Abstract
BCL6 is a transcriptional repressor that is over-expressed due to chromosomal translocations, or other abnormalities, in ∼40% of diffuse large B-cell lymphoma. BCL6 interacts with co-repressor, SMRT, and this is essential for its role in lymphomas. Peptide or small molecule inhibitors, which prevent the association of SMRT with BCL6, inhibit transcriptional repression and cause apoptosis of lymphoma cells in vitro and in vivo. In order to discover compounds, which have the potential to be developed into BCL6 inhibitors, we screened a natural product library. The ansamycin antibiotic, rifamycin SV, inhibited BCL6 transcriptional repression and NMR spectroscopy confirmed a direct interaction between rifamycin SV and BCL6. To further determine the characteristics of compounds binding to BCL6-POZ we analyzed four other members of this family and showed that rifabutin, bound most strongly. An X-ray crystal structure of the rifabutin-BCL6 complex revealed that rifabutin occupies a partly non-polar pocket making interactions with tyrosine58, asparagine21 and arginine24 of the BCL6-POZ domain. Importantly these residues are also important for the interaction of BLC6 with SMRT. This work demonstrates a unique approach to developing a structure activity relationship for a compound that will form the basis of a therapeutically useful BCL6 inhibitor.
Introduction
BCL6 is a transcriptional repressor [1] that accomplishes its effects by binding to DNA through carboxy-terminal zinc fingers and recruitment of co-repressors to its mid-portion and amino-terminus (Figure 1A). Co-repressors NCoR (NCOR1), BCoR (BCOR) and SMRT (NCOR2), which are components of multi-protein complexes that include histone deacetylases, associate with the amino-terminal POZ domain [2]–[4]. SMRT and NCoR share an amino acid sequence (GRSIHEIPR) that is required for binding to the BCL6-POZ domain and is functionally important [5] but in contrast BCoR binding is by means of a different primary sequence (APSSWVVPG) [6]. The binding of co-repressor, SMRT, to the BCL6-POZ domain has been shown to be required for BCL6 function in B-cells, although it may be dispensable for its function in T-cells [7]. SMRT is a scaffold protein that mediates the recruitment of the HDAC3 repression complex to BCL6 and other repressive transcription factors [8].
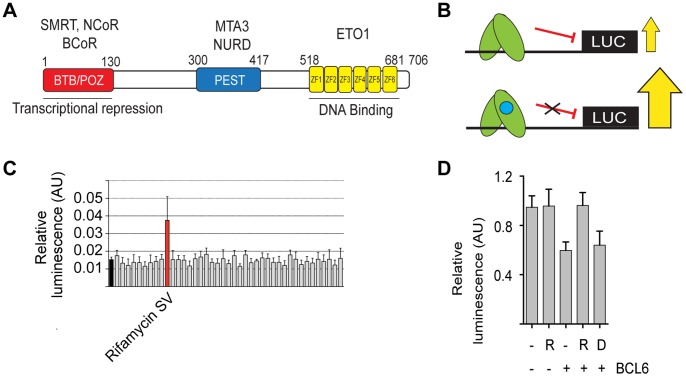
Figure 1. A natural product screen to identify novel inhibitors of BCL6 transcriptional repression.
(A) Schematic of BCL6 showing amino-terminal POZ domain (red), carboxy terminal zinc fingers (yellow) and mid portion containing PEST domains (blue). Different proteins associate with the three portions of BCL6. NCoR, BCoR and SMRT associate with the POZ domain, MTA3 and NuRD with the mid portion and ETO1 with the zinc fingers. (B) Illustration of the screening strategy. BCL6 (green) is shown associating with its binding site cloned upstream of a luciferase reporter gene. Without any compound, or with an inactive compound i.e. one that does not bind BCL6, luciferase output is repressed but in the presence of active compound BCL6 mediated repression is prevented and output of luciferase increases. (C) Screening results for half a plate (40 compounds) from the natural product library. The black bar (furthest left) is the mean negative control i.e. transfected cells without test compound, and the black horizontal line the mean value across the entire screen. The red bar shows rifamycin SV. (D) The effect of rifamycin is due to inhibition of BCL6 transcriptional repression. HEK293T cells were co-transfected with a BCL6 expression construct and a luciferase reporter. Transcriptional repression due to BCL6 was relieved by rifamycin SV (R), but not by an agent that was ineffective in the screen (D).
BCL6 is expressed in normal germinal center B-cells [9] and is essential for high affinity antibody formation [10], [11]. At a cellular level its role may be to allow proliferation and inhibit differentiation to plasma cells [12]. It has been demonstrated that BCL6 promotes the proliferation of primary tonsillar B-cells [13] and prevents terminal differentiation to plasma cells in B-cell lines [12], [14].
BCL6 is involved in chromosomal translocations in ∼25% of all cases of diffuse large B-cell lymphoma (DLBCL) [15] and is, therefore, likely to have a major role in driving lymphomagenesis. This is supported by the finding that mice with constitutive B-cell expression of BCL6 develop lymphomas similar to human DLBCL [16]. Gene expression profiling has been utilized to subtype DLBCL into groups with differing clinical outcomes [17], [18]. The majority of cases with BCL6 translocations are associated with poor prognosis activated B-cell (ABC) DLBCL [19] as defined by the “cell of origin” classification [18], [20]. Other mechanisms causing constitutive expression of BCL6 have been described; mutations disrupting a negative regulatory site in the promoter region of the BCL6 gene occur in 10 to 15% of DLBCL [21], [22] and disruption of normal post-translational regulation of BCL6 by various mechanisms have also been reported and are likely to contribute to deregulated expression [23]–[25]. Overall BCL6 is an important oncogene in DLBCL but it is also expressed from an un-rearranged locus in follicular lymphoma, Burkitt's lymphoma and nodular lymphocyte predominant Hodgkin's lymphoma. Although its role has not been investigated in detail in these diseases it is also likely to contribute to cellular proliferation and survival.
A peptide corresponding to the region of SMRT interacting with the BCL6-POZ domain has been demonstrated to be functionally active in vitro and in vivo [26], [27]. The peptide prevents normal germinal center formation in mice and when administered to BCL6 dependent cell lines or primary lymphoma cells causes apoptosis. A combination of computer assisted drug design and screens of small molecule libraries led to the identification of a compound, 79–6, that binds in the SMRT binding groove in the BCL6 POZ domain [28]. 79–6 is also functionally active in vivo and causes apoptosis of BCL6 dependent lymphoma cell lines. However, there are on-going efforts to carry out further small molecule library screens for BCL6 inhibitors.
Here we report the identification of a direct interaction between the BCL6 POZ domain and members of the ansamycin antibiotic family: rifamycin SV and rifabutin. This represents a novel non-bactericidal activity of the rifamycin family of antibiotics. Rifabutin was found to cause the largest chemical shift perturbations by NMR and a crystal structure of BCL6 in complex with rifabutin reveals new insights into the structure activity relationships required for potential therapeutic agents to disrupt the SMRT/BCL6 interaction.
Materials and Methods
Luciferase reporter screening assay
A BCL6 reporter construct as previously described [12] was transfected into DG75 an EBV negative Burkitt's lymphoma cell line utilising Nucleofector program O-006 (Lonza Group Ltd, Basel, Switzerland). A natural product library (TimTec, Newark, DE, USA) was purchased unsolvated and solvated to a concentration of 10 mM with DMSO. After each compound was effectively solvated, 5 μl was added to the corresponding wells in a daughter plate and diluted with 95 μl of sterile water. From the daughter plate, 3 µl of compound was pipetted into 22 μl of complete RPMI 1640 in quadruplicate into each different assay plate. The compounds were diluted into each assay plate at a concentration of 20 µM. One batch of DG-75 cells was transfected with BCL6 reporter construct with a standard amount of a construct expressing Renilla luciferase as a transfection control. The transfected cells were then incubated at 37°C for 16 to 20 hours. The assay plates were centrifuged and 50 µl of the transfected cells were pipetted into the designated wells on the assay plate. The transfected cells were incubated for 12 hours with the compounds before harvesting and determination of luciferase activity.
HEK293T cells were seeded at 2×104 cells/well in 96-well plates and following 24 hours in culture were co-transfected with a BCL6 reporter vector (100 ng), Renilla luciferase control vector (100 ng) and a full-length BCL6 expression plasmid (200 ng) using polyethylenimine (PEI) (Sigma, St. Louis, MO, USA). Compound (5 µM) was added and cells were lysed, harvested and luciferase activity determined after 24 hours. Each condition was carried out in triplicate.
Protein expression and purification
DNA encoding the POZ domain (residues 7 to 128) of human BCL6 (Figure 1A), with cysteine8 mutated to glutamine, cysteine67 mutated to arginine and cysteine84 mutated to asparagine, was cloned into a vector containing a 58-amino acid GB1 solubility enhancement tag, a 6× histidine affinity tag and a TEV cleavage site (PROTEX, University of Leicester; (http://www2.le.ac.uk/department/biochemistry/research-groups/protex)). Constructs were expressed in the E.coli strain Rosetta (DE3) (Novagen, Merck Chemicals Ltd., Beeston, UK) (Figures S1A and S1B). For preparation of 15N-labelled samples bacteria were cultured 2M9 minimal media containing 1 g of 15N-ammonium chloride per liter. For crystallisation and fluorescence polarisation E. coli were cultured in 2xYT medium. Bacteria were cultured at 37°C BCL6-POZ was purified using Ni-NTA resin and subsequent buffer exchange into 50 mM sodium phosphate pH 6, 300 mM NaCl, 5 mM DTT. Following TEV cleavage overnight at 4°C the sample was further purified by gel filtration using a Superdex S200 column (GE Healthcare, Amersham, UK). Protein concentrations were measured using Bio-Rad Protein Assay (Bio-Rad, Hercules, CA, USA).
Peptide Synthesis and Fluorescence Polarization
Fmoc-protected amino acids were purchased from Novabiochem (Merck Chemicals Ltd, Nottingham, UK) or PolyPeptide Group (Strasbourg, France) (Fmoc-homophenylalanine, Fmoc-Styrylalanine, Fmoc-1-naphthylalanine & Fmoc-2-naphthylalanine) and were used as received. Peptides were synthesized on a CEM Liberty 1 automated microwave-assisted solid-phase peptide synthesizer (CEM Corporation, Buckingham, UK) using a 30 mL Teflon reactor vessel on 0.05 mmol scale using Fmoc-Arg(Pbf)-Wang resin (100–200 mesh) (substitution: 0.63 mmol/g). Peptide solutions were made in PBS containing 1 mM tris-(2-carboxyethylphosphine) and then coupled via the amino-terminal cysteine to the thiol-reactive BODIPY TMR dye (Invitrogen, Paisley, UK) in accordance with manufactures instructions. Unreacted dye was removed by gel filtration using a PD-10 column (GE Healthcare). Fluorescence polarization experiments were performed in a black 96 well assay plate (Corning, Amsterdam, The Netherlands). Titrations were performed using a fixed concentration of SMRT peptide, with increasing concentration of the BCL6-POZ domain protein, in a final volume of 100 μl of assay buffer (PBS, 0.05% (v/v) Triton X-100, 0.1 mg/mL BSA). The plate was mixed by shaking for 1 min and measurements were then taken using a Victor X5 plate reader (Perkin Elmer, Waltham, MA, USA) at room temperature with an excitation wavelength of 531 nm and an emission wavelength of 595 nm. Experiments were performed in triplicate and data were analysed using GraphPad Prism (version 6.0, GraphPad Software, Inc., San Diego, CA, USA). Kd values were calculated by nonlinear curve fitting using a one-site binding (hyperbola).
NMR spectroscopy
All NMR experiments were performed at 303 K using Bruker AVANCE DRX 600 or AVANCE AVII 800 spectrometers both equipped with CryoProbes. Titrations were carried out using 280 μM BCL6-POZ in 50 mM sodium phosphate pH 6, 300 mM NaCl, 5 mM DTT, 5% v/v D2O. Compounds were resuspended in deuterated DMSO (DMSO-d6). 2D 1H15N heteronuclear single-quantum correlation (HSQC) spectra were acquired with transverse relaxation optimization (TROSY) [29] using 32 scans and 92 increments. 1H15N HSQC spectra were collected on BCL6-POZ alone and then with increasing amount of compound. Data were analyzed using CCPN Analysis [30].
Crystallization and X-ray structure determination
Crystals of the BCL6-POZ domain were obtained using the sitting drop vapor diffusion method at room temperature (Figure S1) BCL6-POZ was concentrated to 3.8 mg/ml and crystallised in the presence of rifabutin at a ratio of 1∶8. In detail 1 μl of BCL6-POZ in 50 mM sodium phosphate pH 6, 300 mM NaCl, 5 mM DTT (in the presence or absence of rifabutin) was mixed with 1 μl reservoir solution (20% PEG 6000, 100 mM sodium citrate, pH 5). Crystals grew in the space group P1 21 1. Data were collected to 2.3 Å on the microfocus beam line I24 at the Diamond Light Source, Didcot, Oxfordshire. Data were processed and integrated using XDS, iMosflm, Pointless and Aimless [31], [32]. The structure was solved using molecular replacement using Phaser [33] and the BCL6-POZ domain from the BCL6/SMRT structure (1R2B, [34]). Model fitting and refinement were performed using Coot and Refmac [35], [36]. Statistics of the refinement are presented in Table 1. The Rfree remained higher than expected probably due to the small size of the crystals and slightly streaky nature of the diffraction.
Table 1. Data collection and refinement statistics (Molecular replacement).
| BCL6/Rifabutin | |
| Data Collection | |
| Space Group | P 1 21 1 |
| Cell dimensions | |
| a,b,c (Å) | 35.17, 54.83, 58.16 |
| α,β,γ (°) | 90, 95.21, 90 |
| Resolution (Å) | 39.82–2.3 (2.38–2.3) |
| Rmerge | 10.8 (51.8) |
| I/σI | 9.8 (4.1) |
| Completeness (%) | 97.13 (97) |
| Redundancy | 3.0 (2.9) |
| Refinement | |
| Resolution (Å) | 2.3 |
| No. reflections | 9168 |
| Rwork/Rfree | 20.2/26.9 |
| No. Atoms | 2053 |
| Protein | 1969 |
| Ligand/ion | 61 |
| Water | 23 |
| B-factors | |
| Protein | 27.9 |
| Rifabutin | 48 |
| Water | 24.6 |
| R.M.S. deviations | |
| Bond lengths (Å) | 0.013 |
| Bond angles (°) | 1.885 |
*Highest resolution shell is shown in parenthesis.
Accession numbers
Coordinates and structure factors for the BCL6-POZ domain (residues 7–128) – Rifabutin complex have been deposited in the Protein Data Bank (ID code 4CP3).
Results
Natural Product Screen for inhibitors of the BCL6-SMRT interaction
Natural products form the basis for many drugs in clinical use. Despite their chemical complexity, they have other properties such as cell permeability and relatively high bioavailability that make them attractive starting materials for screens in drug discovery projects. We screened a commercial natural product library consisting of 480 compounds for ability to prevent BCL6 induced transcriptional repression in the Burkitt's lymphoma cell line DG75 (Figure 1B). Nine compounds modified transcriptional activity of a reporter construct bearing BCL6 binding sites in DG75 (3 compounds repressing and 6 compounds enhancing luciferase activity) (Figure 1C and Figures S2 and S3). However, the BCL6 DNA binding sequence shares sequence similarities to that of the STAT family of transcription factors [10] and our results might reflect inhibition of transcription factors other than BCL6. Therefore, to show directly that BCL6 transcription was inhibited we co-transfected a BCL6 expression construct and a luciferase reporter into HEK293T cells that do not express endogenous BCL6. Luciferase expression was repressed by BCL6 and this was relieved by the addition of rifamycin SV (labelled R) (Figure 1D), which was detected in the initial library screen (Figure 1C), but not by the other eight compounds.
Rifamycin SV directly interacts with BCL6-POZ
In order to determine whether a direct interaction with BCL6 in solution was responsible for the observed effects on transcription, we utilized an NMR chemical shift perturbation assay. Work by others has demonstrated that the important interactions of BCL6 with co-repressors occur through the amino-terminal POZ domain, which also mediates homodimerisation [37]. The TROSY 1H15N TROSY-HSQC NMR spectrum of the BCL6-POZ homodimer showed good dispersion and uniform line widths indicative of a stable well-folded protein (Figure S4). Addition of rifamycin SV (Figure 2A), resulted in a subset of peaks shifting in a concentration dependent manner (Figure 2B) supporting the hypothesis that rifamycin interacts directly with the BCL6-POZ domain. However, even at large excesses of rifamycin SV the chemical shift perturbations were relatively small and chemical shift changes continued to be detectable at high concentrations demonstrating that binding had still not reached saturation.
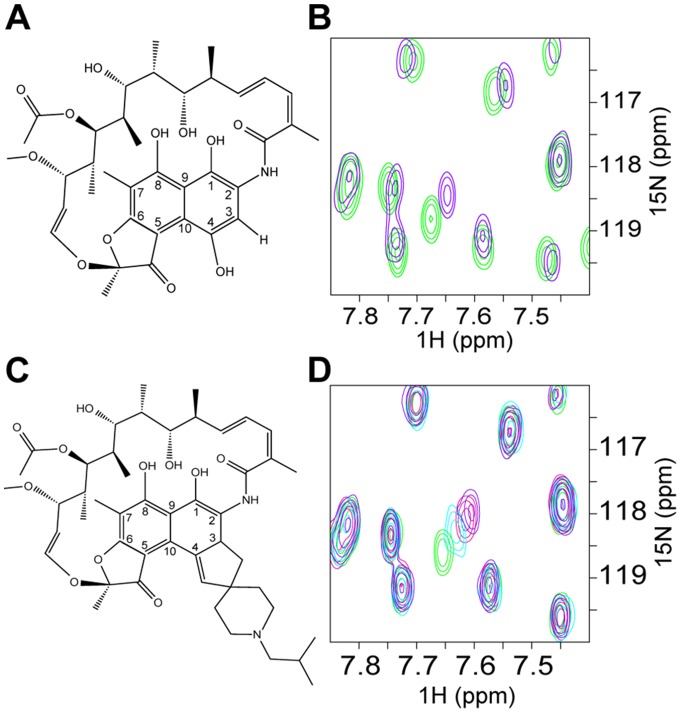
Figure 2. Rifamycin SV and its derivative, rifabutin, bind directly to the BCL6-POZ domain.
Schematic diagram to compare the structures of (A) rifamycin SV and (C) rifabutin. These two compounds differ with respect to the side chains on C-3 and C-4. (B) and (D) TROSY 1H,15N HSQC spectra of 280 μM BCL6-POZ domain. (B) Chemical shift changes due to rifamycin SV. An overlay of the spectra of BCL6-POZ domain alone (green) and in the presence of a 16∶1 molar ratio of rifamycin (purple). (D) Chemical shift changes due to rifabutin with an overlay of the spectra of BCL6-POZ domain alone (green) and in the presence of 4∶1 (light blue), 8∶1 (red) and 16∶1 (purple) molar ratios of rifabutin.
Screening of rifamycin derivatives
Rifamycin SV belongs to a family of ansamycin antibiotics, which have related structures, comprising a napthoquinone ring bridged by an aliphatic chain (Figure 2A and 2C). In order to determine whether other members of the family also bound to the BCL6-POZ domain we analysed the commercially available compounds rifabutin, rifapentine, rifampicin and rifaximin as well as 3-formyl rifamycin, for interaction with the BCL6-POZ domain.
These derivatives all caused spectral changes of varying magnitude with the greatest shifts caused by rifabutin (Figure 2C and 2D). The estimated order of binding from weakest to strongest was: rifaximin, rifapentine, 3-formyl rifamycin, rifampicin, rifamycin SV and rifabutin. Comparison of the BCL6-POZ domain spectra on addition of rifamycin SV and rifabutin (Figures 2B and 2D) showed small differences in the observed shifts of some peaks suggesting minor differences in binding in solution. By plotting the chemical shift change (δΔ) of the most shifted peak, as a function of rifabutin concentration it was possible to estimate the Kd of the interaction as being in the order of ∼1 mM.
Structure of the BCL6-POZ- Rifabutin complex
To explore further the atomic details of the interaction we crystallized the BCL6-POZ domain in complex with rifabutin. Complex crystals were readily obtained and easily identified due to the purple colour of rifabutin (Figure S1C). Molecular replacement with the BCL6-POZ domain (1R2B, [34]) as search molecule produced a clear electron density map with extra density readily identifiable for rifabutin (Figure 3, Table 1). Despite the BCL6-POZ domain being a symmetrical dimer [2], [3] only one molecule of rifabutin is present per dimer. The rifabutin is located at the dimer interface and binds to the surface that overlaps the surface bound by the SMRT and NCoR peptides [2], [34]. The napthoquinone ring of rifabutin occupies the pocket, which is occupied by the residues histidine1426 of SMRT, histidine1352 of NCoR, or tryptophan509 of BCOR, in the three POZ domain co-repressor structures [6]. The most important interaction appears to be a π-stacking interaction with the aromatic ring of tyrosine58 of the BCL6-POZ domain. The aliphatic “handle” or macrocycle of rifabutin makes electrostatic interactions with asparagine21 and arginine24 (Figure 3).
Figure 3. Crystal structure of rifabutin and BCL6-POZ domain.
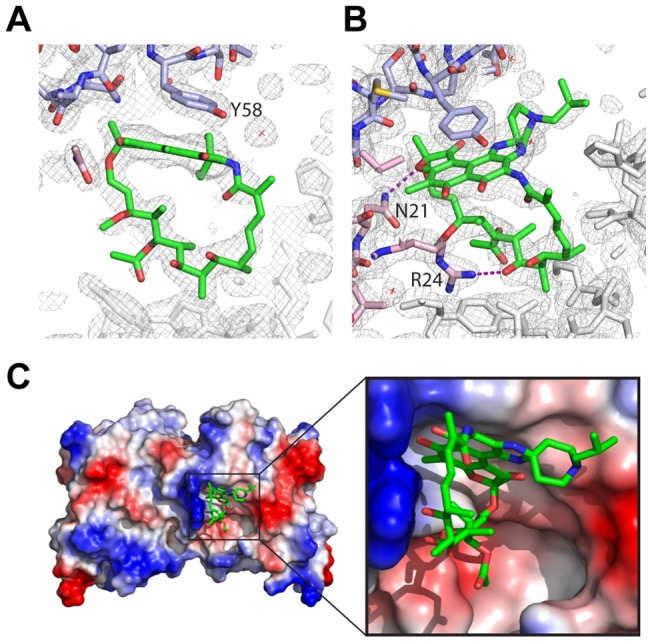
Electron density corresponding to rifabutin, following refinement, in the context of surrounding electron density demonstrating the proximity of (A) tyrosine58 from one monomer of the POZ dimer and (B) asparagine21 and arginine24 from the other monomer. (C) Surface representation of BCL6-POZ with basic residues (including asparagine21 and arginine24) in blue and acidic residues in red. The napthoquinone rings of rifabutin are in proximity to tyrosine58 whilst the aliphatic bridge is adjacent to the basic surface.
A small molecule, 79–6, has been described previously and has been observed to bind in the same pocket at the BCL6:SMRT interface [28]. Comparison of the binding of SMRT peptide, 79–6 and rifabutin demonstrates remarkable similarities (Figure 4). Specifically apolar interactions with tyrosine58 and electrostatic interactions with asparagine21 and arginine24 are involved in the binding of all three molecules.
Figure 4. BCL6-POZ domain in complex with rifabutin, 79–6 and the SMRT peptide.
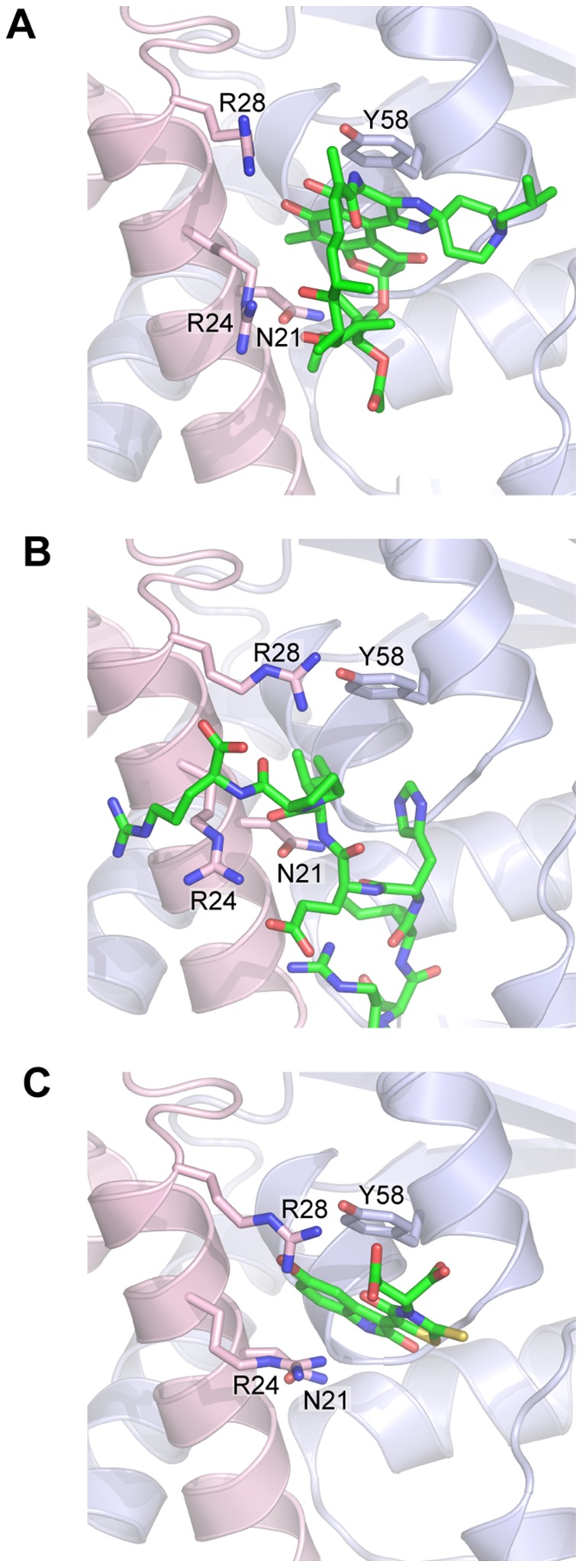
A section of the BCL6-POZ domain structure is shown binding; (A) rifabutin, (B) the SMRT peptide and (C) 79–6.
SMRT peptide containing artificial amino acids to explore binding to BCL6-POZ domain
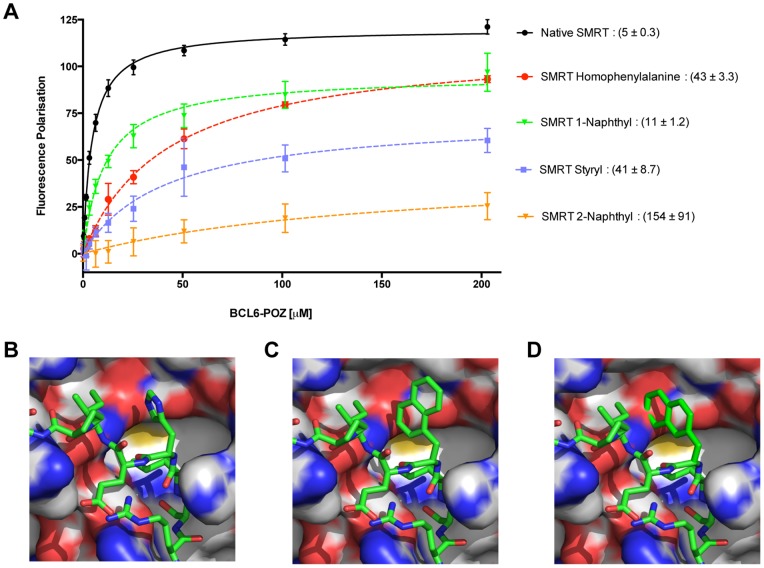
In order to explore the importance of histidine1426 of SMRT or histidine1352 of NCoR for binding to the pocket in the BCL6-POZ domain, which is also occupied by rifabutin and 79–6 we synthesised SMRT/NCoR peptides with artificial amino acids replacing the histidine (Figure S5). By fluorescence polarisation the Kd of binding of the BCL6-POZ domain to labelled wild-type SMRT peptide was determined to be 5 µM. All the artificial amino acids that were employed in the study showed reduced binding, as compared to wild-type peptide, but there were considerable differences in affinity. Whilst the peptide bearing a 1-naphthyl residue had a binding affinity of 11 µM the 2-naphthyl peptide had a much lower affinity of 154 µM (Figure 5A). Homophenylalanine and styryl derivatives had intermediate affinities. Modelling of these artificial amino acids, such that they have the same orientation as tryptophan509 of BCOR and histidine1426 of SMRT [6], suggested explanations for this data (Figure 5B, 5C and 5D). Whilst 1-naphthyl is oriented within the pocket, 2-naphthyl clashes with the BCL6-POZ domain, which is likely to prevent significant binding. Homophenylalanine and styryl side chains were employed to place aromatic rings, potentially capable of interacting with tyrosine58 closer to the BCL6-POZ domain than is histidine1426 in the wild-type structure. Binding affinity was again reduced demonstrating the stringent requirements for compounds that are to be lead molecules as BCL6 inhibitors.
Figure 5. Binding requirements of the SMRT peptide explored utilising artificial amino acids to replace histidine.
(A) Binding curves obtained by fluorescence polarisation for wild-type peptide and 1-napthyl, 2-napthyl, styryl and homophenylalanine substitutions of histidine1426 of SMRT. The Kd in µM (mean±SEM) is presented to the right of the compound name. (B) Structure of wild-type SMRT peptide bound to the BCL6-POZ domain. Molecular modelling of (C) 1-naphthyl SMRT peptide and (D) 2-naphthyl SMRT peptide.
Discussion
There is interest in developing BCL6 inhibitors because this transcription factor is required for proliferation and survival of several types of non-Hodgkin's lymphoma and nodular lymphocyte predominant Hodgkin's lymphoma and proof of principle studies have demonstrated the efficacy of inhibiting BCL6 in diffuse large B-cell lymphoma [27], [28]. The rifamycins and especially rifabutin are attractive starting materials for producing a clinically useful BCL6 inhibitor because of their high lipid solubility, extensive tissue penetrance and long half-life [38].
We have demonstrated that rifamycin SV and rifabutin, members of the ansamycin antibiotic family, that have clinical uses in the prevention or treatment of bacterial infections due to binding and inhibition of bacterial DNA-dependent RNA polymerase, are able to bind the BCL6-POZ domain.
This result is functionally significant as demonstrated by inhibition of BCL6 transcriptional repression by rifamycin SV. The luciferase reporter assay we employed produced 9 “hits” of which only rifamycin SV bound to BCL6. Three of the compounds appeared to enhance transcriptional repression i.e. reduced luciferase production, and for one of these compounds (Figure S2A) the explanation may be that it is a known inhibitor of firefly luciferase (Figure S3B) [39].
Rifamycin SV is derived from rifamycin B (the natural fermentation product of Streptomyces mediterranei) by removal of the glycolic group bound to C-4. The rifamycins are potent inhibitors of bacterial DNA dependent RNA polymerase [40] and are utilised in the treatment of tuberculosis. Another property of these compounds, their high lipid solubility, may help to penetrate the bacterial wall. Structurally, the rifamycins consist of a naphthoquinonic chromophore, which is spanned by an aliphatic bridge between the nitrogen on C-2 and the oxygen on C-12 of the napthoquinone moiety (Figure 2A and 2C). The members of the family differ primarily in the side chains on C-3 and C-4. We demonstrate that rifamycins bind with different strengths to the BCL6 POZ domain. These agents largely differ in the side chain attachments to C-3 and C-4, which do not appear to make significant interactions with the protein in our crystal structure. One possibility is that the side-chains alter the rigidity of the molecule to alter the fit in the lateral groove of the POZ domain dimer. Supporting the view that the C-3/C-4 side chains are important in modulating function, others have shown that rifamycin SV can inhibit amyloid fibril formation through disruption of interactions between fibril aromatic rings that are required for elongation whereas rifaximin does not have this effect [41].
SMRT and NCoR occupy a binding pocket that is present in the apo i.e. unliganded, form of BCL6. The co-repressor, BCOR, associates with BCL6 through interactions with the lateral groove as do SMRT and NCoR. Whilst SMRT and NCoR bind to the BCL6-POZ domain through identical sequences, GRSIHEIPR (residues 1422 to 1430 of SMRT and residues 1348 to 1356 of NCoR), there is no similarity to the BCL6 binding sequence of BCoR, APSSWVVPG. However, binding of peptides derived from SMRT and BCoR have been investigated in detail [6]. BCOR tryptophan509 and SMRT histidine1426 make similar contacts on the BCL6-POZ domain, namely with residues methionine51, cysteine53, serine54, glycine55, asparagine21, arginine24 and arginine28 [6]. The small molecule, 79–6, utilizes a subset of the interactions employed by the SMRT peptide for binding to the BCL6 POZ domain. Our X-ray crystallographic studies show that rifabutin occupies the pocket utilised by histidine in the SMRT and NCoR sequences and tryptophan in BCoR. The binding of rifabutin to the BCL6 POZ domain also appears very similar to that of the small molecule, 79–6, [28]. Collectively it seems likely that rifamycin SV and rifabutin can occupy a druggable pocket in the BCL6-POZ domain.
Macrocyclic compounds, of which rifamycin SV and rifabutin are examples, may have utility in perturbing protein-protein interactions and are also highly membrane permeable. We suggest that the rifamycins will be interesting compounds from which to develop BCL6 inhibitors.
Supporting Information
Protein purification and crystallisation of BCL6. (A) Coomassie stained polyacrylamide gel showing production of BCL6-POZ domain with a GB1 tag and TEV cleavage of the tag. (B) BCL6-POZ domain purified away from the GB1 tag by size exclusion gel filtration. Coomassie stained polyacrylamide gel showing fractions collected. (C) BCL6-POZ crystal mounted in a loop at the Diamond Synchrotron beamline I24 (Red box 25.9 μm2). The crystals are faintly lilac having taken up the coloured rifabutin.
(TIF)
Natural product library screening. Results for all 480 compounds are presented. Column to the left in orange is the mean negative control i.e. transfected cells without test compound, and the orange horizontal line the mean value across the entire screen. Compounds considered “its”are represented as red columns and comprise 6 that relieve BCL6 transcriptional repression and 3, which appear to enhance repression.
(TIF)
Structures of the 9 compounds that alter BCL6 transcriptional repression. The most widely used compound name is presented apart from one compound (A), which has no common name and for which the IUPAC nomenclature is stated. The chemical identifier (CID) from PubChem is also presented. (A to C) Three compounds that reduce luciferase activity. (D to I) Compounds that enhance luciferase activity, including (I) Rifamycin SV.
(TIF)
TROSY 1H, 15N HSQC NMR spectrum of the BCL6-POZ domain.
(TIF)
Sequences of SMRT peptides. Sequences of SMRT peptides utilised in fluorescence polarisation experiments together with the structures of the artificial amino acids at the position of histidine1426 in the wild-type peptide. (A) wild-type SMRT, (B) 1-naphthyl-SMRT, (C) 2-naphthyl-SMRT, (D) homophenylalanine-SMRT and (E) styryl-SMRT.
(TIF)
Acknowledgments
We thank the PROTEX facility at the University of Leicester for generating the BCL6 expression construct and the beamline staff at DIAMOND for help with data collection. 3-formyl-rifamycin was a gift from Professor Colin Fishwick, University of Leeds.
Funding Statement
SEE is supported by a University of Leicester PhD Studentship (http://www.le.ac.uk). The work is supported by a Wellcome Trust Programme Grant WT091820 and Senior Investigator Grant WT100237 to JWRS (http://www.wellcome.ac.uk). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Chang CC, Ye BH, Chaganti RS, Dalla-Favera R (1996) BCL-6, a POZ/zinc-finger protein, is a sequence-specific transcriptional repressor. Proc Natl Acad Sci USA 93: 6947–6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Melnick A, Carlile G, Ahmad KF, Kiang C-L, Corcoran C, et al. (2002) Critical residues within the BTB domain of PLZF and Bcl-6 modulate interaction with corepressors. Mol Cell Biol 22: 1804–1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dhordain P, Albagli O, Lin RJ, Ansieau S, Quief S, et al. (1997) Corepressor SMRT binds the BTB/POZ repressing domain of the LAZ3/BCL6 oncoprotein. Proc Natl Acad Sci USA 94: 10762–10767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dhordain P, Lin RJ, Quief S, Lantoine D, Kerckaert JP, et al. (1998) The LAZ3(BCL-6) oncoprotein recruits a SMRT/mSIN3A/histone deacetylase containing complex to mediate transcriptional repression. Nucleic Acids Res 26: 4645–4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barish GD, Yu RT, Karunasiri MS, Becerra D, Kim J, et al. (2012) The Bcl6-SMRT/NCoR Cistrome Represses Inflammation to Attenuate Atherosclerosis. Cell Metabolism 15: 554–562 10.1016/j.cmet.2012.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ghetu AF, Corcoran CM, Cerchietti L, Bardwell VJ, Melnick A, et al. (2008) Structure of a BCOR corepressor peptide in complex with the BCL6 BTB domain dimer. Mol Cell 29: 384–391 10.1016/j.molcel.2007.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Hatzi K, Melnick A (2013) Lineage-specific functions of Bcl-6 in immunity and inflammation are mediated by distinct biochemical mechanisms. Nat Immunol: 1–11. doi:10.1038/ni.2543. [DOI] [PMC free article] [PubMed]
- 8. Oberoi J, Fairall L, Watson PJ, Yang J-C, Czimmerer Z, et al. (2011) Structural basis for the assembly of the SMRT/NCoR core transcriptional repression machinery. Nat Struct Mol Biol 18: 177–184 10.1038/nsmb.1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allman D, Jain A, Dent A, Maile RR, Selvaggi T, et al. (1996) BCL-6 expression during B-cell activation. Blood 87: 5257–5268. [PubMed] [Google Scholar]
- 10. Dent AL, Shaffer AL, Yu X, Allman D, Staudt LM (1997) Control of Inflammation, Cytokine Expression, and Germinal Center Formation by BCL-6. Science 276: 589–592 10.1126/science.276.5312.589 [DOI] [PubMed] [Google Scholar]
- 11. Ye BH, Cattoretti G, Shen Q, Zhang J, Hawe N, et al. (1997) The BCL-6 proto-oncogene controls germinal centre formation and Th2-type inflammation. Nat Genet 16: 161–170. [DOI] [PubMed] [Google Scholar]
- 12. Reljic R, Wagner SD, Peakman LJ, Fearon DT (2000) Suppression of signal transducer and activator of transcription 3-dependent B lymphocyte terminal differentiation by BCL-6. J Exp Med 192: 1841–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shvarts A, Brummelkamp TR, Scheeren F, Koh E, Daley GQ, et al. (2002) A senescence rescue screen identifies BCL6 as an inhibitor of anti-proliferative p19(ARF)-p53 signaling. Genes Dev 16: 681–686 10.1101/gad.929302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tunyaplin C, Shaffer AL, Angelin-Duclos CD, Yu X, Staudt LM, et al. (2004) Direct repression of prdm1 by Bcl-6 inhibits plasmacytic differentiation. J Immunol 173: 1158–1165. [DOI] [PubMed] [Google Scholar]
- 15. Offit K, Coco Lo F, Louie DC, Ye BH, Jhanwar SC, et al. (1994) Rearrangement of the bcl-6 gene as a prognostic marker in diffuse large-cell lymphoma. N Engl J Med 331: 74–80 10.1056/NEJM199407143310202 [DOI] [PubMed] [Google Scholar]
- 16. Cattoretti G, Pasqualucci L, Ballon G, Tam W, Nandula SV, et al. (2005) Deregulated BCL6 expression recapitulates the pathogenesis of human diffuse large B cell lymphomas in mice. Cancer Cell 7: 445–455 10.1016/j.ccr.2005.03.037 [DOI] [PubMed] [Google Scholar]
- 17. Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, et al. (2002) Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med 8: 68–74 10.1038/nm0102-68 [DOI] [PubMed] [Google Scholar]
- 18. Alizadeh AA, Eisen MB, Ma C, Lossos IS, Rosenwald A, et al. (2000) Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403: 503–511 10.1038/35000501 [DOI] [PubMed] [Google Scholar]
- 19.Iqbal J, Greiner TC, Dave BJ, Smith L, Sanger WG, et al.. (2007) Distinctive patterns of BCL6 molecular alterations and their functional consequences in different subgroups of diffuse large B-cell lymphoma. Leukemia. doi:10.1038/sj.leu.2404856. [DOI] [PMC free article] [PubMed]
- 20. Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, et al. (2004) Prediction of Survival in Diffuse Large-B-Cell Lymphoma Based on the Expression of Six Genes. N Engl J Med 350: 1828–1837 10.1056/NEJMoa032520 [DOI] [PubMed] [Google Scholar]
- 21. Pasqualucci L, Migliazza A, Basso K, Houldsworth J, Chaganti RSK, et al. (2003) Mutations of the BCL6 proto-oncogene disrupt its negative autoregulation in diffuse large B-cell lymphoma. Blood 101: 2914–2923 10.1182/blood-2002-11-3387 [DOI] [PubMed] [Google Scholar]
- 22. Wang X, Li Z, Naganuma A, Ye BH (2002) Negative autoregulation of BCL-6 is bypassed by genetic alterations in diffuse large B cell lymphomas. Proc Natl Acad Sci USA 99: 15018–15023 10.1073/pnas.232581199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Duan S, Cermak L, Pagan JK, Rossi M, Martinengo C, et al. (2011) FBXO11 targets BCL6 for degradation and is inactivated in diffuse large B-cell lymphomas. Nature 481: 90–93 10.1038/nature10688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cerchietti LC, Hatzi K, Caldas-Lopes E, Yang SN, Figueroa ME, et al. (2010) BCL6 repression of EP300 in human diffuse large B cell lymphoma cells provides a basis for rational combinatorial therapy. J Clin Invest 120: 4569–4582 10.1172/JCI42869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pasqualucci L, Dominguez-Sola D, Chiarenza A, Fabbri G, Grunn A, et al. (2011) Inactivating mutations of acetyltransferase genes in B-cell lymphoma. Nature 471: 189–195 10.1038/nature09730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polo JM, Dell'Oso T, Ranuncolo SM, Cerchietti L, Beck D, et al. (2004) Specific peptide interference reveals BCL6 transcriptional and oncogenic mechanisms in B-cell lymphoma cells. Nat Med 10: 1329–1335 10.1038/nm1134 [DOI] [PubMed] [Google Scholar]
- 27. Cerchietti L, Yang S, Shaknovich R, Hatzi K, Polo J, et al. (2008) A peptomimetic inhibitor of BCL6 with potent anti-lymphoma effects in vitro and in vivo. Blood 113: 3997–3405 10.1182/blood-2008-07-168773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cerchietti LC, Ghetu AF, Zhu X, Da Silva GF, Zhong S, et al. (2010) A small-molecule inhibitor of BCL6 kills DLBCL cells in vitro and in vivo. Cancer Cell 17: 400–411 10.1016/j.ccr.2009.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pervushin K, Riek R, Wider G, Wüthrich K (1997) Attenuated T2 relaxation by mutual cancellation of dipole-dipole coupling and chemical shift anisotropy indicates an avenue to NMR structures of very large biological macromolecules in solution. Proceedings of the National Academy of Sciences 94: 12366–12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vranken WF, Boucher W, Stevens TJ, Fogh RH, Pajon A, et al. (2005) The CCPN Data Model for NMR Spectroscopy: Development of a Software Pipeline. Proteins 59: 687–696. [DOI] [PubMed] [Google Scholar]
- 31. Leslie AGW (2006) The integration of macromolecular diffraction data. Acta Crystallogr D Biol Crystallogr 62: 48–57 10.1107/S0907444905039107 [DOI] [PubMed] [Google Scholar]
- 32. Evans PR, Murshudov GN (2013) How good are my data and what is the resolution? Acta Crystallogr D Biol Crystallogr 69: 1204–1214 10.1107/S0907444913000061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCoy AJ, Grosse-Kunstleve RW, Adams PD, Winn MD, Storoni LC, et al. (2007) Phaser crystallographic software. J Appl Crystallogr 40: 658–674 10.1107/S0021889807021206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmad KF, Melnick A, Lax S, Bouchard D, Liu J, et al. (2003) Mechanism of SMRT corepressor recruitment by the BCL6 BTB domain. Mol Cell 12: 1551–1564. [DOI] [PubMed] [Google Scholar]
- 35. Collaborative Computational Project, Number 4 (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50: 760–763 10.1107/S0907444994003112 [DOI] [PubMed] [Google Scholar]
- 36. Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dhordain P, Albagli O, Ansieau S, Koken MH, Deweindt C, et al. (1995) The BTB/POZ domain targets the LAZ3/BCL6 oncoprotein to nuclear dots and mediates homomerisation in vivo. Oncogene 11: 2689–2697. [PubMed] [Google Scholar]
- 38. Kunin CM (1996) Antimicrobial activity of rifabutin. Clin Infect Dis 22 Suppl 1 S3–13–discussionS13–4. [DOI] [PubMed] [Google Scholar]
- 39. Auld DS, Southall NT, Jadhav A, Johnson RL, Diller DJ, et al. (2008) Characterisation of chemical libraries for luciferase inhibitory activity. J Med Chem 51: 2372–2386. [DOI] [PubMed] [Google Scholar]
- 40. Campbell EA, Korzheva N, Mustaev A, Murakami K, Nair S, et al. (2001) Structural mechanism for rifampicin inhibition of bacterial rna polymerase. Cell 104: 901–912. [DOI] [PubMed] [Google Scholar]
- 41. Woods LA, Platt GW, Hellewell AL, Hewitt EW, Homans SW, et al. (2011) Ligand binding to distinct states diverts aggregation of an amyloid-forming protein. Nat Chem Biol 7: 730–739 10.1038/nchembio.635 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protein purification and crystallisation of BCL6. (A) Coomassie stained polyacrylamide gel showing production of BCL6-POZ domain with a GB1 tag and TEV cleavage of the tag. (B) BCL6-POZ domain purified away from the GB1 tag by size exclusion gel filtration. Coomassie stained polyacrylamide gel showing fractions collected. (C) BCL6-POZ crystal mounted in a loop at the Diamond Synchrotron beamline I24 (Red box 25.9 μm2). The crystals are faintly lilac having taken up the coloured rifabutin.
(TIF)
Natural product library screening. Results for all 480 compounds are presented. Column to the left in orange is the mean negative control i.e. transfected cells without test compound, and the orange horizontal line the mean value across the entire screen. Compounds considered “its”are represented as red columns and comprise 6 that relieve BCL6 transcriptional repression and 3, which appear to enhance repression.
(TIF)
Structures of the 9 compounds that alter BCL6 transcriptional repression. The most widely used compound name is presented apart from one compound (A), which has no common name and for which the IUPAC nomenclature is stated. The chemical identifier (CID) from PubChem is also presented. (A to C) Three compounds that reduce luciferase activity. (D to I) Compounds that enhance luciferase activity, including (I) Rifamycin SV.
(TIF)
TROSY 1H, 15N HSQC NMR spectrum of the BCL6-POZ domain.
(TIF)
Sequences of SMRT peptides. Sequences of SMRT peptides utilised in fluorescence polarisation experiments together with the structures of the artificial amino acids at the position of histidine1426 in the wild-type peptide. (A) wild-type SMRT, (B) 1-naphthyl-SMRT, (C) 2-naphthyl-SMRT, (D) homophenylalanine-SMRT and (E) styryl-SMRT.
(TIF)