Abstract
Seven different strains of Aedes aegypti (L.), including a genetically diverse laboratory strain, three laboratory-selected permethrin-resistant strains, a standard reference strain, and two recently colonized strains were fed on human blood containing various concentrations of ivermectin. Ivermectin reduced adult survival, fecundity, and hatch rate of eggs laid by ivermectin-treated adults in all seven strains. The LC50 of ivermectin for adults and the concentration that prevented 50% of eggs from hatching was calculated for all strains. Considerable variation in adult survival after an ivermectin-bloodmeal occurred among strains, and all three permethrin-resistant strains were significantly less susceptible to ivermectin than the standard reference strain. The hatch rate after an ivermectin bloodmeal was less variable among strains, and only one of the permethrin-resistant strains differed significantly from the standard reference strain. Our studies suggest that ivermectin induces adult mortality and decreases the hatch rate of eggs through different mechanisms. A correlation analysis of log-transformed LC50 among strains suggests that permethrin and ivermectin cross-resistance may occur.
Keywords: Aedes aegypti, ivermectin, permethrin, cross-resistance
A number of in vitro and in vivo studies have demonstrated that, when imbibed in a bloodmeal, ivermectin causes a significant reduction in adult female mosquito survival, fecundity, and decreased egg hatch rate (Pampiglioni et al. 1985; Iakubovich et al. 1989; Tesh and Guzman 1990; Cartel et al. 1991; Focks 1991; Focks et al. 1991; Mahmood et al. 1991; Gardner et al. 1993; Bockarie et al. 1999; Foley et al. 2000; Fritz et al. 2009; Kobylinski et al. 2010, 2011). Ivermectin, a macrocyclic lactone, is a broad-spectrum drug which is widely used for the treatment of a number of parasitic infections, including the control of onchocerciasis and lymphatic filariasis. Mass drug administration (MDA) of ivermectin through the African Programme for On-chocerciasis Control and the Global Program for the Elimination of Lymphatic Filariasis distributes ivermectin to over 80 million people annually across the globe (Amazigo 2008, Ottesen et al. 2008). There is considerable geographic overlap of locales treated by MDA with ivermectin and areas endemic for mosquito-borne diseases such as malaria and dengue. It has been proposed that in addition to controlling nematode infections, more frequent MDA could also be effective in controlling mosquito-borne diseases (Wilson 1993; Kobylinski et al. 2010, 2011; Foy et al. 2011).
Two field-based studies have demonstrated that MDA using ivermectin significantly reduces the survivorship of adult field-caught mosquitoes (Bockarie et al. 1999, Sylla et al. 2010). Models have shown that even modest reductions in the daily probability of mosquito survival may have a significant impact on the transmission of mosquito-borne disease such as dengue and malaria (Garrett-Jones 1964, Billingsley et al. 2008, Sylla et al. 2010, Foy et al. 2011). Further, MDA of ivermectin is effective in disrupting malaria transmission (Kobylinski et al. 2011).
Despite a growing body of literature on the effects of ivermectin in mosquitoes, the variation in susceptibility to ivermectin among strains of the same species has not been investigated, nor have insecticide resistant strains been evaluated for ivermectin cross-resistance. We chose Aedes aegypti (L.) as a model to study variation in susceptibility to ivermectin when imbibed in a bloodmeal. Seven strains of Ae. aegypti, including three laboratory-selected permethrin resistant strains, were administered bloodmeals containing various concentrations of ivermectin through an artificial membrane feeding system. The concentrations at which ivermectin could affect adult survivorship, fecundity, and hatch rate were evaluated and compared among the seven mosquito lines.
Materials and Methods
Mosquitoes
The Solidaridad (SLD) strain originated from Mexico was collected as previously described (Flores et al. 2006, Kobylinski et al. 2011), and the Iquitos strain (IQT) originating from Iquitos, Peru were collected by Dr. Amy Morrison, and then sent to Colorado State University for further studies. The SLD and IQT strains were used to generate permethrin-resistant laboratory strains of mosquitoes (SLD-PR and IQT-PR, respectively), as described elsewhere (Saavedra-Rodriguez 2012). The Isla Mujeres strain (IMU-PR), was collected from the field and exhibited high levels of pyrethroid and temephos resistance without laboratory selection, and was also selected for eight consecutive generations for permethrin resistance (Saavedra-Rodriguez et al. 2008). The genetically diverse laboratory strain (GDLS) was constructed as described (Wise de Valdez et al. 2010). The standard laboratory reference strain, New Orleans (NO), was kindly provided by the Center for Disease Control and Prevention (CDC), Atlanta, GA. All of the mosquitoes were reared at 28 ± 2°C, 80% humidity under a photoperiod of 14:10 (L:D). Larvae were raised in 28 liters containers filled with ≈ 15 liters of tap water. Larval density was maintained at 500 – 600 mosquito larvae per container to ensure uniform development and size. Mosquito larvae were fed a diet of ground Tetramin fish food mixed with ground mouse food. Adult mosquitoes were provided with water and raisins as a sugar source ad libitum.
Ivermectin
A powdered formulation of ivermectin was obtained from Sigma-Aldrich (St. Louis, MO) and dissolved in dimethylsulfoxide (DMSO) to a concentration of 10 mg/ml. Multiple aliquots were stored, frozen, at −20°C A fresh aliquot was used for each blood feed.
In Vitro Blood Feeds
Human blood was used for all in vitro blood feeds. Blood was drawn from a human volunteer into 3.2% sodium citrate blood collection tubes by a phlebotomist at the Colorado State University Health Network Medical Clinic in Fort Collins, CO. Blood was drawn from the same volunteer for all of the experiments described, and was no more than 1 wk postdrawn at the time of the bloodfeed. Ivermectin diluted in DMSO was thawed, and then serially diluted into phosphate buffered saline (PBS) to a concentration 10 times greater than the final concentration desired, which was finally diluted 1:10 into blood to reach the final concentrations provided to mosquitoes. At the time of bloodfeed, mosquitoes were 3–5 d postemergence. Twenty-four hours before the blood feed, adult mosquitoes were placed into 4 liters plastic containers. Mosquitoes were starved of sugar and water for 12 and 3 hr, respectively, before the blood feed. Glass membrane feeders (Lillie, Glass Blowers, Smyrna, GA) were covered by securing hog sausage casing to the feeder with a rubber band, and then heated to 37°C with a heated water circulator. Mosquitoes were allowed to feed for 30 min. After the feed, mosquitoes were cold-anesthetized in a refrigerator, placed onto a glass petri dish maintained on ice and sorted for the presence of abloodmeal. Only fully engorged mosquitoes were retained for survival analysis, fecundity, and embryo survival studies.
LC50(adult) Determination
The concentration of ivermectin required to kill 50% of adults (LC50(adult)) was determined by feeding the following concentrations of the drug to mosquitoes: 800, 400, 200, 100, 75, 50, 35, and 0 ng/ml. Control mosquitoes were fed PBS containing a concentration of DMSO equivalent to the highest concentration fed to experimental mosquitoes. For each of the mosquito strains, the LC50(adult) was determined from three experimental replicates (n = 45–55 mosquitoes per concentration per replicate) using a nonlinear mixed model with probit analysis (Kobylinski et al. 2010).
Estimation of Fecundity
A subset of five fully engorged females were reserved from each of the blood feeds. Mosquitoes of similar body and bloodmeal size were placed into 500 ml ice cream containers. A 10 ml oviposition cup lined with a paper towel was filled with ≈8 ml of water and placed into each container. Containers were covered with organdy fabric, and mosquitoes had access to raisins as a sugar source. Two days after the blood feed, surviving mosquitoes were counted and recorded. Five days postblood feed, mosquitoes were anesthetized using carbon dioxide, and the oviposition cup was removed. Eggs laid on the water surface were collected by filtering the water through a coffee filter. Eggs were allowed to dry inside of aplastic container covered with organdy fabric that was maintained in the insectary for 3 d. Eggs were counted using a stereoscopic dissecting microscope. The number of eggs laid per female mosquito was estimated by dividing the total number of eggs by the number of surviving mosquitoes at 2 d postblood feed. Eggs were then placed into plastic bags and maintained in the insectary for five additional days.
LC50(hatch) Determination
The ability of ivermectin in a maternal bloodmeal to prevent 50% of eggs from hatching (LC50(hatch)) was assessed by submerging a subset of 50–60 eggs from mosquitoes fed each different concentration of ivermectin in 500 ml of tap water. To minimize error associated with installment hatching (Gillett et al. 1977), water was de-oxygenated by bubbling nitrogen gas into the hatch container for 10 min. Hatch cups were maintained in the insectary, and 3 d after hatching all larvae were counted. The number of eggs that failed to hatch was calculated by subtracting the number of larvae counted from the number of eggs submerged in water. The LC50(hatch) for each mosquito strain was calculated using the nonlinear model described above.
Permethrin Resistance Bioassays
Adults from each strain were subjected to bioassays to estimate the LC50 for permethrin exposure (47.6% cis – 50.4% trans; Chem Service, West Chester, PA). The bottle bioassay was a minor modification of the assay described by Brogen and McAllister (Brogdon and McAllister, 1998). Briefly, the insides of 250 ml glass bottles were coated with 1 ml of acetone containing five different concentrations of permethrin. Twenty-five adults per replicate (three replicates tested) were exposed in the bottle for 1 h, transferred into a clean cardboard one-pint carton, and mortality was scored after 24 h. LC50s were estimated using a logistic regression model in R version 2.11.1 (http://cran.r-project.org/).
Statistical Analysis
To compare calculated LC50(adult) and LC50(hatch) estimates among the seven different mosquito lines, LC50(adult) and LC50(hatch) estimates were calculated for each experimental replicate. Data from all seven strains was compared using a one-way analysis of variance (ANOVA) followed by multiple pairwise comparisons. Statistical analysis was carried out using PROC GLM and least squares means (SAS version 9.2, SAS Institute, Cary, NC). Resistance ratios for the LC50(adult) and LC50(hatch) were calculated relative to the susceptible NO strain.
The effect of ivermectin on the fecundity for each mosquito line was assessed using a one-way ANOVA, and the mean number of eggs laid at each of the concentrations of ivermectin fed to mosquitoes was compared with the PBS-DMSO control group. The significance level was set at P < 0.05, and statistical analysis was carried out using PROC GLM and least squares means (SAS version 9.2).
The effect of each ivermectin concentration on the egg hatch rate was assessed for each mosquito strain by comparing the hatch rate from each concentration to the corresponding PBS-DMSO control. Data from all three replicates were pooled and then analyzed using a Fisher Exact Test using R (http://www.r-project.org/).
Results
Adult LC50 Determination
Ivermectin reduced the survivorship of all strains of adult Ae. aegypti mosquitoes. The LC50(adult) estimates and the corresponding 95% fiducial limits are reported in Table 1 and depicted in Fig. 1. Calculated LC50(adult) ivermectin estimates across all mosquito lines ranged from 187.17 ng/ml to 576.43 ng/ml. The fit of the NLM for calculating the LC50(hatch) estimate for each strain is provided in Supp Fig. 1 (online only), and the fit of the NLM for the LC50(adult) of the NO is provided as a sample in Fig. 2a. The LC50(adult) estimates of ivermectin for the IMU-PR, IQT-PR, and SLD-PR strains differed significantly from the NO standard reference strain (P < 0.05). The SLD and IQT strains did not differ from the NO standard reference strain (P > 0.05). The GDLS did not differ significantly from any of the other mosquito strains. Pairwise comparisons of strains IQT-PR and SLD-PR with their respective selection free strains revealed significant differences in LC50(adult) estimates.
Table 1. Effect of imbibed ivermectin on adult survival and hatch rate of seven different Ae. aegypti strains.
| Strain | LC50(adult) | LC50(hatch) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Ivermectin conc. ng/ml | 95% fiducial limits | RR | Slope ± SE | Intercept ± SE | Ivermectin conc. ng/ml | 95% fiducial limits | RR | slope ± SE | Intercept ± SE | |
| IMU-PR | 576.43 | (458.80, 722.96) | 3.08 | 6.19 ± 0.48 | −11.58 ± 3.07 | 334.48 | (3.64, 645.12) | 7.61 | 6.69 ± 0.87 | −10.84 ± 5.14 |
| SLD-PR | 536.35 | (402.11, 822.64) | 2.87 | 3.63 ± 0.31 | −8.16 ± 1.89 | 60.52 | (43.87, 76.83) | 1.38 | 4.66 ± 0.32 | −6.32 ± 1.39 |
| IQT-PR | 487.39 | (397.36, 605.13) | 2.60 | 5.29 ± 0.32 | −10.3 ± 1.97 | 125.03 | (79.96, 178.23) | 2.84 | 2.44 ± 0.16 | −4.28 ± 0.84 |
| GDLS | 355.64 | (297.04, 424.66) | 1.90 | 4.06 ± 0.18 | −8.21 ± 1.04 | 110.82 | (75.65, 156.59) | 2.52 | 2.66 ±0.17 | −4.59 ± 0.86 |
| IQT | 284.59 | (242.93, 331.32) | 1.52 | 4.31 ± 0.17 | −8.28 ± 0.99 | 75.58 | (57.28, 92.43) | 1.72 | 2.23 ±0.17 | −4.59 ± 0.86 |
| SLD | 284.04 | (242.03, 333.89) | 1.52 | 4.10 ± 0.15 | −7.9 ± 0.8 | 52.75 | (40.44, 68.54) | 1.20 | 10.18 ± 0.55 | −9.21 ± 2.27 |
| NO | 187.17 | (162.79, 215.75) | – | 5.10 ± 0.15 | −8.5 ± 0.81 | 43.95 | (34.80, 50.95) | – | 10.49 ± 0.43 | −8.91 ± 1.73 |
Fig. 1.
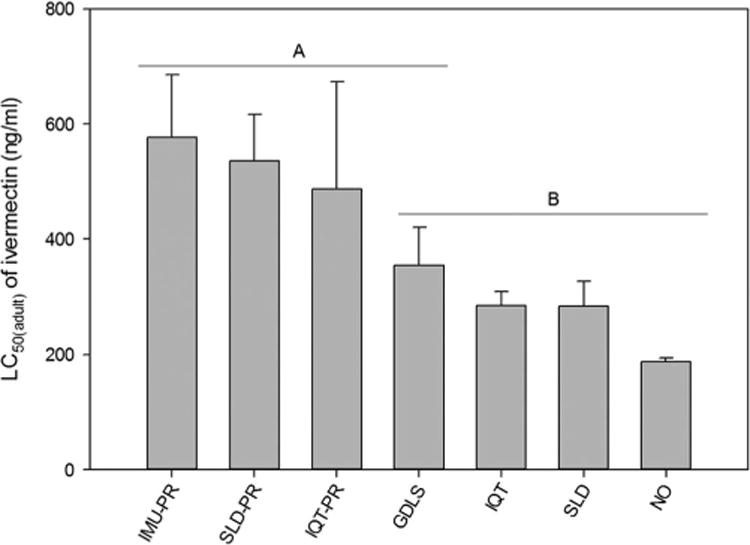
Graphical comparison of LC50(adult) for ivermectin between seven strains of Ae. aegypti. Error bars indicate experiment-wise standard error of the mean LC50 value of three replicate experiments. Strains that did not differ significantly in the LC50(adult) for ivermectin are denoted with the same letter (α = 0.05; Least Squares Means procedure).
Fig. 2.
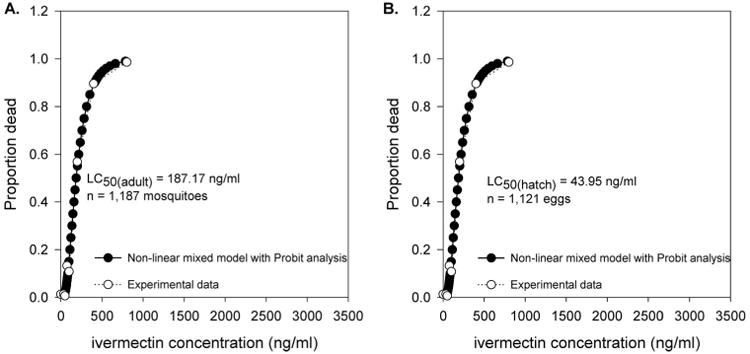
Fit of the nonlinear model used to calculate LC50(adult) (A)and LC50(hatch) (B) for orally ingested ivermectin in the NO Ae. aegypti strain. A plot of the observed mortality versus the ivermectin concentration imbibed by mosquitoes was overlaid with a plot of the probit mortality versus the predicted ivermectin concentration from the nonlinear model.
Effect of Ivermectin on Hatch Rate
The hatch rate of eggs from all mosquito strains was reduced after a maternal bloodmeal containing ivermectin. The LC50(hatch) estimates and the corresponding 95% fiducial limits are reported in Table 1; Fig. 3. Calculated LC50(hatch) ivermectin estimates across all mosquito lines ranged from 43.95 ng/ml to 334.45 ng/ml. The fit of the NLM for calculating the LC50(hatch) estimate for each strain is provided in Supp Fig. 2 (online only), and the fit of the NLM for the LC50(hatch) of the NO is provided as a sample in Fig. 2b. Hatch rates for each concentration of ivermectin recorded by mosquito strain are presented in Table 2. The IMU-PR mosquito strain was the only strain that differed significantly from the susceptible NO strain. Pairwise comparisons of strains IQT-PR and SLD-PR with their respective selection-free strains did not reveal significant differences.
Fig. 3.

Graphical comparison of LC50(htch) for IVM between seven strains of Ae. aegypti. Error bars indicate experiment-wise standard error of the mean LC50 value from three replicate experiments. Strains that did not differ significantly in the LC50(hatch) for ivermectin are denoted with the same letter (α = 0.05; Least Squares Means procedure).
Table 2. Hatch rate of seven different Ae. aegypti strains after a maternal bloodmeal containing ivermectin.
| Mosquito strain | Conc. Of ivermectin fed to female mosquito, ng/ml | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Percent hatch ratea | ||||||||
|
| ||||||||
| 800 | 400 | 200 | 100 | 75 | 50 | 35 | PBS-DMSO | |
| IMU-PR | 4.2 ± 4.2 | 34.0 ± 31.1 | 77.0 ±9.0 | 83.0 ± 9.4 | 98.0 ± 2.0 | 78.5 ±14.8 | 92.7 ± 3.4 | 95.3 ± 2.7 |
| SLD-PR | 0 | 1.7 ± 1.7 | 0 | 16.6 ± 8.9 | 47.2 ± 9.6 | 55.1 ± 10.6 | 76.7 ± 11.8 | 95.3 ± 2.7 |
| IQT-PR | 0 | 6.1 ± 4.0 | 43.6 ± 14.0 | 68.5 ± 1.5 | 58.3 ± 13.0 | 70.3 ± 8.7 | 76.1 ± 4.5 | 94.6 ± 2.8 |
| GDLS | 0 | 16.7 ± 11.2 | 22.7 ± 16.2 | 39.2 ± 12.4 | 70.3 ± 13.9 | 80.1 ± 4.2 | 81.2 ± 4.2 | 96.0 ± 4.0 |
| IQT | – | 0.7 ± 0.7 | 0 | 34.7 ± 19.1 | 44.9 ± 5.1 | 81.0 ± 4.2 | 82.2 ± 10.2 | 96.8 ± 3.2 |
| SLD | – | 0 | 0 | 16.3 ± 16.3 | 12.6 ± 0.7 | 37.3 ± 19.3 | 93.9 ± 3.0 | 98.7 ± 1.3 |
| NO | – | – | 0 | 12 ± 7.6 | 32.7 ± 16.5 | 32.7 ± 16.5 | 55.2 ± 1.7 | 82.5 ±1.3 |
–, denotes a conc. where no mosquitoes survived to lay eggs.
Percentage of larvae hatching from eggs ± SEM. Numbers denoted in bold font correspond with an overall significant reduction in hatch rate are denoted in bold font (χ2 test for significance P < 0.05).
Fecundity of Ae. aegypti Mosquitoes After a Blood-meal Containing Ivermectin
A two-way ANOVA revealed that ivermectin concentration had a significant effect on the average number of eggs laid per female mosquito (F = 32.89; df = 7, 112; P < 0.001), and that the mean number of eggs laid per female differed significantly among mosquito strains (F = 4.487; df = 6,112; P < 0.001). There was no significant interaction effect between ivermectin concentration and mosquito strain (F = 0.780; df = 42, 112; P = 0.819). Because the average number of eggs laid per female differed significantly among mosquito strains, we analyzed the effects of ivermectin on the mean number of eggs produced per female for each mosquito strain separately (Table 3). A significant reduction in the mean number of eggs laid by the IQT-PR and the SLD-PR strains was seen only at the 800 and 400 ng/ml concentrations of ivermectin. In the corresponding selection free strains, no mosquitoes survived to oviposit after feeding on ivermectin at a concentration of 800 ng/ml, and significant reductions in the mean number of eggs laid were seen after a bloodmeal containing 400 and 200 ng/ml of ivermectin (Table 2).
Table 3. Fecundity of varied strains of Ae. aegypti after ingestion of a bloodmeal containing ivermectin.
| Mosquito strain | Conc. of ivermectin fed to female mosquito, ng/ml | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| No. eggs laid/femalea | ||||||||
|
| ||||||||
| 800 | 400 | 200 | 100 | 75 | 50 | 35 | PBS-DMSO | |
| IMU-PR | 11.2 ± 10.4 | 54.39 ± 33.6 | 60.5 ± 11.4 | 117.3 ± 22.1 | 120.1 ± 11.5 | 111.7 ± 13.0 | 70.6 ± 8.5 | 128.1 ± 21.0 |
| SLD-PR | 38 ± 38 | 30.8 ± 10.1 | 122.7 ± 48.4 | 105.6 ± 5.1 | 113.5 ± 3.2 | 133.1 ±4.1 | 112.5 ± 4.7 | 122.4 ± 17.4 |
| IQT-PR | 5.3 ± 5.3 | 34.6 ± 26.7 | 90.1 ± 13.0 | 91.0 ± 12.8 | 85.6 ± 28.1 | 113.9 ± 16.9 | 103.3 ± 12.0 | 125.0 ± 6.5 |
| GDLS | 1.8 ± 1.8 | 28.4 ± 14.3 | 53.4 ± 18.0 | 64.4 ± 12.1 | 83.6 ± 22.2 | 97.0 ± 11.6 | 92.4 ± 10.9 | 88.9 ± 5.1 |
| IQT | – | 7.9 ± 7.9 | 18.8 ± 12.7 | 92.7 ± 30.0 | 81.3 ± 8.4 | 99.1 ± 12.1 | 90.9 ± 12.1 | 77.3 ± 21.1 |
| SLD | – | 9.3 ± 9.3 | 39.6 ± 28.3 | 81.0 ± 21.5 | 92.7 ± 14.0 | 134.7 ± 25.1 | 126.4 ± 26.7 | 118.7 ± 12.8 |
| NO | – | – | 59.3 ± 26.7 | 65.2 ± 37.8 | 81.9 ± 17.3 | 98.9 ± 14.2 | 105.5 ± 7.2 | 118.4 ± 25.7 |
–, denotes a conc. where no mosquitoes survived to lay eggs.
Mean no. of eggs ± SEM. For each strain of mosquito, the mean no. of eggs laid by mosquitoes at each concn of ivermectin was compared with the corresponding control. Significant reductions in fecundity are denoted in bold font (χ2test for significance [P < 0.05]).
Correlation Between LC50 of Permethrin and LC50(adult) of Ivermectin
We observed a significant correlation between the LC50 for permethrin (measured in micrograms/bottle) and the LC50 for ivermectin (Pearson's product-moment correlation = 0.97, P = 0.002). The LC50 estimates for each of the mosquito strains are published elsewhere (Saavedra-Rodriguez et al. 2008, 2012). A plot of the log-transformed LC50 of permethrin versus the log-transformed LC50(adult) for ivermectin is shown in Fig. 4. The GDLS was omitted from correlation analysis because the LC50 for permethrin was not available for this strain.
Fig. 4.

Regression of LC50 of permethrin against the LC50(adult) of ivermectin in six strains of Ae. aegypti.
Correlation Between LC50(adult) and LC50(hatch) of Ivermectin
There was not a correlation observed between the LC50(adult) and LC50(hatch) of ivermectin (Pearson's product-moment correlation = 0.70, P = 0.08).
Discussion
Three outcomes (adult survival, fecundity, and hatch rate) were used to evaluate the susceptibility of seven different lines of Ae. aegypti mosquitoes to different concentrations of ivermectin contained in a bloodmeal. While we and others have reported on the effects of ivermectin on Ae. aegypti (Pampiglioni et al. 1985, Tesh and Guzman 1990, Mahmood et al. 1991, Focks et al. 1995, Kobylinski et al. 2010), this is the first analysis of variation in IVERMECTIN susceptibility among mosquito strains of the same species. This is also the first study to determine the LC50(adult) and LC50(hatch) of ivermectin in mosquitoes with known insecticide resistance.
We chose to use Ae. aegypti as a model in the current study because of our possession of multiple different mosquito strains that have been previously characterized, including the GDLS and three permethrin-resistant mosquito strains, two of which we also possessed their parent nonselected strains. Further, the effects of ivermectin on fecundity and hatch rate are temporary, and diminish after a second bloodmeal that does not contain ivermectin (Tesh and Guzman 1990). An advantage to using Ae. aegypti is that oogenesis is completed after one bloodmeal, whereas Anopheles gambiae (Giles) has been shown to often require more than one bloodmeal for completion of oogenesis (Fernandes and Briegel 2005).
The in vitro feeding strategy used in this study allowed for the consistent administration of varied concentrations of ivermectin to all of the mosquito strains, thus allowing for direct comparisons of the LC50(adult) and LC50(hatch) for ivermectin among the different mosquito strains. After oral ingestion of ivermectin in bloodmeal, we observed a large degree of variation in the LC50(adult) of ivermectin among the mosquito strains.
The LC50(adult) estimates for all of the laboratory-selected permethrin-resistant mosquito lines (IMU-PR, SLD-PR, and IQT-PR) were significantly higher than the standard laboratory reference strain (NO). A selection-free line of IMU could not be maintained in the laboratory; therefore, a contrast between the IMU-PR strain and the corresponding selection free strain could not be made. However, the LC50(adult) for ivermectin in the SLD-PR and IQT-PR strains differed significantly from the corresponding selection free strains, and the LC50 of permethrin was positively correlated with the LC50(adult) for ivermectin. Collectively, these results indicate that a cross-resistance mechanism could be responsible for the increased tolerance to ivermectin by the IMU-PR, SLD-PR, and IQT-PR Ae. aegypti mosquito strains. These results were indeed quite surprising and unexpected.
Ivermectin is an allosteric agonist of glutamategated chloride (GluCl) anion channels. In parasitic worms, ivermectin binds the GluCl receptor causing an increased permeability to chloride ions, which then leads to hyperpolization of the nerve-cell membrane, leading to flaccid paralysis and death of the parasite (Cleland 1996, Wolstenholme and Rogers 2005). However, permethrin pyrethroid that delays the normal closing of voltage-gated sodium channels of arthropods, resulting in depolarization of nerve-cell membranes ultimately leading to excessive neuroex-citation and death (Soderlund and Bloomquist 1989). Given the disparate modes of action and target sites for these two compounds, cross-resistance is more likely because of metabolic mechanisms. Permethrin-induced cross-resistance to abamectin, a macrocyclic lactone differing from ivermectin only by the presence of a double-bond, has been reported in house fies (Scott 1989, Geden et al. 1992) and German cockroaches (Scott 1991). In one study, permethrin-resistant Musca domestica (L.) were observed to have a 25-fold cross-resistance to abamectin that was temporarily suppressed by the mixed-function oxidase inhibitor, piperonyl butoxide (Scott 1989). Reports of permethrin-induced cross-resistance to avermectin are conflicting. Others have reported that permethrin-resistant house flies (Roush and Wright 1986) and permethrin-resistant German cockroaches (Cochran 1990) are fully susceptible to avermectin, and recently permethrin-resistant head lice were shown to be susceptible to ivermectin (Strycharz et al. 2008). In all of these studies, avermectin or ivermectin was applied topically, which differs from our methods in which ivermectin was orally imbibed.
While it is possible that the increased tolerance to ivermectin observed in any one of the permethrin-resistant Ae. aegypti mosquito strains is an artifact of laboratory-selection, it is interesting that adult mosquitoes of all three permethrin-resistant strains were approximately two-fold less susceptible to the effects of ivermectin than any of the permethrin-susceptible lines. Pyrethroid resistance in field populations of mosquitoes is well documented (Santolamazza et al. 2008, Garcia et al. 2009). In light of the recent report and models that demonstrate MDA of ivermectin can disrupt the transmission of human malaria parasites (Sylla et al. 2010, Kobylinski 2011), the question of whether pyrethroid resistance can result in cross-resistance to ivermectin is clearly an area that needs to be further explored.
The hatch rate of all mosquito strains was decreased after a maternal bloodmeal containing ivermectin. These data are consistent with the findings of other reports of ivermectin in Ae. aegypti (Tesh and Guzman 1990, Mahmood et al. 1991), however the LC50(hatch) of ivermectin for all of the mosquito strains we evaluated are notably higher than the previous reports. Tesh and Guzman (1990) reported a LC50(hatch) of 3.4 ng/ml using the Rock strain.
With the exception of the IMU-PR strain, no significant differences occurred among strains with respect to the LC50(hatch) of ivermectin. These results are strikingly different from those for the LC50(adult) estimates, where all permethrin-resistant strain estimates were significantly higher than standard susceptible strain. In addition, there was no correlation between the LC50(adult) and LC50(hatch) of ivermectin. Collectively, these results suggest that ivermectin induces adult mortality and decreases the hatch rate of eggs through different mechanisms.
Mahmood et al. (1991) blood-fed Ae. aegypti mosquitoes on sublethal concentrations of ivermectin, and also observed a large decrease in hatch rate of the eggs from treated mosquitoes. Many of the un-hatched eggs contained live larvae that failed to hatch despite multiple submersions in water (Mahmood et al. 1991). In the same report, the authors propose that residual amounts of ivermectin may be deposited in the egg and prevent eclosion. Such a mechanism could explain why we did not see alarge variations in the LC50(hatch) of the mosquito strains. Our experiments were not designed to elucidate the mechanism through which ivermectin interferes with egg hatching, but clearly this is an area that should be further explored.
The maximal concentrations of ivermectin found in human venous plasma after a standard MDA dose of ivermectin (150 μg/kg) ranges from 9 to 75 ng/ml, with a mean maximal concentration of ≈46 ng/ml (Elkassaby 1991). The LC50(adult) of ivermectin in Ae. aegypti reported here and elsewhere (Tesh and Guzman 1990, Kobylinski et al. 2010) are far greater than the serum concentrations expected in humans after ingestion of the 150 μg/kg dose of ivermectin typically used in MDA (Elkassaby 1991), thus it is unlikely that MDA administration of ivermectin will be effective in controlling diseases transmitted by Ae. aegypti. Nonetheless, Ae. aegypti may prove to be a useful laboratory model for studying the mechanisms of ivermectin induced pathology in the mosquito as well as potential mechanisms of resistance that could develop in the mosquito.
In summary, we found that in Ae. aegypti, adult survival after ingestion of ivermectin in a bloodmeal varies largely by mosquito strain, whereas the effect of maternal ingestion of ivermectin on the ability of eggs to hatch varies among strains to afar lesser extent. Our results also support that cross-resistance to ivermectin may develop in permethrin-resistant Ae. aegypti mosquitoes, although this is clearly an area that necessitates additional research.
Supplementary Material
Acknowledgments
We would like to acknowledge the help of Michael Salasek for providing the eggs to generate the GDLS laboratory strain. We would also like to acknowledge Brenn Wheeler and Molly Perres for their assistance in the insectary. This research was supported by grant R21 AI079528, and by contract N01-(AI)-25489 from the U.S. National Institutes of Allergy and Infectious Diseases, and by the Grand Challenges Explorations grant 51995 from the Bill and Melinda Gates Foundation.
References Cited
- Amazigo U. The African Programme for Onchocerciasis Control (APOC) Ann Trop Med Parasitol. 2008;102(Suppl. 1):19–22. doi: 10.1179/136485908X337436. [DOI] [PubMed] [Google Scholar]
- Billingsley PF, Foy B, Rasgon JL. Mosquitocidal vaccines: a neglected addition to malaria and dengue control strategies. Trends Parasitol. 2008;24:396–400. doi: 10.1016/j.pt.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Bockarie MJ, Hii JL, Alexander ND, Bockarie F, Dagoro H, Kazura JW, Alpers MP. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med Vet Entomol. 1999;13:120–123. doi: 10.1046/j.1365-2915.1999.00159.x. [DOI] [PubMed] [Google Scholar]
- Brogdon WG, McAllister JC. Simplification of adult mosquito bioassays through use of time-mortality determinations in glass bottles. J Am Mosq Control Assoc. 1998;14:159–164. [PubMed] [Google Scholar]
- Cartel JL, Sechan Y, Spiegel A, Nguyen L, Barbazan P, Martin PM, Roux JF. Cumulative mortality rates in Aedes polynesiensis after feeding on polynesian Wuchereria bancrofti carriers treated with single doses of ivermectin, diethylcarbamazine and placebo. Trop Med Parasitol. 1991;42:343–345. [PubMed] [Google Scholar]
- Cleland TA. Inhibitory glutamate receptor channels. Mol Neurobiol. 1996;13:97–136. doi: 10.1007/BF02740637. [DOI] [PubMed] [Google Scholar]
- Cochran DG. Efficacy of abamectin fed to German cockroaches (Dictyoptera: Blattellidae) resistant to pyrethroids. J Econ Entomol. 1990;83:1243–1245. doi: 10.1093/jee/83.4.1243. [DOI] [PubMed] [Google Scholar]
- Elkassaby MH. Ivermectin uptake and distribution in the plasma and tissue of Sudanese and Mexican patients infected with Onchocerca volvulus. Trop Med Parasitol. 1991;42:79–81. [PubMed] [Google Scholar]
- Fernandes L, Briegel H. Reproductive physiology of Anopheles gambiae and Anopheles atroparvus. J Vector Ecol. 2005;30:11–26. [PubMed] [Google Scholar]
- Flores AE, Grajales JS, Salas IF, Garcia GP, Becerra MH, Lozano S, Brogdon WG, Black WCT, Beaty B. Mechanisms of insecticide resistance in field populations of Aedes aegypti (L.) from Quintana Roo, Southern Mexico. J Am Mosq Control Assoc. 2006;22:672–677. doi: 10.2987/8756-971X(2006)22[672:MOIRIF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Focks DA. Impact of minor reductions in adult and larval survival, fecundity and hatch on the population dynamics of Psorophora columbiae: a simulation study. J Am Mosq Control Assoc. 1991;7:476–480. [PubMed] [Google Scholar]
- Focks DA, McLaughlin RE, Linda SB. Effects of ivermectin (MK-933) on the reproductive rate of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1991;28:501–505. doi: 10.1093/jmedent/28.4.501. [DOI] [PubMed] [Google Scholar]
- Focks DA, Daniels E, Haile DG, Keesling JE. A simulation model of the epidemiology of urban dengue fever: literature analysis, model development, preliminary validation, and samples of simulation results. Am J Trop Med Hyg. 1995;53:489–506. doi: 10.4269/ajtmh.1995.53.489. [DOI] [PubMed] [Google Scholar]
- Foley DH, Bryan JH, Lawrence GW. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans R Soc Trop Med Hyg. 2000;94:625–628. doi: 10.1016/s0035-9203(00)90211-6. [DOI] [PubMed] [Google Scholar]
- Foy BD, Kobylinski KC, Silva IM, Rasgon JL, Sylla M. Endectocides for malaria control. Trends Parasitol. 2011;27(10):423–428. doi: 10.1016/j.pt.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz ML, Siegert PY, Walker ED, Bayoh MN, Vulule JR, Miller JR. Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann Trop Med Parasitol. 2009;103:539–547. doi: 10.1179/000349809X12459740922138. [DOI] [PubMed] [Google Scholar]
- Garcia GP, Flores AE, Fernandez-Salas I, Saavedra-Rodriguez K, Reyes-Solis G, Lozano-Fuentes S, Guillermo Bond J, Casas-Martinez M, Ramsey JM, Garcia-Rejon J, et al. Recent rapid rise of a permethrin knock down resistance allele in Aedes aegypti in Mexico. PLoS Negl Trop Dis. 2009;3:e531. doi: 10.1371/journal.pntd.0000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K, Meisch MV, Meek CL, Biven WS. Effects of ivermectin in canine blood on Anopheles quadrimaculatus, Aedes albopictus and Culex salinarius. J Am Mosq Control Assoc. 1993;9:400–4002. [PubMed] [Google Scholar]
- Garrett-Jones C. Prognosis for interruption of malaria transmission through assessment of the mosquitoÕs vectorial capacity. Nature. 1964;204:1173–1175. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- Geden CJ, Rutz DA, Scott JG, Long SJ. Susceptibility of house flies (Diptera: Muscidae) and five pupal parasitoids (Hymenoptera: Pteromalidae) to abamectin and seven commercial insecticides. J Econ Entomol. 1992;85:435–440. doi: 10.1093/jee/85.2.435. [DOI] [PubMed] [Google Scholar]
- Gillett JD, Roman EA, Phillips V. Erratic hatching in Aedes eggs: a new interpretation. Proc R Soc Lond B Biol Sci. 1977;196:223–232. doi: 10.1098/rspb.1977.0038. [DOI] [PubMed] [Google Scholar]
- Iakubovich V, Zakharova NF, Alekseev AN, Alekseev EA. Evaluation of the action of ivermectin on blood-sucking mosquitoes. Med Parazitol (Mosk) 1989:60–64. [PubMed] [Google Scholar]
- Kobylinski KC, Deus KM, Butters MP, Hongyu T, Gray M, da Silva IM, Sylla M, Foy BD. The effect of oral anthelmintics on the survivorship and refeeding frequency of anthropophilic mosquito disease vectors. Acta Trop. 2010;116:119–126. doi: 10.1016/j.actatropica.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobylinski KC, Sylla M, Chapman PL, Sarr MD, Foy BD. Ivermectin mass drug administration to humans disrupts malaria parasite transmission in senegalese villages. Am J Trop Med Hyg. 2011;85:3–5. doi: 10.4269/ajtmh.2011.11-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood F, Walters LL, Guzman H, Tesh RB. Effect of ivermectin on the ovarian development of Aedes aegypti (Diptera: Culicidae) J Med Entomol. 1991;28:701–707. doi: 10.1093/jmedent/28.5.701. [DOI] [PubMed] [Google Scholar]
- Ottesen EA, Hooper PJ, Bradley M, Biswas G. The global programme to eliminate lymphatic filariasis: health impact after 8 years. PLoS Negl Trop Dis. 2008;2:e317. doi: 10.1371/journal.pntd.0000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pampiglioni S, Majori G, Petrangeli G, Romi R. Avermectins, MK-933 and MK-936, for mosquito control. Trans R Soc Trop Med Hyg. 1985;79:797–799. doi: 10.1016/0035-9203(85)90121-x. [DOI] [PubMed] [Google Scholar]
- Roush RT, Wright JE. Abamectin: toxicity to house flies (Diptera: Muscidae) resistant to synthetic organic insecticides. J Econ Entomol. 1986;79:562–564. doi: 10.1093/jee/79.3.562. [DOI] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Strode C, Flores Suarez A, Fernandez Salas I, Ranson H, Hemingway J, Black WCT. Quantitative trait loci mapping of genome regions controlling permethrin resistance in the mosquito Aedes aegypti. Genetics. 2008;180:1137–1152. doi: 10.1534/genetics.108.087924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K, Flores Suarez A, Fernandez Salas I, Strode C, Ranson H, Hemingway J, Black WC. Transcription of detoxification genes following permethrin selection in the mosquito Aedes aegypti. Insect Mol Biol. 2012;21(1):31–77. doi: 10.1111/j.1365-2583.2011.01113.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santolamazza F, Calzetta M, Etang J, Barrese E, Dia I, Caccone A, Donnelly MJ, Petrarca V, Simard F, Pinto J, della Torre A. Distribution of knock-down resistance mutations in Anopheles gambiae molecular forms in west and west-central Africa. Malar J. 2008;7:74. doi: 10.1186/1475-2875-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JG. Cross-resistance to the biological insecticide abamectin in pyrethroid-resistant house flies. Pestic Biochem Physiol. 1989;34:27–31. [Google Scholar]
- Scott JG. Toxicity of abamectin and hydramethylnon to insecticide-susceptible and resistant strains of German cockroach (Dictyoptera: Blattellidae) J Agric Entomol. 1991;8:77–82. [Google Scholar]
- Soderlund DM, Bloomquist JR. Neurotoxic actions of pyrethroid insecticides. Annu Rev Entomol. 1989;34:77–96. doi: 10.1146/annurev.en.34.010189.000453. [DOI] [PubMed] [Google Scholar]
- Strycharz JP, Yoon KS, Clark JM. A new ivermectin formulation topically kills permethrin-resistant human head lice (Anoplura: Pediculidae) J Med Entomol. 2008;45:75–81. doi: 10.1603/0022-2585(2008)45[75:aniftk]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Sylla M, Kobylinski KC, Gray M, Chapman PL, Sarr MD, Rasgon JL, Foy BD. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J. 2010;9:365. doi: 10.1186/1475-2875-9-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB, Guzman H. Mortality and infertility in adult mosquitoes after the ingestion of blood containing ivermectin. Am J Trop Med Hyg. 1990;43:229–233. doi: 10.4269/ajtmh.1990.43.229. [DOI] [PubMed] [Google Scholar]
- Wilson ML. Avermectins in arthropod vector management-prospects and pitfalls. Parasitol Today. 1993;9:83–87. doi: 10.1016/0169-4758(93)90210-7. [DOI] [PubMed] [Google Scholar]
- Wise de Valdez MR, Suchman EL, Carlson JO, Black WC. A large scale laboratory cage trial of Aedes densonucleosis virus (AeDNV) J Med Entomol. 2010;47:392–399. doi: 10.1603/me09157. [DOI] [PubMed] [Google Scholar]
- Wolstenholme AJ, Rogers AT. Glutamate-gated chloride channels and the mode of action of the avermectin/milbemycin anthelmintics. Parasitology. 2005;131(Suppl):S85–S95. doi: 10.1017/S0031182005008218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


