
| 20 | 56.89±0.21h | 60.89±0.12ef | 69.61±0.17a | 60.28±0.32fg | 54.56±0.37i | |
| 30 | 55.44±0.39i | 62.83±0.42c | 62.0±0.65cd | 51.33±0.09j | 51.33±0.49j | |
Data were recorded at 10 DAP. Value represented a mean of three replicates with SD of each treatment, which consisted of 10 cuttings. 0 min soaking duration of different NAA dosages (0 mg/l, 100 mg/l, 200 mg/l, 300 mg/l and 400 mg/l) was without treatment and considered as control. Within the same characteristics, means followed by the same letters are not significantly different at P<0.05 according to LSD tests.
Effect of NAA-treatment on POD activity
POD activity was influenced by NAA dosages for different soaking durations of cuttings excised from plants in 2011 and 2012 (Figure 2B, Table 2). 100 mg/l and 200 mg/l NAA treatments caused a significant increase in POD activity, and the most remarkable promotion of POD activity occurred at 200 mg/l NAA treatment, but 400 mg/l NAA treatment caused a significant reduction compared to the control. POD activity was promoted significantly with increasing soaking duration for 100 mg/l NAA. Additionally, the highest POD activity was for the 20 min soaking duration, followed by 10 min, and then 30 min under 200 mg/l NAA, which were significantly higher than the control by 25.73%, 23.24% and 9.80% in 2011, and 52.19%, 34.30% and 13.90% in 2012, respectively. On the contrary, POD activity was reduced with increasing soaking durations under 300 mg/l and 400 mg/l NAA treatments.
Effect of NAA-treatment on PPO activity
PPO activity was significantly affected by the NAA dosages for different soaking durations of cuttings excised from plants in 2011 and 2012 (Figure 2C, Table 2). PPO activity was significantly increased in the cuttings treated with 100 mg/l and 200 mg/l NAA, and the highest PPO activity was observed in 200 mg/l NAA treatment. However, PPO activity was significantly decreased in cuttings treated with 400 mg/l NAA. Additionally, the PPO activity values were maximized when soaked at 100 mg/l NAA for 30 min soaking duration and soaked at 200 mg/l NAA for 20 min soaking duration in both years. The most remarkable promotion occurred at 200 mg/l NAA×20 min soaking duration, respectively 20.99% and 17.64% higher compared to the control in 2011 and 2012.
Discussion
This study showed that adventitious root formation was influenced by NAA dosage and soaking duration for cuttings obtained in 2011 and 2012, but the value of rooting characteristics was higher for the 2012 cuttings than for the 2011 cuttings. This may be because the stem cuttings in 2012 were more mature. Similarly, Dick and Magingo [28] reported that rooting ability of cuttings varied among clones and node positions. Of the NAA dosages tested, lower dosage of NAA caused a significant increase in rooting ability as compared with the control, and 200 mg/l was found to be the best at enhancing rooting ability, while higher doses of NAA caused a significant decrease in root formation. This verified Hentig and Gruber’s [29] report that hormonal doses could induce the best rooting when being just below the toxic level. Similar results were obtained in Sun and Hong’s [17] research, which found that lower concentrations of NAA (1.0 mg/l and 2.0 mg/l) in the callus induction medium had a stronger effect on successive plant regeneration of the halophyte Leymus chinensis than higher concentrations. In contrast, the range of effective NAA concentration was much lower compared with our experiment, this may be due to the difference between cuttings cultured both in vitro and ex vitro [30].
Furthermore, auxin is widely applied in the vegetative propagation of various plants [31], [32], and there are many deviations in the range of effective NAA dosage on cuttings of different plant species. Cui and Mao [33] reported that the best way to apply auxin was to dip quickly with 3,000 mg/l NAA for root growth and cutting propagation of Zizphus spinosus. In the case of teak stem cuttings, the maximum number of roots per cutting was produced by quick dipping in 4,000 mg/l NAA [22]. In the present study, treatment with 200 mg/l NAA for 20 min soaking duration produced the highest rooting percentage and the maximum number of adventitious roots per cutting in whip grass, followed by treatment with 200 mg/l NAA for 10 min soaking duration (Figure 1, Table 1). This showed that the effective NAA dosage in cuttings of trees was higher than that of grass cuttings.
The stimulatory effects of NAA on rooting of plant stem cuttings have been reported by many researchers, and promotion of adventitious root formation has been considered as one of the characteristics of auxin [13]. There is considerable evidence that indole-3-acetic acid (IAA) is involved in adventitious root formation. Liu and Reid [34] reported that high levels of IAA were associated with the promotion of adventitious root. In Arabidopsis, high concentrations of exogenous IAA triggered pericycle cells adjacent to protoxylem poles to become part of a lateral root primordium [35]. Kowalczyk et al. [36] reported that the IAA levels increased after treatment in NAA solution of mesocotyl and coleoptile segments of maize. In addition, IAAO activity might play a crucial role in regulating endogenous IAA levels [9], and the relationship between the levels of IAA and IAAO activity is negatively correlated [37]. In the present study, the lowest IAAO activity during the induction period was obtained in the cuttings treated with 200 mg/l NAA for 20 min soaking duration (Figure 2A, Table 2), resulting in the highest rooting ability. The results indicated that the lower IAAO activity during the induction period in lower NAA dosage treated cuttings appeared to be responsible for better development of adventitious roots, possibly being related to higher endogenous IAA content [9]. Similarly, the soybean hypocotyls of NAA-treated cuttings grew significantly higher numbers of adventitious roots with an increase in endogenous IAA levels that corresponded with a decrease in IAAO activity examined [38].
The formation of adventitious root involves the process of redifferentiation during which predetermined cells switch from their morphogenetic path to act as mother cells for the root primordia [39]. Among these changes, POD activity is known to be involved in auxin metabolism as well as in lignification process in the cell wall synthesis an obligatory step in root formation [13], [23]. The present results showed that an increase in POD activity was observed at the induction phase in 100 mg/l and 200 mg/l NAA-treated cuttings, and the highest POD activity in the cuttings treated with 200 mg/l NAA for 20 min soaking duration was consistent with the rooting ability (Figure 2B, Table 2), indicating that POD activity was an indicator of better rooting ability and might serve as a good marker for rooting ability in cuttings [40]. Likewise, auxin-induced changes in POD during the rooting process have also been reported [38], [41]. These results postulated that the POD acts as antioxidants, thereby protecting IAA from oxidation and plant tissue from oxidative stress due to wounding.
In addition, PPO catalyzes the oxidation of polyphenols and the hydroxylation of monophenols and lignification of plant cells during the rooting process [24]. It is positively correlated with lignin content in both shoot and fruit of eggplant, and the higher amount of PPO and lignin contents improved rooting and resistance to biotic stress [25]. Our results showed that the PPO activity was increased with lower NAA dosage, which was consistent with Rout’s data [16], indicating that the increase in the activity of PPO during the induction phases might be associated with better rooting ability in treated cuttings. The present results also showed that PPO activity was highest in 200 mg/l NAA for 20 min soaking duration treated cuttings, which was significantly higher than that of 200 mg/l NAA for 10 min soaking duration and 100 mg/l NAA for 30 min soaking duration (Figure 2C, Table 2). These results suggested that PPO activity metabolism of whip grass cuttings was sensitive to NAA dosage and soaking duration, similar to the results of Hunt et al. [42] and James [43], which found that the concentration of auxin and the timing of its application were key factors in successful rooting and associated physiological changes.
Conclusion
In our study, 100 mg/l and 200 mg/l NAA increased rooting ability, POD and PPO activity, but decreased IAAO activity during the rooting induction phase in whip grass cuttings. The changes in physiological parameters by the use of NAA seemed related to the changes of morphological traits, as NAA induced adventitious roots, with a concomitant increase in dry weight of the roots per cutting. Higher activities of POD and PPO and lower IAAO activity in the cuttings might be among the critical factors to improve rooting, and to prevent membrane damage. The present studies indicated that 200 mg/l NAA for 20 min soaking duration had a significant effect on root regeneration in whip grass cuttings. Thus, these results would be useful for mass-scale propagation and conservation of whip grass, future experiments should be conducted to examine the validity of these results with other forage grass.
Funding Statement
This work was supported by the earmarked fund for Modern Agro-Industry Technology Research System (#CARS-35-05) and the National Science & Technology Pillar Program in the Twelfth Five-Year Plan (#2011BAD17B03). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yang CH, Li XL, Han JG, Chen LZ, He F (2010) Mixtures of whip grass with cool-season annual forages to extend the forage production season in south-west China. Tropical Grasslands 44: 47–54. [Google Scholar]
- 2. Huang LK, Zhang XQ, Cheng L, Chen LZ (2012) Evaluation on the waterlogging resistance of whip grass germplasm by subordinate function. Advanced Sci. Letters 7: 328–331. [Google Scholar]
- 3. Hu ZD, Ye C, Hu TX (2008) Growth status of Hemarthria compressa and its influence on soil nutrients. . Res. Soil & Water Conser. 15: 120–123. [Google Scholar]
- 4. Li F, Zhang XQ, Ma X, Huang LK, Chen YX (2009) Comparative study on the agronomic traits of new whipping grass lines. . Hubei Agri. Sci. 48: 1210–1213. [Google Scholar]
- 5. Kaur S, Cheema SS, Chhabra BR, Talwar KK (2002) Chemical induction of physiological changes during adventitious root formation and bud break in grapevine cuttings. . Plant Growth Regul. 37: 63–68. [Google Scholar]
- 6.Gaspar T, Kevers C, Hausman JF (1997) Indissociable chief factors in the inductive phase of adventitious rooting. In: Altman, Waisel, editors. Biology of root formation and development. New York: Plenum Press.
- 7. Klerk GJD, Brugge JT (1992) Factors affecting adventitious root formation in microcuttings of Malus. Agronomy 12: 747–755. [Google Scholar]
- 8. Klerk GJD (1996) Marker of adventitious root formation. Agronomy 16: 609–616. [Google Scholar]
- 9. Nag S, Saha K, Choudhuri MA (2001) Role of auxin and polyamines in adventitious root formation in relation to changes in compounds involved in rooting. . J. Plant Growth Regul. 20: 182–194. [Google Scholar]
- 10. Hausman JF, Evers D, Kevers C, Gaspar T (1997) Internal controls of root induction in poplar shoots raised in vitro. . Angew. Bot. 71: 104–107. [Google Scholar]
- 11. Kevers C, Hausman JF, Faivre-Rampant O, Evers D, Gaspar T (1997) Hormonal control of adventitious rooting: progress and questions. . J. Appl. Bot. 71: 71–79. [Google Scholar]
- 12. Klerk GJD, Krieken WVD, Jong JCD (1999) The formation of adventitious roots: new concepts, new possibilities, In Vitro Cell Dev. Biol. -Plant 35: 189–199. [Google Scholar]
- 13. Rout GR (2006) Effect of auxins on adventitious root development from single node cuttings of Camellia sinensis (L.) Kuntze and associated biochemical changes. Plant Growth Regul. 48: 111–117. [Google Scholar]
- 14. Mishra BS, Singh M, Aggrawal P, Laxmi A (2009) Glucose and auxin signaling interaction in controlling Arabidopsis thaliana seedlings root growth and development. Plos One 4(2): e4502 doi:10.1371/journal.pone.0004502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartmann HT, Kester DE, Davies FT (1968) Plant propagation: principles and practices. India: Prentice Hall.
- 16.Srivastava LM (2002) Plant growth and development: hormones and environment. San Diego (CA): Academic.
- 17. Sun YL, Hong SK (2010) Effects of plant growth regulators and L-glutamic acid on shoot organogenesis in the halophyte Leymus chinensis (Trin.). Plant Cell Tiss Organ Cult 100: 317–328. [Google Scholar]
- 18. Raju NL, Prasad MNV (2010) Influence of growth hormones on adventitious root formation in semi-hardwood cuttings of Celasturs paniculatus Willd. : a contribution for rapid multiplication and conservation management. . Agroforest Syst. 79 249–52. [Google Scholar]
- 19. Khan S, Bi TB (2012) Direct shoot regeneration system for date palm (Phoenix dactylifera L.) cv. Dhakki as a means of micropropagation. . Pak. J. Bot. 44 1965–71. [Google Scholar]
- 20. Yan YH, Zhang XQ, Gong Y, Ma YM, Yang WY, et al. (2013) Influence of different dosages of naphthalene acetic acid (NAA) and soaking time on stem cuttings of Hemarthria compressa. Res. on Crops 14: 907–914. [Google Scholar]
- 21. Husen A, Pal M (2007) Metabolic changes during adventitious root primordium development in Tectona grandis Linn. f. (teak) cuttings as affected by age of donor plants and auxin (IBA and NAA) treatment. New Forests 33: 309–323. [Google Scholar]
- 22. Sato Y, Sugiyama M, Gorecki RJ, Fukuda H, Komamine A (1993) Interrelationship between lignin deposition and the activities of peroxidase isoenzymes in differentiating tracheary elements of Zinnia . Planta 189: 584–589. [Google Scholar]
- 23. Rivero RM, Ruiz JM, Garcia PC, Lopez-Lefebre LR, Sanchez E, et al. (2001) Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 160: 315–321. [DOI] [PubMed] [Google Scholar]
- 24. Khorsheduzzaman AKM, Alam MZ, Rahman MM, Khaleque MMA, Ismail HMM (2010) Biochemical basis of resistance in eggplant (Solanum melongena L.) to Leucinodes orbonalis Guenee and their correlation with shoot and fruit infestation. Bangladesh J. . Agril. Res. 35: 149–155. [Google Scholar]
- 25. Beffa R, Martin HV, Pilet PE (1990) In vitro oxidation of indoleacetic acid by soluble auxin-oxidases and peroxidases from maize roots. . Plant Physiol. 94: 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li Z, Peng Y, Ma X (2013) Different response on drought tolerance and post-drought recovery between the small-leafed and the large-leafed white clover (Trifolium repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol. Plant 35: 213–222. [Google Scholar]
- 27. Wang L, Deng F, Ren W-J, Yang W-Y (2013) Effects of Shading on Starch Pasting Characteristics of Indica Hybrid Rice (Oryza sativa L.). Plos One 8: e68220 doi:10.1371/journal.pone.0068220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dick J, Magingo F (1998) Rooting ability of Leucaena leafy and leafless stem cuttings in a non-mist propagator. In: Shelton HM, Gutteridge RC, Mullen BF, Bray RA, (eds), Leucaena-Adaptation, Quality and Farming Systems. ACIAR Proceedings No. 86. Canberra, Australia, pp. 188–192.
- 29. Hentig W, Gruber G (1987) Influence of rooting hormones on the propagation of several ‘‘new’’ ornamentals. Acta Hortic 226: 479–488. [Google Scholar]
- 30. Mori Y, Miyahara F, Tsutsumi Y, Kondo R (2011) Effects of combinational treatment with ethephon and indole-3-butyric acid on adventitious rooting of Pinus thunbergii cuttings. . Plant Growth Regul. 63: 271–278. [Google Scholar]
- 31. Husen A, Pal M (2006) Variation in shoot anatomy and rooting behaviour of stem cuttings in relation to age of donor plants in teak (Tectona grandis Linn. f.), New Forest 31: 57–73. [Google Scholar]
- 32. Hunt RW, Chinnasamy S, Bhatnagar A, Das KC (2010) Effect of biochemical stimulants on biomass productivity and metabolite content of the microalga, Chlorella sorokiniana . Appl Biochem Biotechnol 162: 2400–2414. [DOI] [PubMed] [Google Scholar]
- 33. Cui XD (2010) MaoXH (2010) Effects of the plant growth regulators on cutting propagation of Zizphus spinosus . Northern Hortic 2: 10–13. [Google Scholar]
- 34. Liu JH, Reid DM (1992) Adventitious rooting in hypocotyls of sunflower (Helianthus annuus) seedlings. IV. The role of changes in endogenous free and conjugated indole-3-acetic acid. Physiol. Plant 86: 285–292. [Google Scholar]
- 35. Laskowski MJ, Williams ME, Nusbaum HC, Sussex IM (1995) Formation of lateral root meristems is a two-stage process. Development 121: 3303–3310. [DOI] [PubMed] [Google Scholar]
- 36. Kowalczyk S, Jakubowska A, Bandurski RS (2002) 1-Naphtalene acetic acid induces indole-3-ylacetylglucose synthase in Zea mays seedling tissue. . Plant Growth Regul. 38: 127–134. [Google Scholar]
- 37. Wiesman Z, Riov J, Epstein E (1988) Comparison of movement and metabolism of indole-3-acetic acid and indole-3-butyric acid in mung bean cuttings. Physiol. Plant 74: 556–560. [Google Scholar]
- 38. Liu ZH, Hsiao IC, Pan YW (1996) Effect of naphthalene acetic acid on endogenous indole-3-acetic acid, peroxidase and auxin oxidase in hypocotyl cuttings of soybean during root formation. . Bot. Bull. Acad. Sin. 37: 247–253. [Google Scholar]
- 39. Aeschbacher RA (1994) Schiefelbein JW, Benfey PN (1994) The genetic and molecular basis of root development. . Annu. Rev. Plant Physiol. Plant Mol. Biol. 45: 25–45. [Google Scholar]
- 40. Gaspar T, Kevers C, Hausman JF, Berthon JY, Ripetti V (1992) Practical uses of peroxidase activity as a predictive marker of rooting performance of micropropagated shoots. Agronomy 12: 757–765. [Google Scholar]
- 41. Klerk GJD (1996) Markers of adventitious root formation. Agronomy 16: 609–616. [Google Scholar]
- 42. Hunt MA, Trueman SJ, Rasmussen A (2011) Indole-3-butyric acid accelerates adventitious root formation and impedes shoot growth of Pinus elliottii var. elliottii×P. caribaea var. hondurensis cuttings. New Forests 41: 349–360. [Google Scholar]
- 43. James DJ (1983) Adventitious root formation “in vitro” in apple rootstocks (Malus pumila). II. Uptake and distribution of indol-3yl-acetic acid during the auxin-sensitive phase in M.9 and M.26. . Physiol. Plant. 57: 154–58. [Google Scholar]


