Abstract
The proper control of tissue growth is essential during normal development and an important problem in human disease. Merlin, the product of the Neurofibromatosis 2 tumor suppressor gene, has been extensively studied to understand its functions in growth control. Here we describe experiments in which we used Drosophila as an in vivo system to test the functions of the normal human NF2 gene products and patient-derived mutant alleles. Although the predominant NF2 gene isoform, isoform 1, could functionally replace the Drosophila Merlin gene, a second isoform with a distinct C-terminal tail could not. Immunofluorescence studies show that the two isoforms have distinct subcellular localizations when expressed in the polarized imaginal epithelium, and function in genetic rescue assays correlates with apical localization of the NF2 protein. Interestingly, we found that a patient-derived missense allele, NF2L64P, appears to be temperature sensitive. These studies highlight the utility of Drosophila for in vivo functional analysis of highly conserved human disease genes.
Introduction
Neurofibromatosis 2 (NF2) is a dominantly inherited tumor predisposition syndrome characterized by the formation of schwannomas, meningiomas and other nervous system tumors [1]. In addition, mutations in the NF2 gene have been associated with non-neuronal, sporadic cancers such as mesothelioma, suggesting that NF2 has tumor suppressor functions in a variety of tissues. NF2 encodes a membrane-associated cytoplasmic protein, called Merlin, which contains a Four-point-one, Ezrin-Radixin-Moesin (FERM) domain and is most closely related to the ERM (Ezrin-Radixin-Moesin) proteins [2]–[4]. FERM domains are believed to interact with the cytoplasmic tails of transmembrane proteins and bind other membrane-associated proteins [4], [5]. Thus Merlin likely acts at the cytoplasmic face of the plasma membrane [6], though some studies have suggested nuclear functions for Merlin as well [7].
Previous studies have identified a well-conserved NF2 homologue, called Merlin (Mer), in Drosophila [8]. Like its mammalian counterparts, Drosophila Merlin has growth suppressive functions as revealed by somatic mosaic analyses [9]. In addition, studies have demonstrated that Merlin protein is associated with endocytic processes, has functional interactions with Expanded, another FERM domain containing tumor suppressor, and works antagonistically to EGFR and other signaling pathways that function to promote proliferation in developing epithelia [10]–[12]. Recent work has also implicated Merlin and Expanded as upstream regulators of the Hippo pathway [13], [14], a well-conserved kinase-signaling cascade that is believed to control tissue growth during development and regeneration. Studies in mice indicate that Merlin also functions upstream of Hippo signaling in mammals [15], although the mechanisms by which Merlin regulates Hippo signaling might vary between different organisms. In addition, it is currently unclear whether all of Merlin's roles in tumor suppression are carried out through its ability to regulate Hippo signaling, or whether it might have some functions, such as regulation of EGFR accessibility at the cell surface and junctional organization, that are independent of the Hippo pathway [16], [17].
Given the similarities in structure and function between fly and mammalian genes and the utility of Drosophila genetics for studying gene function, we reasoned that Drosophila would be a valuable system in which to study the effects of splicing variants and disease associated mutations in human NF2. Here we describe functional analyses of an array of NF2 forms using transgenic Drosophila as an in vivo ‘test tube’ for protein function. We show that although the predominant NF2 isoform (Iso1) is able to rescue the lethality associated with Drosophila Merlin null mutations, a splice variant with a unique C-terminal tail does not. We find that like Drosophila Merlin, human NF2 truncations still retain some, albeit weak, function. In addition, we provide evidence that a well-described severe patient derived mutation, NF2L64P, is a temperature sensitive allele that retains significant functionality at non-restrictive temperatures. Together these studies provide insights into NF2 function and display the utility of Drosophila as an orthologous system for analysis of human disease genes.
Materials and Methods
NF2 transgenes and genetic rescue experiments
Transgenes expressing the indicated NF2 proteins were cloned with N-terminal Flag epitope tags in the pUASt transformation vector. P-element transformation [18] was used to generate multiple stable transgenic lines for each NF2 allele tested. For genetic rescue tests, at least three independent autosomal insertion lines for each allele were crossed to female flies of the following genotype, y w Mer4/FM7; T80-Gal4, and for each of these lines, a sufficient number of offspring was scored to ensure that the predicted rescue class would number at least 40 flies. Percentage genetic rescue was calculated from the ratio of surviving y w Mer4 hemizygous males over half of the total female offspring, thereby avoiding the semi-lethality associated with the FM7 balancer chromosome in males. The Mer4 chromosome was marked with y to allow us to distinguish between rescued males (with yellow body color) and males (normal body color) generated by patroclinous X chromosome inheritance due to meiotic non-disjunction in FM7 females.
Immunofluorescence
Tissue dissection, fixation and antibody staining were performed as previously described [19]. Mouse anti-Flag (Sigma-Aldrich) was used at 1∶20,000 and guinea-pig anti-Merlin [8] was used at 1∶5000. Secondary antibodies (Jackson ImmunoResearch) were diluted 1∶1000 and tissues were mounted in Prolong Antifade (Molecular Probes). Confocal images were taken on either a Zeiss LSM 410 or LSM 510 microscope using Zeiss acquisition software (Carl Zeiss MicroImaging).
Analysis of wing phenotypes
The effect of ectopic expression of the NF2 isoforms under the control of the apterous-Gal4 driver was examined in adult wings. Wings were prepared by incubating flies in 70% EtOH, rinsing in water, and then mounting the dissected wings in Aquamount (BDH Laboratory Supplies). Wings were imaged under brightfield optics on a Zeiss Axioplan 2ie compound microscope and recorded using a Spot digital camera.
Quantitative PCR
Total RNA was prepared from 20 wing imaginal discs from larvae of the following genotypes – ApGal4/+ (control), ApGal4/P[hNF2Iso1]; P[hNF2Iso1]/+, ApGal4/+; (2X)P[hNF2Iso2]/+ - using Trizol (Invitrogen). cDNA was produced using the Taqman kit (ABI) and quantitative PCR performed using the LightCycler FastStart SYBR green system (Roche).
Results
Genetic rescue of Merlin mutations by NF2 alleles
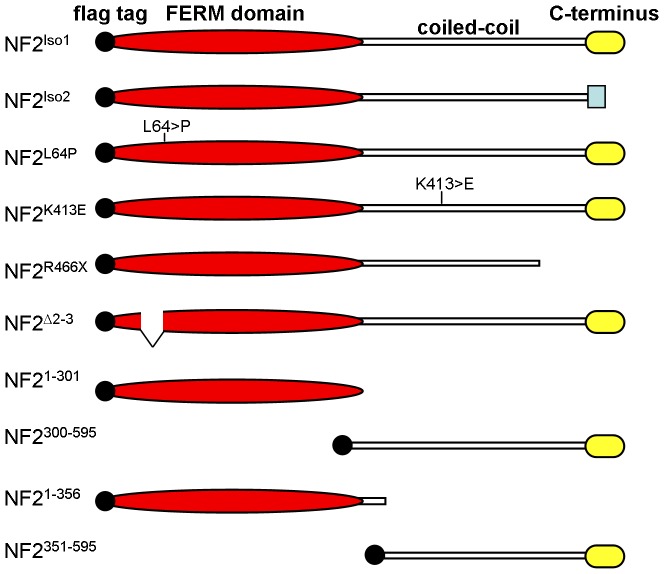
We first asked if expression of the two known isoforms, NF2Iso1 and NF2Iso2 [20], could functionally replace the endogenous Merlin gene in a transgenic rescue assay (Fig. 1). Isoform 1 has homology with Drosophila Merlin and the ERM proteins along its entire length. In contrast, isoform 2, which is formed by the insertion of an exon near the 3′ end of the coding sequence (exon 16) that replaces the C-terminal 16 amino acids of isoform 1 with a novel stretch of 11 amino acids, has a unique C-terminal tail. All transgenic constructs were Flag epitope tagged at the amino terminus (Fig. 1; Materials and Methods). Ability to rescue was tested using ubiquitous expression via the Gal4/UAS system in animals hemizygous for the Mer4 null allele, which severely truncates the Merlin protein [9]. In all rescue experiments, at least three independent NF2 transgenic lines of each construct were tested to control for expression differences due to insertion site. Also, immunostaining using anti-Flag antibody (Fig. 2) and immunoblot analysis (data not shown) were used to confirm expression of all NF2 forms.
Figure 1. Diagrams of hNF2 proteins used in this study.
The known protein domains, including the FERM domain, the coiled-coil domain, and the C-terminal FERM binding domain, are indicated as shown. All constructs are N-terminally tagged with the FLAG epitope. Details of the mutant alleles are provided in the text.
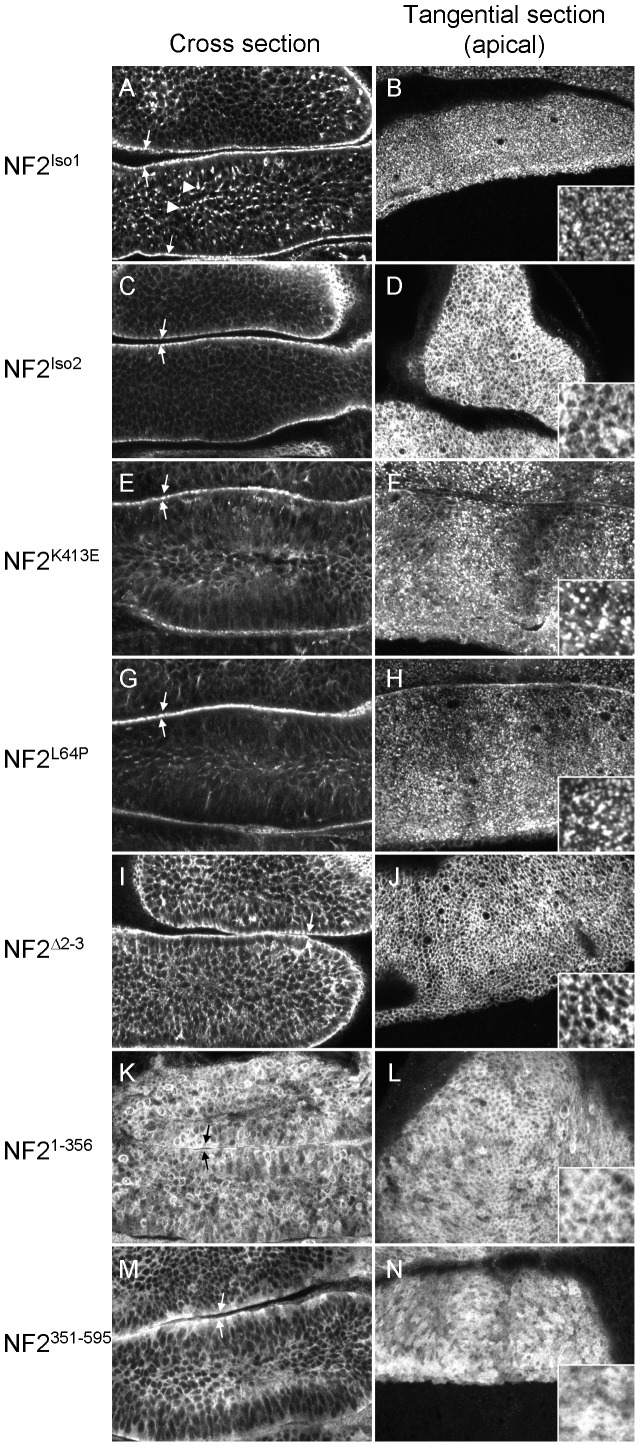
Figure 2. Mutations in hNF2 alter the subcellular localization of the Merlin protein expressed in Drosophila.
Confocal optical sections of wing imaginal epithelia, either cutting deeply into the epithelium (‘Cross section’) or tangentially through the apical surface of the epithelium, are presented for each allele or isoform. A–B) NF2 isoform 1 is primarily localized to the apical membrane (arrows) of these polarized epithelial cells, and adopts a punctate appearance at the apical cortex that is not clearly associated with cell boundaries (see inset at right). In addition, some punctate staining is observed basally (arrowheads in A). C–D) In contrast isoform 2, while still primarily apical (arrows, C), appears associated with cell boundaries (outlining the periphery of the apical ends of cells in D) and does not form punctae. The localizations of the K413E (E–F) and L64P (G–H) point mutations appear similar to that of isoform 1. The Δ2-3 deletion allele (I-J) appears localized to the lateral cell cortex, somewhat similar to isoform 2, but less strongly apically localized (arrows mark apical; also note staining throughout the basolateral domain in I). NF21-356 (K–L) is substantially cytoplasmic and fails to accumulate apically while NF2351-595 (M–N) shows some apical enhancement (arrows in M). Both truncations display substantial cytoplasmic localizations, as indicated by the tangential views (compare L and N to J).
As shown in Table 1, expression of a transgene expressing NF2Iso1 using the ubiquitously expressed T80 Gal4 driver [21] substantially rescued the lethality of the null Mer4 allele, while NF2Iso2 provided very poor rescue. Interestingly, the rescue provided by NF2Iso1 improved slightly with increased genetic dose, while NF2Iso2 rescue seemed to decrease with increasing dosage. This behavior would be expected for an allele with dominant negative effects on the low levels of maternally encoded Merlin found in Mer4 zygotes, and clearly indicates that isoform 2 has significant functional differences from isoform 1 in this assay. This conclusion is consistent with a recent study demonstrating an isoform 2-specific neural function in the mouse [22].
Table 1. Genetic rescue by hNF2 alleles.
| Allele | % rescue (1 dose1) | %rescue (2 doses2) |
| Isoform 1 | 59±7 | 72 |
| Isoform 2 | 8±4 | 0 |
| L64P | 73±6 | 71 |
| K413E | 36±10 | 62 |
| R466X | 0±0 | 11 |
| Δ2–3 | 0±0 | 0 |
| 1–301 | 0±0 | 0 |
| 300–595 | 2±1 | 0 |
| 1–356 | 0.2±0.2 | 0 |
| 351–595 | 0±0 | 0 |
Average of three or more independent lines.
Using two of the insertions tested in the one-dose experiments.
Studies of NF2 patient families have identified a number of point mutations in the NF2 gene. Most are nonsense mutations, but there are a small number of known disease-causing missense mutations that we reasoned could be used to provide insights into structure/function relationships in the Merlin protein [23]. Given that NF2Iso1 provides significant rescue of Mer null mutations in flies, we generated transgenes carrying these disease-associated NF2 alleles and tested their function in the rescue assay (Fig. 1; Table 1). Interestingly, the NF2L64P missense mutation, which has been proposed to be strongly inactivating [24], provided complete genetic rescue in our assay. Another disease-associated mutation, NF2K413E, showed reduced rescue ability in a dose sensitive fashion, suggesting that it is hypomorphic for Merlin function.
To further analyze structure/function relationships in NF2, we generated deletion transgenes and tested their ability to rescue Merlin lethality. Multiple C-terminal deletions were tested - deletions of the C-terminal half of Merlin (NF21-301, NF21-356), and a disease associated nonsense mutation (NF2R466X). While NF21-301 and a slightly larger form NF21-356 failed to provide rescue at any dose, the NF2R466X allele, which encodes more of the C-terminal domain, rescued lethality moderately when expressed at the higher level afforded by two copies of the transgene (Table 1). This observation suggests that this allele is hypomorphic and is consistent with previous studies of Drosophila Merlin that indicate that the C-terminal region of the protein contains non-essential regulatory sequences [9], [14]. In contrast, expression of the C-terminal domain alone (NF2300-595 and NF2351-595) was not sufficient to provide rescue. Expression of a form that lacks the coding sequences provided by exons 2 and 3 (NF2 Δ2-3), which has been proposed to have dominant-negative activity [25], similarly failed to provide any genetic rescue.
Subcellular localizations of NF2 proteins
To learn more about the functions of these NF2 transgenes, we examined the subcellular localization of their products when expressed in the larval imaginal discs. These structures, which produce adult appendages such as wings and legs during metamorphosis, consist of a single layered columnar epithelium that displays a highly structured apical/basal epithelial polarity. Merlin functions to control proliferation in these tissues, and the Merlin protein is primarily localized to the apical membrane and junctional domain [8], [9]. NF2Iso1 expressed in the wing imaginal epithelium under the apterous Gal4 (apGal4) driver was highly concentrated at the apical domain, in a distinctly punctate pattern (Fig. 2A–B). In addition this protein localized to punctate structures below the apical surface, consistent with previous studies indicating that Merlin is associated with endocytic compartments [8], [26]. NF2Iso2 was also apical, but lacked the punctate appearance and instead was associated with cell boundaries at the apical ends of epithelial cells (Fig. 2C–D). As observed in the genetic rescue experiments (Table 1), this result suggests significant functional differences between these two NF2 isoforms. Consistent with this, the two other proteins that provided significant rescue, NF2L64P and NF2K413E, had subcellular distributions that were indistinguishable from that of NF2Iso1 (Fig. 2E–H). In contrast, NF2Δ2-3 was primarily associated with the lateral cell cortex (Fig. 2 I–J) and the N- and C-terminal truncations were more cytoplasmic in localization (Fig. 2 K–N). Similar localizations were observed when the each transgene was present in either one or two doses, indicating that differences in expression level do not affect subcellular localization.
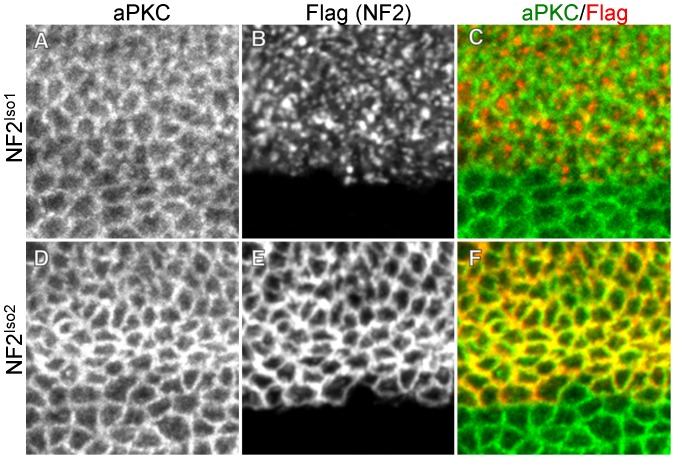
To better characterize the localizations of isoforms 1 and 2, we co-stained for these proteins and atypical protein kinase C (aPKC) in the wing imaginal epithelium. Previous studies have shown that aPKC is concentrated in the marginal zone, a region of the lateral cell membrane just apical to the adherens junction, in these cells [27], [28]. As expected for a marginal zone component, aPKC localized junctionally in the apical region of wing imaginal disc cells (Fig. 3A,D). Strikingly, while NF2Iso1 was also predominantly localized apically, it did not show the same ‘fishnet’ staining pattern observed for aPKC, and did not strongly colocalize with aPKC (Fig. 3A–C). In contrast, NF2Iso2 staining was quite similar to aPKC, and strongly colocalized with aPKC (Fig. 3D–F). These staining patterns indicate that NF2Iso1 is distributed in a punctate, non-junctional pattern across the apical cell cortex, while NF2Iso2 localizes primarily in the apical junctional region. Thus, there was a correlation between punctate, apical localization and ability to provide genetic rescue, suggesting that this localization is important for Merlin function.
Figure 3. NF2 isoforms 1 and 2 display distinct localizations within the apical domain.
Wing imaginal discs expressing Flag-tagged NF2Iso1 (A–C) or NF2Iso2 (D–F) under the ApGal4 driver. Images are taken along the dorsal-ventral boundary to include the expression boundary of ApGal4, thereby demonstrating the specificity of anti-Flag staining in these tissues. Comparison to aPKC, a marker for the marginal zone in these cells (A,D), demonstrates that NF2Iso1 (B–C) is expressed in the apical cell cortex but is not closely associated with the marginal zone. In contrast, NF2Iso2 (E–F) is highly concentrated in the marginal zone together with aPKC.
Effect of NF2 expression on endogenous Merlin
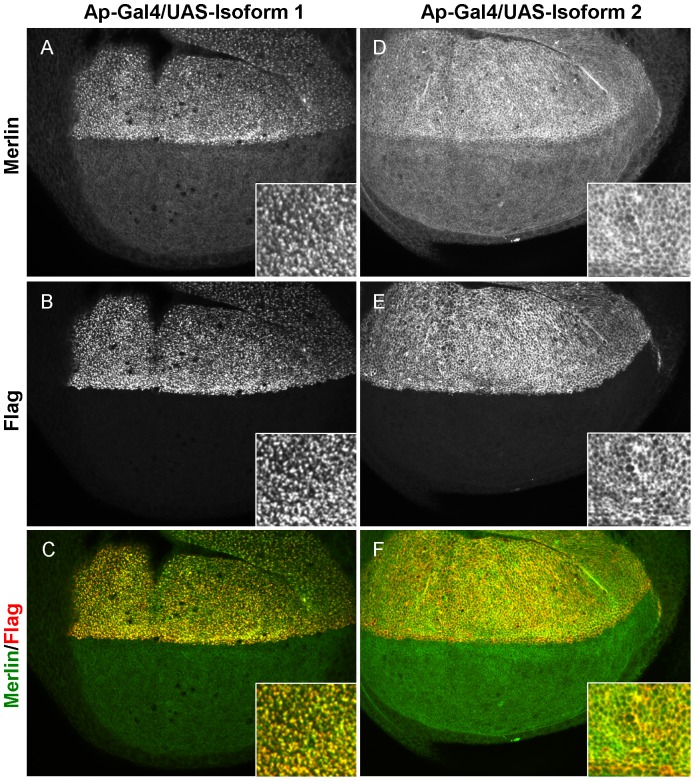
Previous studies of Merlin and the closely related ERM proteins have demonstrated that these proteins display both homotypic and heterotypic intermolecular interactions in vitro [29], [30]. This led us to ask if NF2 expression resulted in alterations in expression or localization of endogenous Drosophila Merlin in these tissues. Expressing NF2 in the dorsal half of the epithelium under the apGal4 driver, allowed us to make side-by-side comparisons of Merlin in cells that did or did not express NF2. Control experiments, in which isoform 1 was expressed in a Mer4 null mutant background, demonstrated that our anti-Drosophila Merlin antiserum (raised against the less conserved carboxyl terminal half of the protein [8]) specifically recognized the fly protein and did not cross react with human Merlin (data not shown). We then used this antibody to compare Merlin localization in cells that express NF2 ectopically to Merlin localization in normal epithelial cells. Expression of NF2Iso1, which strongly rescued null Mer mutations (Table 1), affected both the apparent amount and localization of endogenous Merlin. As shown in Fig. 4, endogenous Merlin staining was much more abundant in cells that express NF2Iso1. In addition, although Drosophila Merlin is normally primarily junctional in imaginal epithelial cells [8], it appeared localized to the apical cell cortex with NF2Iso1 in cells expressing both proteins. This degree of redistribution and colocalization suggests that Drosophila Merlin and NF2Iso1 interact when expressed in these tissues. Imaginal discs expressing NF2Iso2 also showed increased endogenous Merlin staining in the dorsal compartment, though in this case Merlin was predominantly associated with cell boundaries in the apical region of cells, mirroring the difference we observed between NF2Iso1 and NF2Iso2. Consistent with the idea that the effects of NF2 expression on endogenous Merlin were posttranscriptional, quantitative PCR analysis failed to show any detectable effect on Merlin transcription in wing imaginal discs expressing either NF2 isoform under the apGal4 driver (Table 2).
Figure 4. Expression of hNF2 isoforms affects expression and localization of endogenous Drosophila Merlin.
Larval wing imaginal discs are shown stained for hNF2 (A, C) or Drosophila Merlin (B, D) proteins. hNF2 expression is driven specifically in the dorsal half of the wing epithelium by the apterous-Gal4 driver. Images are projections of a series of tangential confocal optical sections, showing protein primarily localized to the apical surface of the epithelium. Expression of isoform 1 (A–B) results in increased Merlin protein levels and redistribution into punctate structures at the apical surface. In contrast, expression of isoform 2, while also causing increased expression of Merlin, does not cause redistribution away from the junctional region. Insets at lower right of each panel show higher magnification views of subcellular localization.
Table 2. qPCR analysis of effect of hNF2 expression on endogenous Merlin expression.
| Experimental replicate | net ΔMerlin1 | |
| ApGal4-2x[NF2Iso1] | ApGal4-2x[NF2Iso2] | |
| 1 | 1.39 | 0.93 |
| 2 | 0.84 | 0.65 |
| 3 | 1.01 | 0.97 |
| Mean | 1.08 | 0.85 |
| SD | 0.28 | 0.18 |
Fold expression change compared to control genotype, ApGal4/+.
Dominant negative effects of NF2Iso2
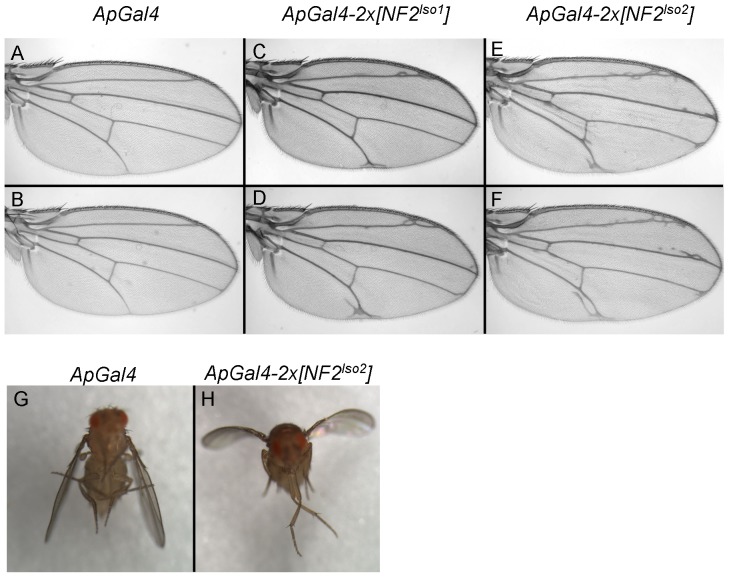
To further test the functions of these NF2 transgenes in developing Drosophila tissues, we examined their adult phenotypes when expressed under the control of the apGal4 driver, which is expressed only in the dorsal half of the wing imaginal disc. Our previous work with Drosophila Merlin showed that overexpression of a Mer+ transgene in the wing has no phenotype [9]. However, overexpression of NF2Iso1 did show some dominant phenotypes in the wing, most typically ectopic vein material along veins II and V (Fig. 5C–D). Expression of NF2Iso2 produced qualitatively similar ectopic wing vein material (Fig. 5E–F). However unlike NF2Iso1, we observed a marked downward ‘cupping’ of the wing blade when NF2Iso2 was expressed in the dorsal half of the wing (Fig. 5G–H). This phenotype is characteristic of overproliferation in the dorsal wing compartment, and has been seen previously with a dominant-negative Merlin transgene expressed by the apGal4 driver [9]. This result suggests that NF2Iso2 dominantly interferes with endogenous Merlin function, consistent with the results from rescue assays (Table 1). In support of this notion, the severity of NF2Iso2 induced wing vein phenotypes was enhanced in Mer4/Mer+ females in comparison to Mer+/Mer+ flies (data not shown).
Figure 5. Ectopic expression of hNF2 isoforms 1 and 2 has different effects on Drosophila wing development.
Two transgenic copies of each isoform were expressed in the dorsal half of the developing wing blade under the control of the apterous-Gal4 driver. The apterous-Gal4 driver itself has little or no effect on wing development (A–B), while expression of isoform 1causes some ectopic vein formation, particularly along the second and fifth wing veins (C–D). Expression of isoform 2 (E–F) causes more severe ectopic vein formation that includes veins 2, 3 and 5. In addition, expression of this isoform causes a downward cupping of the wing blade (data not shown).
The NF2L64P allele is temperature sensitive
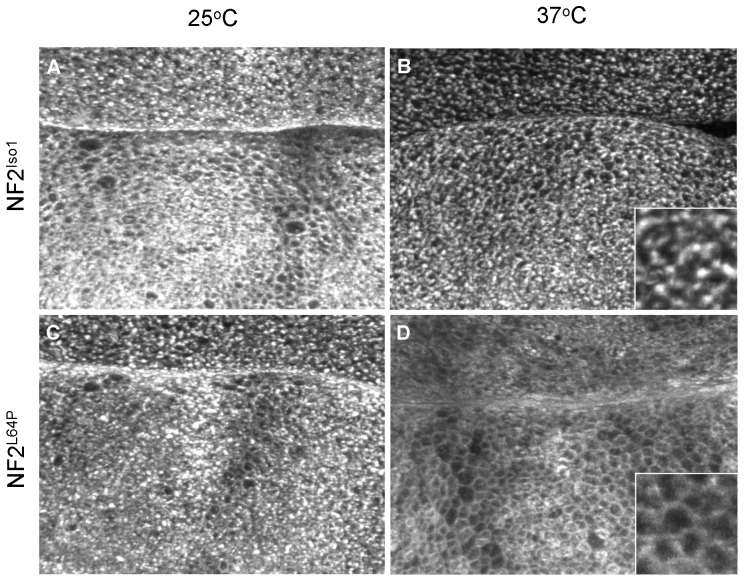
It was surprising to find that the NF2L64P mutation, a patient-derived mutation [24], appeared to function equivalently to wild-type NF2 in the genetic rescue assay (Table 1). This result is particularly unexpected in light of studies that have reported significant differences between wild-type and NF2L64P mutant protein in mammalian cell-based functional assays due to misfolding of the protein [23], [24]. For these reasons, we speculated that NF2L64P might be temperature sensitive and that the differences between observed NF2L64P function in mammalian and Drosophila cells relate to the difference in temperature at which these organisms normally function – our in vivo Drosophila assays are conducted at 25°C while mammalian cells are typically cultured at 37°C. Because flies cannot survive when raised at 37°C, to test this possibility we expressed the NF2L64P transgene in larval wing imaginal epithelial cells, pulsed the animals at 37°C for two hours, and then used immunofluorescence staining to determine the subcellular localization of the expressed protein. Side by side comparison with either NF2L64P expressing animals maintained at 25°C, or with animals expressing NF2Iso1 similarly pulsed at 37°C, showed an obvious mislocalization of the mutant protein at 37°C (Fig. 6). In particular, the punctate apical staining that is characteristic of NF2Iso1 was disrupted when animals expressing the NF2L64P protein were maintained at 37°C for two hours, and instead the mutant protein appeared largely cytoplasmic as observed previously in mammalian cells [31]. Given the correlation between apical, punctate localization and functionality in the rescue assay described above, these results strongly suggest that NF2L64P is functional at 25°C but not at 37°C, and therefore that this allele is temperature sensitive.
Figure 6. The NF2L64P mutation affects subcellular localization in a temperature sensitive fashion.
Subcellular localizations of expressed NF2 isoform 1 (A–B) and the L64P mutation (C–D) are compared in wing imaginal discs kept at 25°C (A,C) or 37°C (B,D) for two hours prior to dissection. While the L64P mutant protein shows normal apical, punctate localization at 25°C (C), it is much more cytoplasmic and evenly distributed at 37°C (D). In contrast, the higher temperature has no apparent effect on subcellular distribution of the wild type isoform 1 protein, which was apical and punctate at both temperatures (A–B). Insets in B and D show higher magnification views of representative areas.
Discussion
Taken together, the results presented here reveal several aspects of NF2/Merlin function relevant to its role in tumor suppression and the evolutionary conservation of its functions. Our in vivo studies in the fly reveal clear differences in genetic function and subcellular localization in epithelial tissues between NF2 isoforms 1 and 2, which differ only in the C-terminal 16 amino acid residues. This is particularly interesting in light of a recent study showing that genetically deleting either isoform alone does not result in lethality or increased tumor formation in mice [22] indicating substantial functional redundancy between the two isoforms. In contrast, in flies isoform 2 is unable to compensate for loss of endogenous Merlin, and appears to dominantly interfere with endogenous Merlin activity in restricting proliferation in the wing epithelium. Thus, our results suggest that isoform 2 could, at least in some contexts, antagonize isoform 1 function in proliferating tissues. This activity could be regulated through interactions with other proteins, consistent with previous studies that have identified isoform-specific NF2 binding partners [22], [32], [33]. Indeed, Schulz et al. [22] demonstrate that isoform 2, but not isoform 1, forms a complex with RhoGDI and promotes RhoA activation in neurons. This function is also unlikely to be recapitulated in fly cells because the phenotypes in the wing we observed from ectopic expression of isoform 2 (Fig. 4) do not resemble those previously described for increased Rho1 function [34].
An interesting implication of these studies relates to the question of how well Merlin function has been conserved evolutionarily. At the protein level, Merlin is 55% identical between flies and mammals [8], and as we have shown here, NF2 can genetically rescue null Merlin mutations in flies. Recent work in flies has strongly implicated Merlin as an upstream regulator of the Hippo pathway, and suggests that all Merlin functions in growth control are mediated through its effects on Hippo signaling. In mammalian cells it is less clear whether all Merlin functions are directly related to the Hippo pathway [17], though it seems that at least some of them are [15]. Whether some of this functional diversity is due to isoform 2, which is unique to mammals, remains to be seen, but its unique properties are consistent with this possibility.
Strikingly, the NF2L64P allele, which is reported to be a strongly inactivating allele in humans [24], displays essentially wild-type genetic rescue and subcellular localization in Drosophila reared at 25°C, suggesting that this missense mutation is temperature sensitive. Consistent with this, we found that while normal at 25°C, localization of NF2L64P was dramatically altered at 37°C. Similar behavior has been seen previously for other disease-causing mutations [35], [36]. If correct, then our model for NF2L64P suggests that approaches based on correcting protein folding defects might be therapeutically effective for treating this disease causing mutation in the NF2 gene. A second disease related allele we examined, NF2K413E, appeared hypomorphic in our in vivo assays, while a previous study in mammalian cells indicated that it severely affects NF2 function [24]. This allele could also be temperature sensitive, though it is also possible that this functional distinction simply reflects the differences in the assays used in the two studies.
Our studies suggest a strong relationship between subcellular localization in polarized epithelial cells and proper NF2 protein function. All NF2 proteins providing genetic rescue in flies, NF2Iso1, NF2K413E, and NF2L64P, localize to the apical cell cortex in the imaginal epithelium. Interestingly, we observed that two dominant negative NF2 proteins, NF2Iso2 and NF2Δ2-3 [25], are predominantly localized to the lateral plasma membrane, as is true for the dominant negative delta-blue-box allele of fly Merlin [9]. Based on these results, and previous studies in mammals that indicate that Merlin functions to regulate both receptor availability [11], [37] and Hippo pathway activity [38], [39] at cell junctions, we infer that normal Merlin function is dependent on its ability to localize, at least transiently, to both the apical membrane and the junctional region, and that Merlin might normally traffic between these membrane domains.
Acknowledgments
We are grateful to members of the Fehon laboratory for comments during the course of this work.
Funding Statement
This work was supported by grants from the National Institutes of Health (NS034783) and the U.S. Army Neurofibromatosis Research Program (DAMD17-97-1-7345) to RGF. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McClatchey AI, Giovannini M (2005) Membrane organization and tumorigenesis—the NF2 tumor suppressor, Merlin. Genes Dev 19: 2265–2277. [DOI] [PubMed] [Google Scholar]
- 2. Trofatter JA, MacCollin MM, Rutter JL, Eldridge R, Kley N, et al. (1993) A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell 72: 791–800. [DOI] [PubMed] [Google Scholar]
- 3. Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, et al. (1993) Alteration in a new gene encoding a putative membrane-organizing protein causes neurofibromatosis type 2. Nature 363: 515–521. [DOI] [PubMed] [Google Scholar]
- 4. Fehon RG, McClatchey AI, Bretscher A (2010) Organizing the cell cortex: the role of ERM proteins. Nat Rev Mol Cell Biol 11: 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bretscher A, Edwards K, Fehon RG (2002) ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol 3: 586–599. [DOI] [PubMed] [Google Scholar]
- 6. McClatchey AI, Fehon RG (2009) Merlin and the ERM proteins—regulators of receptor distribution and signaling at the cell cortex. Trends Cell Biol 19: 198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li W, Cooper J, Karajannis MA, Giancotti FG (2012) Merlin: a tumour suppressor with functions at the cell cortex and in the nucleus. EMBO reports 13: 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCartney BM, Fehon RG (1996) Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophila homologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol 133: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. LaJeunesse DR, McCartney BM, Fehon RG (1998) Structural analysis of Drosophila Merlin reveals functional domains important for growth control and subcellular localization. J Cell Biol 141: 1589–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCartney BM, Kulikauskas RM, LaJeunesse DR, Fehon RG (2000) The Neurofibromatosis-2 homologue, Merlin, and the tumor suppressor expanded function together in Drosophila to regulate cell proliferation and differentiation. Development 127: 1315–1324. [DOI] [PubMed] [Google Scholar]
- 11. Maitra S, Kulikauskas RM, Gavilan H, Fehon RG (2006) The tumor suppressors Merlin and Expanded function cooperatively to modulate receptor endocytosis and signaling. Curr Biol 16: 702–709. [DOI] [PubMed] [Google Scholar]
- 12. LaJeunesse DR, McCartney BM, Fehon RG (2001) A systematic screen for dominant second-site modifiers of Merlin/NF2 phenotypes reveals an interaction with blistered/DSRF and scribbler . Genetics 158: 667–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hamaratoglu F, Willecke M, Kango-Singh M, Nolo R, Hyun E, et al. (2006) The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat Cell Biol 8: 27–36. [DOI] [PubMed] [Google Scholar]
- 14. Yin F, Yu J, Zheng Y, Chen Q, Zhang N, et al. (2013) Spatial Organization of Hippo Signaling at the Plasma Membrane Mediated by the Tumor Suppressor Merlin/NF2. Cell 154: 1342–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang N, Bai H, David KK, Dong J, Zheng Y, et al. (2010) The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev Cell 19: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yi C, Kissil JL (2010) Merlin in organ size control and tumorigenesis: Hippo versus EGFR? Genes Dev 24: 1673–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Benhamouche S, Curto M, Saotome I, Gladden AB, Liu CH, et al. (2010) Nf2/Merlin controls progenitor homeostasis and tumorigenesis in the liver. Genes Dev 24: 1718–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rebay I, Fehon RG, Artavanis-Tsakonas S (1993) Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell 74: 319–329. [DOI] [PubMed] [Google Scholar]
- 19. Neisch AL, Formstecher E, Fehon RG (2013) Conundrum, an ARHGAP18 orthologue, regulates RhoA and proliferation through interactions with Moesin. Mol Biol Cell 24: 1420–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hara T, Bianchi AB, Seizinger BR, Kley N (1994) Molecular cloning and characterization of alternatively spliced transcripts of the mouse neurofibromatosis 2 gene. Cancer Res 54: 330–335. [PubMed] [Google Scholar]
- 21. Wilder EL, Perrimon N (1995) Dual functions of wingless in the Drosophila leg imaginal disc. Development 121: 477–488. [DOI] [PubMed] [Google Scholar]
- 22. Schulz A, Baader SL, Niwa-Kawakita M, Jung MJ, Bauer R, et al. (2013) Merlin isoform 2 in neurofibromatosis type 2-associated polyneuropathy. Nat Neurosci 16: 426–433. [DOI] [PubMed] [Google Scholar]
- 23. Gutmann DH, Sherman L, Seftor L, Haipek C, Hoang Lu K, et al. (1999) Increased expression of the NF2 tumor suppressor gene product, merlin, impairs cell motility, adhesion and spreading. Hum Mol Genet 8: 267–275. [DOI] [PubMed] [Google Scholar]
- 24. Gutmann DH, Geist RT, Xu H, Kim JS, Saporito-Irwin S (1998) Defects in neurofibromatosis 2 protein function can arise at multiple levels. Hum Mol Genet 7: 335–345. [DOI] [PubMed] [Google Scholar]
- 25. Giovannini M, Robanus-Maandag E, Niwa-Kawakita M, van der Valk M, Woodruff JM, et al. (1999) Schwann cell hyperplasia and tumors in transgenic mice expressing a naturally occurring mutant NF2 protein. Genes Dev 13: 978–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hughes SC, Fehon RG (2006) Phosphorylation and activity of the tumor suppressor Merlin and the ERM protein Moesin are coordinately regulated by the Slik kinase. J Cell Biol 175: 305–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tepass U (2012) The apical polarity protein network in Drosophila epithelial cells: regulation of polarity, junctions, morphogenesis, cell growth, and survival. Annual review of cell and developmental biology 28: 655–685. [DOI] [PubMed] [Google Scholar]
- 28. Guilgur LG, Prudencio P, Ferreira T, Pimenta-Marques AR, Martinho RG (2012) Drosophila aPKC is required for mitotic spindle orientation during symmetric division of epithelial cells. Development 139: 503–513. [DOI] [PubMed] [Google Scholar]
- 29. Grônholm M, Sainio M, Zhao F, Heiska L, Vaheri A, et al. (1999) Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin. J Cell Sci 112: 895–904. [DOI] [PubMed] [Google Scholar]
- 30. Nguyen R, Reczek D, Bretscher A (2001) Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J Biol Chem 276: 7621–7629. [DOI] [PubMed] [Google Scholar]
- 31. Sher I, Hanemann CO, Karplus PA, Bretscher A (2012) The tumor suppressor merlin controls growth in its open state, and phosphorylation converts it to a less-active more-closed state. Dev Cell 22: 703–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Scoles DR, Huynh DP, Morcos PA, Coulsell ER, Robinson NG, et al. (1998) Neurofibromatosis 2 tumour suppressor schwannomin interacts with betaII- spectrin. Nat Genet 18: 354–359. [DOI] [PubMed] [Google Scholar]
- 33. Goutebroze L, Brault E, Muchardt C, Camonis J, Thomas G (2000) Cloning and characterization of SCHIP-1, a novel protein interacting specifically with spliced isoforms and naturally occurring mutant NF2 proteins. Mol Cell Biol 20: 1699–1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Denholm B, Brown S, Ray RP, Ruiz-Gomez M, Skaer H, et al. (2005) crossveinless-c is a RhoGAP required for actin reorganisation during morphogenesis. Development 132: 2389–2400. [DOI] [PubMed] [Google Scholar]
- 35. Yang K, Fang K, Fromondi L, Chan KW (2005) Low temperature completely rescues the function of two misfolded K ATP channel disease-mutants. FEBS Lett 579: 4113–4118. [DOI] [PubMed] [Google Scholar]
- 36. McClatchey AI, Van den Bergh P, Pericak-Vance MA, Raskind W, Verellen C, et al. (1992) Temperature-sensitive mutations in the III-IV cytoplasmic loop region of the skeletal muscle sodium channel gene in paramyotonia congenita. Cell 68: 769–774. [DOI] [PubMed] [Google Scholar]
- 37. Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI (2007) Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol 177: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cockburn K, Biechele S, Garner J, Rossant J (2013) The Hippo pathway member Nf2 is required for inner cell mass specification. Curr Biol 23: 1195–1201. [DOI] [PubMed] [Google Scholar]
- 39. Hirate Y, Hirahara S, Inoue K, Suzuki A, Alarcon VB, et al. (2013) Polarity-dependent distribution of angiomotin localizes hippo signaling in preimplantation embryos. Curr Biol 23: 1181–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]