Abstract
Agropyron cristatum (L.) Gaertn. (2n = 4x = 28, PPPP) not only is cultivated as pasture fodder but also could provide many desirable genes for wheat improvement. It is critical to obtain common wheat–A. cristatum alien disomic addition lines to locate the desired genes on the P genome chromosomes. Comparative analysis of the homoeologous relationships between the P genome chromosome and wheat genome chromosomes is a key step in transferring different desirable genes into common wheat and producing the desired alien translocation line while compensating for the loss of wheat chromatin. In this study, six common wheat–A. cristatum disomic addition lines were produced and analyzed by phenotypic examination, genomic in situ hybridization (GISH), SSR markers from the ABD genomes and STS markers from the P genome. Comparative maps, six in total, were generated and demonstrated that all six addition lines belonged to homoeologous group 6. However, chromosome 6P had undergone obvious rearrangements in different addition lines compared with the wheat chromosome, indicating that to obtain a genetic compensating alien translocation line, one should recombine alien chromosomal regions with homoeologous wheat chromosomes. Indeed, these addition lines were classified into four types based on the comparative mapping: 6PI, 6PII, 6PIII, and 6PIV. The different types of chromosome 6P possessed different desirable genes. For example, the 6PI type, containing three addition lines, carried genes conferring high numbers of kernels per spike and resistance to powdery mildew, important traits for wheat improvement. These results may prove valuable for promoting the development of conventional chromosome engineering techniques toward molecular chromosome engineering.
Introduction
Transferring desirable genes from wild relatives into common wheat is an important strategy for wheat breeding. Since McFadden [1] first transferred a stem rust resistance gene from Triticum dicoccum into common wheat, breeders and geneticists have transferred numerous useful genes into wheat through genetic manipulation. The range of hybridizations between wheat and its wild relatives has been extended continuously. All the genera of the tribe Triticeae have been successfully hybridized with wheat, including 23 genomes and more than 100 alien genes that have been transferred into wheat and named [2], [3]. However, due to insufficient compensation or genetic drag, the alien genes with important roles in wheat breeding involve only 5 linkage groups, including the following 15 genes: Lr24/Sr24, Sr26 from Thinopyrum elongatum, Sr36/Pm6 from Triticum timopheevii, Lr26/Sr31/Yr9/Pm8 from the translocation line T1BL·1R#1S, Gb2/Pm17 from T1AL·1R#2S of Secale cereale, and Yr17/Lr37/Sr38/Cre5 from Aegilops ventricosa [4], [5], [6].
Producing wheat–alien chromosome disomic addition lines and analyzing their genetic constitutions is a key step for the effective transfer of useful genes. Since the 1950s, many wheat–alien chromosome disomic addition lines have been produced, such as rye [7], barley [8], Dasypyrum villosum [9], and several species of the genera Agropyron [25], [26], Aegilops [10], and Thinopyrum [11]. These addition lines are often used as bridge materials to transfer desirable genes to wheat. Understanding the genetic constitutions of addition lines could be helpful for producing compensating translocations for the transfer of genes from alien chromosomes into the wheat genomes. Disomic addition lines can be identified by means such as morphological analysis, chromosome banding, in situ hybridization, and molecular markers. Molecular markers have been widely used to determine the homoeologous relationships between alien and wheat chromosomes [27], [62], [63], [64], [65].
Agropyron Gaertn. (P genome), a perennial genus of the tribe Triticeae, not only is cultivated as pasture fodder but also could provide many desirable genes for wheat improvement [12], [13], [14]. Previous wide crosses between common wheat and Agropyron have been considered unsuccessful [15], [16]. It may not be possible to transfer genes from Agropyron to Triticum even if the intergeneric hybrids can be obtained [12]. However, the application of embryo rescue has enabled the successful hybridization of common wheat with Agropyron species, such as A. cristatum, A. desertorum, A. fragile and A. michnoi [17], [18], [19], [20], [21], [22], [23], [24]. In our laboratory, the common wheat Fukuhokomugi was used as the maternal parent in a hybridization with the A. cristatum accession Z559, which originated from Xinjiang, China [23]. Following several generations of backcrossing or selfing, a series of disomic addition lines was obtained [25], [26], including the stable disomic addition line 4844-12, which possessed large spikes with multiple florets and grains and was identified as a 6P disomic addition line by genetic control analysis [27]. The successful distant hybridization between common wheat and A. cristatum, as well as the development of addition lines, are the foundation of the transfer of desirable genes from A. cristatum to wheat.
In this study, six wheat–A. cristatum disomic addition lines were identified using GISH, SSR and STS molecular markers, and their genetic constitutions and morphology were comparatively analyzed. The aim of this work was to provide guidance for transferring desirable compensating genes and for improving the utilization efficiency of wheat breeding through distant hybridization.
Materials and Methods
Our experiment was carried out at the CAAS Experiment Station, and the studies did not involve the protected area of land and endangered or protected species.
Plant Materials
Six wheat–A. cristatum alien addition lines were obtained after several generations of backcrossing or selfing following the hybridization of the common wheat Fukuhokomugi with A. cristatum accession Z559 (2n = 4x = 28, PPPP). The accession numbers of these lines are 4844-12, 5113, 5114, 5106, II-26, and II-29-2i (Table 1), and one of them (4844-12) was 6P disomic addition line identified previously by Wu et al. [27].
Table 1. Six addition lines from Triticum aestivum and A. cristatum.
| Accession no. | Original F1 hybrids | Generations |
| 4844-12 | FC-1 | F6 |
| 5113 | FC-1 | BC1F2 |
| 5114 | FC-1 | BC1F2 |
| 5106 | FC-1 | BC1F2 |
| II-26 | FC-2 | BC3F1 |
| II-29-2i | FC-2 | BC3F1 |
Morphology of Addition Lines
During the 2010–2011 wheat growing season, these lines were planted in a field trial with two replicates at the CAAS Experiment Station in Beijing, China. Each line was planted in three 2-m rows spaced 30 cm apart, with 30 seeds in each row. For each replicate, ten plants were harvested to evaluate their agronomic traits, including grain number per spikelet, grain number per spike, and thousand-grain weight, etc. The means for the different lines were compared using Fisher’s LSD (P<0.01) in the SAS package (V8.1, SAS Institute Inc., Cary, NC, USA). The evaluation of powdery mildew resistance was performed under field conditions by inoculating the adult plants with Erysiphe graminis f. sp. tritici (Egt) isolate E09. The scale described by Li et al. [28] was used to score the infection types as HR (highly resistant), R (resistant), MR (moderately resistant), S (susceptible), or HS (highly susceptible).
Chromosome Preparation and GISH
To determine whether there were A. cristatum chromosomes in each line, the chromosome composition was confirmed using GISH analysis. The methods for preparing chromosomes from the plant root tip cells and pollen mother cells (PMCs) were described in Cuadrado et al. [29]. The cytological observations were performed using a BX51 Olympus phase-contrast microscope (Olympus Corp., Tokyo, Japan).
A. cristatum genomic DNA was used as a probe to detect the P genome chromosome, and Fukuhokomugi genomic DNA was used for blocking. GISH was performed on the root tip cells and PMCs in accordance with the basic method described by Cuadrado et al. [29] and the improved procedure described by Liu et al. [30]. The in situ hybridization images were obtained using an Olympus AX80 (Japan) fluorescence microscope and were processed using Photoshop CS 3.0 (Adobe, San Jose, CA, USA).
Molecular Marker Analysis
The SDS method was used to extract genomic DNA from A. cristatum (Z559), the recipient parent Fukuhokomugi and the plants that had been identified as disomic addition lines [31].
In total, 904 SSR primer pairs and 422 EST-SSR primer pairs [32], [33], [34], [35], [36] were used to analyze the homoeologous relationships between the P genome chromosomes and the wheat genome chromosomes. In total, 2,815 sequence-tagged site (STS) markers were designed based on A. cristatum expressed sequence tags (ESTs) and suppression subtractive hybridization between 4844-12 and the recipient parent, Fukuhokomugi. These STS markers were used to analyze the genetic constitutions of the six addition lines. PCR was performed as previously described by Luan et al. [37]. The amplification products were separated on 6% denaturing polyacrylamide gels and were visualized by silver staining.
A cluster analysis of the six addition lines, based on the STS markers, was performed to generate a dendrogram using the unweighted pair group method with arithmetic averages (UPGMA) in the SAS software package (V8.1, SAS Institute Inc., Cary, NC, USA).
Construction of Chromosome 6P Comparative Maps
Comparative maps of chromosome 6P were constructed based on the relative locations of the STS markers specific to the 6P disomic addition lines. The relative locations of the 6P-specific markers were determined by comparing the ESTs corresponding to the markers with the deletion-mapped ESTs from hexaploid wheat using local BLASTN and TBLASTX algorithms. The sequence comparison was performed as previously described [38], [39]. The high-scoring pairs (HSPs) with an E-value greater than 1E-5 were rejected. ESTs with matched wheat ESTs that were not mapped or that mapped only to a chromosome or chromosome arm without clear loci, were excluded from the analysis [40], [41]. The chromosomal breakpoints were determined by deletion mapping [42], [43]. Homoeologous group 6 of the A, B, and D wheat genomes was combined into a single consensus wheat genome to construct the group 6 consensus chromosome bin map, as previously described [44]. The breakpoints on this consensus chromosome bin map divided the chromosome into a larger number of bins than the individual chromosomes. By comparison, the specific markers that contained location information were used to construct the comparative maps. Details of the mapped wheat ESTs can be found at http://wheat.pw.usda.gov/cgi-bin/westsql/map_locus.cgi.
Results
GISH Detection of the Six Addition Lines
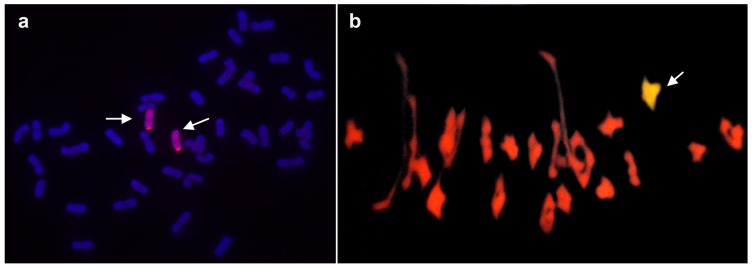
GISH was performed to detect A. cristatum chromosomes in the six addition lines 4844-12, 5113, 5114, 5106, II-26, and II-29-2i. Mitotic GISH revealed that the root-tip cells contained two A. cristatum chromosomes of the addition line II-26 (Figure 1a). Meiotic GISH indicated that two P genome chromosomes were paired in the PMCs at meiotic metaphase I of the addition line II-26 (Figure 1b). Observations from the other addition lines were the same with that from the addition line II-26 (data not shown), which confirmed that all the materials were wheat–A. cristatum disomic addition lines (2n = 44).
Figure 1. GISH patterns of wheat-A. cristatum addition line II-26.
(a) Chromosomes in red are A. cristatum chromosomes. The arrows indicate two alien P chromosomes in the addition line at mitotic metaphase. (b) Chromosomes in yellow-green are A. cristatum chromosomes. The arrow indicates a ring bivalent formed between the two P chromosomes at meiotic metaphase I.
Homoeology of A. cristatum Chromosomes with Wheat Chromosomes
A total of 904 SSR and 422 EST-SSR markers distributed over seven wheat homoeologous groups were used to analyze Z559 and Fukuhokomugi. The marker analysis showed that 334 (37.0%) of the SSR and 229 (54.3%) of the EST-SSR markers were polymorphic between Z559 and Fukuhokomugi (Table 2). Markers specific to A. cristatum accounted for 42.5% of the total and were distributed over seven wheat homoeologous groups. The 563 polymorphic SSR and EST-SSR markers were further used to analyze the six wheat–A. cristatum disomic addition lines and their parents, Z559 and Fukuhokomugi. Of the polymorphic markers, fifty-one successfully amplified P genome-specific bands in the addition lines (Table 3), e.g., Xgwm113 and Cfd80 on chromosomes 4B and 6A/6D, respectively (Figure 2). The largest number of specific markers, 20 (39.2%), belonged to homoeologous group 6. Among the other markers, 7 (13.7%) belonged to homoeologous group 1, 8 (15.7%) to group 2, 4 (7.8%) to group 3, 4 (7.8%) to group 4, 4 (7.8%) to group 5, and 4 (7.8%) to group 7.
Table 2. Polymorphism of SSR and EST-SSR markers between A. cristatum and Fukuhokomugi.
| Type of marker | Series | Number of detected markers | Number of polymorphic markers | Proportion (%) |
| SSR | Xgwm | 233 | 76 | 32.62 |
| Xgdm | 69 | 34 | 49.28 | |
| Barc | 206 | 75 | 36.41 | |
| Wmc | 261 | 104 | 39.85 | |
| Cfd | 109 | 36 | 33.03 | |
| Cfa | 26 | 9 | 34.62 | |
| Total | 904 | 334 | 36.95 | |
| EST-SSR | Ksum | 51 | 20 | 39.21 |
| Cnl | 19 | 7 | 36.84 | |
| Cwem | 26 | 12 | 46.15 | |
| Cfe | 156 | 73 | 46.79 | |
| Swes | 170 | 117 | 68.82 | |
| Total | 422 | 299 | 54.27 | |
| Total | 1326 | 563 | 42.46 |
Table 3. PCR amplification of P chromatin in the six addition lines using SSR and EST-SSR markers.
| Primers | Chromosome | Z559 | Fukuhokomugi | II-29-2i | 5113 | II-26 | 5106 | 4844-12 | 5114 |
| Wmc716 | 1AL | + | − | + | + | + | + | + | + |
| Xgwm268 | 1BL | + | − | − | − | − | − | + | − |
| Swes215 | 1B | + | − | + | + | + | + | + | + |
| Xgwm232 | 1DL | + | − | − | − | − | + | − | − |
| Cfe26 | 1A1B | + | − | − | + | + | + | + | − |
| Swes145 | 1A1B | + | − | − | − | − | + | − | − |
| Swes98 | 1B1D | + | − | + | + | + | + | + | + |
| Xgwm265 | 2AL | + | − | + | + | + | + | + | + |
| Xgwm339 | 2AL | + | − | + | + | + | + | + | + |
| Xgwm425 | 2AL | + | − | + | + | + | + | + | + |
| Xgwm614 | 2AS | + | − | + | − | − | + | + | + |
| Xgdm107 | 2DS | + | − | − | − | − | + | − | − |
| Xgdm35 | 2DS | + | − | + | + | + | + | + | + |
| Xgwm455 | 2DS | + | − | + | + | + | + | + | + |
| Barc335 | 2D | + | − | + | + | + | + | + | + |
| Xgwm674 | 3AS | + | − | + | + | + | + | + | + |
| Wmc612 | 3BS | + | − | − | − | − | − | + | − |
| Wmc623 | 3BS | + | − | + | + | + | + | + | + |
| Xgdm38 | 3DL | + | − | + | + | + | + | + | + |
| Xgwm113 | 4BL | + | − | + | + | + | + | + | + |
| Ksum154 | 4BL | + | − | − | − | − | + | + | − |
| Xgdm61 | 4DL | + | − | + | + | + | + | + | + |
| Wmc617 | 4AL4BL4DS | + | − | + | + | + | + | + | + |
| Swes71 | 5B | + | − | − | + | + | − | − | − |
| Xgdm116 | 5DL | + | − | − | − | − | − | + | − |
| Xgwm654 | 5DL | + | − | + | + | + | + | + | + |
| Cnl142 | 5BS | + | − | + | + | + | + | + | + |
| Cfe132 | 6A | + | − | + | + | + | + | + | + |
| Cfe179 | 6A | + | − | + | + | + | + | + | + |
| Xgdm147 | 6B | + | − | − | + | + | − | − | − |
| Cfe32 | 6B | + | − | + | + | + | + | + | + |
| Cnl64 | 6BS | + | − | + | + | + | + | + | + |
| Cnl113 | 6BS | + | − | + | + | + | + | + | + |
| Swes180 | 6BS | + | − | + | + | + | + | + | + |
| Barc79 | 6BL | + | − | + | + | + | + | + | + |
| Xgwm608 | 6BL | + | − | + | + | + | + | + | + |
| Xgwm644 | 6BL | + | − | + | + | + | + | + | + |
| Xgdm61 | 6BL | + | − | − | + | + | − | − | − |
| Xgwm311 | 6BL2A | + | − | + | + | + | + | + | + |
| Barc123 | 6DS | + | − | + | + | + | + | + | + |
| Xgwm469 | 6DS | + | − | + | + | + | + | + | + |
| Barc21 | 6DL | + | − | + | + | + | + | + | + |
| Barc175 | 6D | + | − | + | + | + | + | + | + |
| Barc146 | 6A6B | + | − | + | + | + | + | + | + |
| Cfd80 | 6A6D | + | − | + | + | + | + | + | + |
| Swes2 | 6A6BL6DS | + | − | − | − | − | − | + | − |
| Cfe2 | 6A6B6D | + | − | − | − | − | − | + | − |
| Barc1167 | 7A | + | − | + | + | + | + | + | + |
| Xgwm282 | 7AL | + | − | + | + | + | + | + | + |
| Wmc76 | 7BL | + | − | − | − | − | + | − | − |
| Swes157 | 7A7D | + | − | − | − | − | + | − | − |
+, − represent positive and negative amplification of SSR and EST-SSR markers, respectively.
Figure 2. PCR amplification profiles using primers Xgwm113 (a) and Cfd80 (b).
M: DNA ladder; Z: A. cristatum (Z559); F: Fukuhokomugi; 1: II-29-2i; 2∶5113; 3: II-26; 4∶5106; 5∶4844-12; 6∶5114. The arrows indicate the diagnostic bands of P chromatin.
According to the genetic maps of SSR and EST-SSR [32], [33], [34], [35], [36], 32 markers which amplified specific bands in the wheat–A. cristatum addition lines were located on 21 pairs of wheat chromosomes. A map of chromosome 6P was generated based on the locations of the above markers on wheat chromosomes A, B, and D (Figure 3). This map indicated that 21.9% of the SSR and EST-SSR markers specific to the P genome were located near the pericentromeric region of 6A, 6B, and 6D; the remaining specific markers were distributed in the distal region of the chromosome (Figure 3). The homoeologous group of one chromosome could be determined by the markers clustered in the pericentromeric region, for the sequence of this region was highly conserved. Therefore, the A. cristatum chromosomes in the six addition lines (4844-12, 5113, 5114, 5106, II-26, and II-29-2i) were homoeologous to group 6 of common wheat and were thus designated as 6P. The mapped molecular markers specific to addition lines were primarily related to homoeologous group 6 (43.8%); the remaining markers (total 56.2%) were related to other homoeologous groups. This finding indicates that chromosome 6P may have undergone rearrangements.
Figure 3. A map of the A. cristatum chromosomes in the six addition lines.
a, b, c, d, e, and f represent the A. cristatum chromosomes in the disomic addition lines 4844-12, 5113, 5114, 5106, II-29-2i, and II-26, respectively. The colored strips and the numbers 1, 2, 3, 4, 5, 6, and 7 represent the homoeologous groups 1 to 7 in wheat. The markers in the dotted box indicate the same loci in the pericentromeric region of the six addition lines.
Construction of Chromosome 6P Comparative Maps and Further Confirmation of Rearrangements
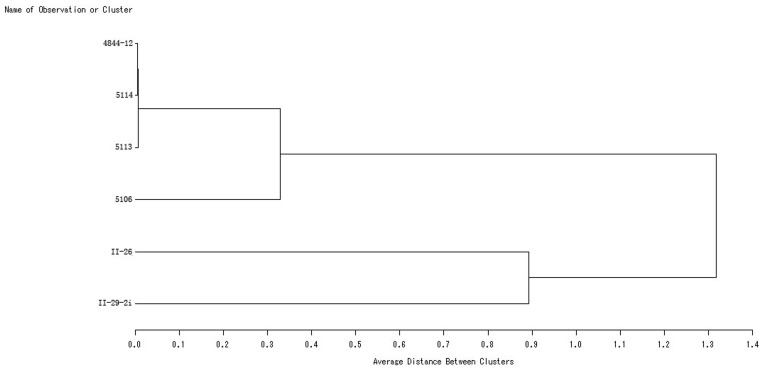
2,815 STS markers were used to analyze A. cristatum accession Z559, common wheat Fukuhokomugi and the six wheat–A. cristatum 6P disomic addition lines (4844-12, 5113, 5114, 5106, II-29-2i, and II-26). The results demonstrated that 688 primer pairs amplified P genome-specific bands, and the amplification results differed among the addition lines. There were 14 types of results (Table 4). Type 1 (Figure 4a) were the largest group (38.5%), followed by the II-29-2i-specific markers (27.2%). Although 44 markers were common to all six addition lines (Figure 4b), these represented only 6.4% of the total, and even though there were many common specific markers for 4844-12, 5113, and 5114, the minor differences could also be found by molecular marker analysis. This result indicated that the six addition lines differed in terms of chromosome 6P and genetic constitution. The cluster analysis performed using UPGMA (SAS V8.1 software) indicated that the six addition lines could be divided into four groups (Figure 5): 6PI (4844-12/5113/5114), 6PII (5106), 6PIII (II-29-2i), and 6PIV (II-26).
Table 4. Types of STS markers specific to chromosome 6P in wheat–A. cristatum addition lines.
| Type | Z559 | Fukuhokomugi | 4844-12 | 5113 | 5114 | 5106 | II-26 | II-29-2i | Number of markers | Percentage (%) |
| 1 | + | − | + | + | + | + | − | − | 265 | 38.52 |
| 2 | + | − | + | + | + | + | + | + | 44 | 6.40 |
| 3 | + | − | + | + | + | + | + | − | 26 | 3.78 |
| 4 | + | − | + | + | + | + | − | + | 15 | 2.18 |
| 5 | + | − | − | − | − | − | − | + | 187 | 27.18 |
| 6 | + | − | − | − | − | − | + | − | 29 | 4.22 |
| 7 | + | − | − | − | − | + | + | − | 69 | 10.03 |
| 8 | + | − | − | − | − | + | − | + | 23 | 3.34 |
| 9 | + | − | − | − | − | + | + | + | 20 | 2.91 |
| 10 | + | − | − | − | − | + | − | − | 6 | 0.87 |
| 11 | + | − | + | − | + | − | + | + | 1 | 0.15 |
| 12 | + | − | + | − | + | + | − | − | 1 | 0.15 |
| 13 | + | − | + | − | + | − | − | − | 1 | 0.15 |
| 14 | + | − | − | + | + | + | − | + | 1 | 0.15 |
| Total | 688 | 0 | 353 | 351 | 354 | 470 | 189 | 290 | 688 | 100 |
+, − represent positive and negative amplification of STS markers, respectively.
Figure 4. PCR amplification profiles using STS markers Agc70446 (a) and Agc13186 (b).
M: DNA ladder; Z: A. cristatum (Z559); F: Fukuhokomugi; 1∶4844-12; 2∶5113; 3∶5114; 4∶5106; 5: II-26; 6: II-29-2i. The arrows indicate diagnostic bands of P chromatin.
Figure 5. Cluster tree of the six addition lines based on STS markers and generated using UPGMA.
A large number of the ESTs that were physically mapped in wheat chromosome bins provided valuable information about the chromosome or genome constitution. A sequence comparison was performed between 688 corresponding ESTs of the STS markers specific to the wheat–A. cristatum addition lines and wheat ESTs with known physical locations [42], [43]. 160 ESTs of A. cristatum could match mapped wheat ESTs by this comparison. The locations of the 6P-specific markers were determined based on the information derived from the matched wheat ESTs.
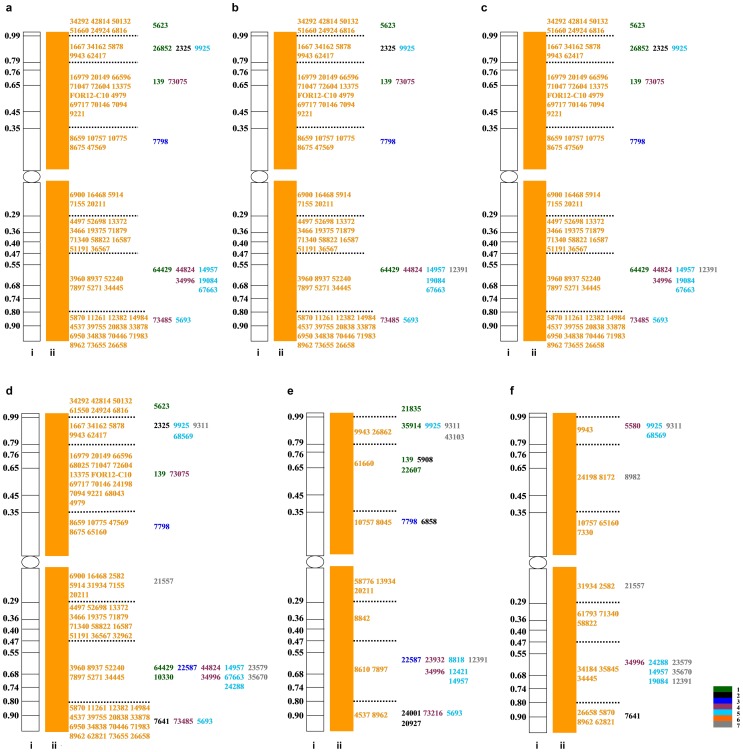
Using the wheat homoeologous group 6 consensus chromosome bin map as a reference, chromosome 6P was divided into 8 bins (6S-0.00–0.35, 6S-0.35–0.79, 6S-0.79–0.99, and 6S-0.99–1.00; 6L-0.00–0.29, 6L-0.29–0.47, 6L-0.47–0.80, and 6L-0.80–1.00) based on the number and distribution pattern of the 6P-specific STS markers. The STS markers that mapped to a portion of a chromosome arm containing more than one bin were excluded from the analysis. Thus, 124 of 160 markers were mapped to different chromosome 6P bins, and six different comparative maps were constructed (Figure 6). There were 80, 79, 81, 94, 34, and 32 markers along the six 6P chromosomes. It was found on the maps that most of the STS markers (81.3%, 82.3%, 80.3%, 76.6%, 38.2%, and 56.3%) belonged to homoeologous group 6, and the remaining markers which distributed mainly in distal regions (18.8%, 17.7%, 19.8%, 23.4%, 61.8%, and 43.8%) belonged to other homoeologous groups. Therefore, these results are consistent with those from the wheat molecular markers and further confirmed that the A. cristatum chromosomes had a homoeologous relationship with group 6 of wheat.
Figure 6. Comparative maps of A. cristatum chromosome 6P in the six addition lines.
a, b, c, d, e, and f represent the A. cristatum chromosomes in the disomic addition lines 4844-12, 5113, 5114, 5106, II-29-2i, and II-26, respectively. i: Consensus bin map of wheat group 6; ii: The specific markers distributed in different bins along the chromosome 6P. The colored strips and the numbers 1, 2, 3, 4, 5, 6, and 7 represent the homoeologous groups 1 to 7. The colored markers which belonged to different homoeologous groups were consistent with the colored strips. The ‘Agc’ of the marker names were ellipsis in the maps.
The six addition lines were divided into four types (6PI, 6PII, 6PIII, and 6PIV) based on a cluster analysis. There were large differences among the different chromosome 6P types in terms of both the number of markers and the distribution density on chromosome 6P. Although all of the added A. cristatum chromosomes were chromosome 6P, their molecular markers of homoeologous group 6 exhibited varying degrees of difference. The markers in the dotted box of Figure 3 showed the same loci in the pericentromeric region of the six addition lines. However, Figure 6 showed the homoeologous group 6 markers in the pericentromeric region were not completely identical among types 6PI–6PIV; moreover, there were several markers belonging to other homoeologous groups. The results further demonstrated that the genetic constitution of chromosome 6P differed among the six addition lines. Additionally, the results indicated that different types of chromosome 6P underwent obvious structural rearrangements compared with the wheat genome, and these rearrangements occurred mainly in the distal region of chromosome 6P.
Chromosomal Locations of Desirable Alien Genes in Addition Lines
The main agronomic traits of the six wheat–A. cristatum addition lines are shown in table 5. All of agronomic traits were analyzed by an analysis of variance with repeated measures (ANOVAR, P<0.01) [45]. We found significant differences in grain number per spikelet and grain number per spike; 6PI (comprising three addition lines: 4844-12, 5113, and 5114) was significantly higher than the wheat parent, Fukuhokomugi, with no infertile spikelets (Figure 7a). Previous study has reported that a gene(s) controlling high numbers of kernels per spike is located on chromosome 6P of the wheat–A. cristatum addition line 4844-12 [27]. A further comparative analysis of the genetic constitutions of 5113, 5114, and 4844-12 revealed that their A. cristatum chromosomes were all 6PI, suggesting that the added chromosome 6PI of 4844-12, 5113, and 5114 carried a gene(s) conferring high numbers of kernels per spike.
Table 5. Comparison of main agronomic traits among the six wheat–A. cristatum disomic addition lines.
| Addition line | Chromosome composition | Spike length (cm) | Spikelet number per spike | Grain number per spikelet | Grain number per spike | Thousand-grain weight | Powdery mildew resistance |
| Fukuhokomugi | 42Wa | 7.36Eb | 14.80C | 4.00C | 40.50B | 24.60BC | HS |
| 4844-12 | 42W+2PI | 10.39BC | 22.00A | 6.40A | 95.70A | 43.19A | HR |
| 5113 | 42W+2PI | 11.88A | 22.30A | 5.40B | 88.40A | 20.64C | HR |
| 5114 | 42W+2PI | 10.54B | 20.70A | 6.30A | 87.50A | 29.23B | HR |
| 5106 | 42W+2PII | 8.83D | 17.70B | 2.90D | 25.20C | 37.62A | HS |
| II-29-2i | 42W+2PIII | 7.13E | 17.60B | 3.10D | 32.60BC | 28.86B | S |
| II-26 | 42W+2PIV | 9.36CD | 18.50B | 2.70D | 33.60BC | 38.11A | HS |
P and W indicate A. cristatum and wheat chromosomes, respectively.
Means followed by the same letter are not significantly different (P<0.01) based on Fisher’s LSD.
Figure 7. Morphological comparison of the six wheat-A. cristatum disomic addition lines.
(a) Spike morphology (b) Morphology of flag leaves. (c) Symptoms of powdery mildew resistance. The materials from left to right in a, b, and c are 4844-12, 5113, 5114, 5106, II-26, and II-29-2i, respectively.
The evaluation of the six addition lines for resistance to powdery mildew showed that the addition lines differed in their degree of powdery mildew resistance. 4844-12, 5113, and 5114 were highly resistant to Egt isolate E09, whereas II-29-2i was susceptible and II-26 and 5106 were highly susceptible to E09 (Figure 7c). Comparison of the powdery mildew resistance of the recipient wheat Fukuhokomugi (highly susceptible) and the alien donor A. cristatum (immune) indicated that 6PI carried genes conferring not only high numbers of kernels per spike but also resistance to powdery mildew.
In addition, the sizes of the flag leaves in the six addition lines differed; II-29-2i had an especially slender flag leaf (Figure 7b), which was similar to that of A. cristatum. Moreover, there were no significant differences between II-29-2i and the wheat parent, Fukuhokomugi, in grain number per spike or thousand-kernel weight (Table 5). This specific trait may be useful in wheat improvement programs.
Discussion
In contrast to conventional disomic addition lines, this study identified 4 types of wheat–A. cristatum 6P disomic addition lines based on the alien chromosome (6P) rearrangements. Theoretically, the number of a complete set of wheat–A. cristatum disomic addition lines should be seven, with one pair of alien chromosomes corresponding to one disomic addition line, and there should be only one 6P disomic addition line. In fact, six wheat–A. cristatum 6P disomic addition lines in this study were obtained. They were derived from two F1 hybrids and could be divided into four types by molecular marker analysis, indicating that the two F1 hybrids likely contained a different PP combination from the tetraploid parent, Agropyron cristatum. Agropyron are cross-pollinating plants. The tetraploid A. cristatum originates from derivatives of hybridizations between diploid A. cristatum and A. mongolicum. Although the diploid A. cristatum and A. mongolicum contain the same basic P genome, their P genomes exhibited rearrangements and variation [46], [47]. The two P genomes exhibit segmental autosomy in the tetraploid A. cristatum [48] and are distinguished from each other by structural rearrangements [46]. Studies of genetic diversity have shown that there are genetic variations among different individuals within one population [66], [67], thus there might be large genetic differences between the P chromosomes of the two F1 hybrids. We accordingly speculated that the major rearrangements of P-genome chromosomes might have been present in the tetraploid A. cristatum before the hybridization of wheat with A. cristatum. This phenomenon of rearrangements can also be found in the genomes of other wild relatives of wheat [62], [63], [64], [65]. Besides the genetic rearrangements of P-genome in A. cristatum, genomic rearrangements may occur in wheat–A. cristatum addition line due to the instability of addition line chromosome. As found in Alkhimova et al. [68] and Szakács et al. [69], the variability of wheat–rye addition line led to many rearrangements of rye chromosomes. Bento et al. [70] and Tomás et al. [71] discovered that genomic rearrangements occurred during the wheat–rye addition lines creation and appeared in the form of elimination of DNA sequences. These studies seem to suggest that some genetic differences of 6P chromosomes in this paper may result from elimination of DNA sequences. Sequence elimination could be reflected by the disappearance of many A. cristatum specific bands from some 6P addition lines. On this account, there were minor differences among 4844-12, 5113, and 5114, which belong to 6PI. In addition, we found that elimination of repetitive sequences on some chromosomes of ABD genomes was present in 4844-12 (unpublished results), and this situation could also be found in wheat–rye monosomic addition lines [72]. Elimination of DNA sequences might be the common form of rearrangements in addition line. Recombination between wheat genome and P genome did not occur in the six addition lines from the results of meiotic GISH, but we could not rule out the possibility of recombination in F1 hybrids. Furthermore, mobilization of active transposons induced by wide hybridization may be present, a phenomenon not examined in the present study. All in all, the genetic differences of the six wheat–A. cristatum addition lines might be a result of many aspects.
The genetic constitutions of different types of addition lines provide important guidance for transferring desirable compensating genes into wheat through wide hybridization. Transferring alien desirable genes and improving utilization efficiency in wheat improvement are formidable tasks due to linkage drag or insufficient compensation for the loss of wheat chromatin. To overcome this difficulty, it is necessary to identify the alien chromosomal regions of target genes and to analyze their homoeologous relationships with wheat chromosomes. Only well-compensating translocations or introgressions produced by recombination between alien chromosomal regions and homoeologous wheat chromosomes are beneficial for wheat improvement. Indeed, the 15 useful alien genes (Lr24/Sr24, Sr26, Sr36/Pm6, Lr26/Sr31/Yr9/Pm8, Gb2/Pm17, and Yr17/Lr37/Sr38/Cre5) that significantly contribute to agriculture are, without exception, compensating translocations [4], [5]. In our study, chromosome 6P contained many fragments that did not belong to homoeologous groups 6. If producing compensating translocations, different wheat homoeologous groups may be involved. We found that the marker Agc26852 was specific to 6PI (4844-12/5114) by analyzing the comparative maps. Marker Agc26852 belonged to homoeologous group 1 and mapped to bin 6PS-0.79–0.99; therefore, we inferred that this marker might be associated with the chromosomal region conferring high numbers of kernels per spike or powdery mildew resistance and this region also might belong to homoeologous group 1. Previous QTL mapping results have shown that a major QTL controlling high numbers of kernels per spike was located on 1AS of Pubing 3228, which was derived from 4844-12 [52]. The results not only suggested that genes conferring high numbers of kernels per spike had been transferred into common wheat but also were consistent with our prediction. Thus, understanding the genetic constitutions of different types of addition lines may be helpful for producing compensating translocations for gene transfer from 6P into the wheat genome.
The efficiency and precision of conventional breeding could be increased by means of the marker-assisted selection of objective traits [49], [50]. When breeders perform wide crosses, they have no idea of the locations of the targeted genes. They usually reduce the target region via repeated backcrossing and the use of markers linked to objective traits to accelerate the breeding process [51]. Different types of chromosome 6P possess different desirable genes due to rearrangements. We deduced that the marker Agc26852 specific to 6PI (4844-12/5114) was related to the chromosomal region containing genes conferring high numbers of kernels per spike and powdery mildew resistance and mapped to bin 6PS-0.79–0.99. To physically locate these genes, numerous translocation lines were obtained by inducing 4844-12 using 60Co-γirradiation and gametocidal chromosomes [37], [53]. The marker Agc26852 may be useful in selecting translocations with the characteristic of high numbers of kernels per spike. A large number of studies have shown that further improving wheat yield mainly depends on increasing the grain number per spike and an increasing number of specialists focus on transferring genes related to yield from wild relatives to wheat [54], [55]. Addition line II-29-2i may possess high photosynthetic efficiency because of its especially slender flag leaf and no decreasing in grain number per spike or thousand-kernel weight compared to the wheat parent, Fukuhokomugi. The improvement in photosynthesis per unit leaf area was correlated with increases in the harvest index and kernel number per square meter [56]. When other constraint conditions are not restricted, enhancing photosynthesis improves crop yield [57]. There were 187 markers specific to 6PIII (II-29-2i), and 27 markers matched to mapped wheat ESTs. The latter 27 markers were distributed across different chromosomal bins and belonged to different homoeologous groups, making it difficult to determine the location and homoeologous group of the gene conferring high photosynthetic efficiency. This difficulty may be related to the rearrangements of 6PIII being more complex than those of the other three chromosome 6P types. However, these markers may contribute to producing compensating translocations. In addition, structural rearrangements led to different phenotypes in the six addition lines. There were significant phenotypic differences between 6PI (4844-12/5113/5114) and the other three types, which is consistent with the results related to their genetic constitutions. However, there were no differences among 6PII (5106), 6PIII (II-29-2i), and 6PIV (II-26) types, especially in spikelet number per spike, grain number per spikelet and grain number per spike. The reason why the phenotypes were different from the genetic constitution results may be that the differences in genetic constitution did not reflect the traits we focused on. Therefore, a greater understanding of the genetic constitutions of the six addition lines would contribute to the ability to transfer useful genes from 6PI and 6PIII into the wheat genome and improve the utilization efficiency of these genes in germplasm enhancement programs.
The development of comparative maps of chromosome 6P will promote the development of conventional chromosome engineering toward molecular chromosome engineering. Comparative genomics has shown that gene content and order are highly conserved between related plant species [58], [59], [60]. The conservation of the content and order of genes among different species has improved the effectiveness and predictive value of information transfer. In this study, we utilized the conserved synteny between common wheat and A. cristatum to identify six addition lines as wheat–A. cristatum 6P disomic addition lines and to develop six comparative maps of chromosome 6P. The locations of STS markers on chromosome 6P were determined based on the locations of mapped wheat ESTs. However, the reliability of these maps should be verified using a deletion bin map of chromosome 6P. After all, we could not rule out the possibility of non-homologous translocation, duplication or intrachromosomal inversion of P chromosomes like other genome chromosomes of Triticeae [64], [65], [73]. The locations of STS markers may be not consistent with their actual physical locations. Molecular markers clustered together can effectively detect the chromosomal fragment that contains a desirable gene. Niu et al. [61] efficiently eliminated a large amount of Aegilops speltoides chromatin surrounding Sr39 using DNA marker-assisted chromosome engineering. In our lab, we have produced many translocation lines of chromosome 6P, and these maps were used to determine the size of alien chromatin and which bin they were from (unpublished data). A similar study was conducted in the identification of 4VS translocations [74]. The development of comparative maps of chromosome 6P in this study may facilitate the transfer and elimination of alien chromatin (6P) tagged by molecular markers, allowing researchers to overcome the inefficiency and randomness of conventional chromosome engineering.
Acknowledgments
The authors gratefully acknowledge Dr. Steven S. Xu (USDA-ARS) for revising the manuscript.
Funding Statement
The financial support was provided by the National Basic Research Program of China (Grant No. 2011CB100104; URL: http://www.973.gov.cn/AreaAppl.aspx) and the National High Technology Research and Development Program of China (Grant No. 2011AA100102; URL: http://www.863.gov.cn/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. McFadden ES (1930) A successful transfer of emmer characters to vulgare wheat. Journal of the American Society of Agronomy 22: 1020–1034. [Google Scholar]
- 2.Wang RRC (2011) Chapter 2. Agropyron and Psathyrostachys. In: Chittaranjan Kole (ed.), Wild Crop Relatives: Genomic and Breeding Resources, Cereals. Springer-Verlag, Berlin and Heidelberg. pp. 77–108.
- 3.Mujeeb-Kazi A, Kazi AG, Dundas I, Rasheed A, Kishii M, et al.. (2013) Genetic diversity for wheat improvement as a conduit to food security. In Sparks D (ed.) Advances in Agronomy, Vol 122, AGRON, UK: Academic Press, pp. 179–258.
- 4. Friebe B, Jiang J, Raupp W, McIntosh R, Gill B (1996) Characterization of wheat-alien translocations conferring resistance to diseases and pests: current status. Euphytica 91: 59–87. [Google Scholar]
- 5. Gill BS, Friebe B, Raupp WJ, Wilson DL, Cox TS, et al. (2006) Wheat genetics resource center: The first 25 years. Advances in Agronomy 89: 73–136. [Google Scholar]
- 6. Jahier J, Abelard P, Tanguy M, Dedryver F, Rivoal R, et al. (2001) The Aegilops ventricosa segment on chromosome 2AS of the wheat cultivar ‘VPM1’ carries the cereal cyst nematode resistance gene Cre5. Plant Breeding 120: 125–128. [Google Scholar]
- 7.Chapman V, Riley R (1955) Disomic addition of rye chromosome II to wheat. Nature 175, 1091–1092.
- 8. Islam A, Shepherd K, Sparrow D (1981) Isolation and characterization of euplasmic wheat-barley chromosome addition lines. Heredity 46: 161–174. [Google Scholar]
- 9. Uslu E, Reader S, Miller T (1999) Characterization of Dasypyrum villosum (L.) Candargy chromosomes by fluorescent in situ hybridization. Hereditas 131: 129–134. [Google Scholar]
- 10. Schneider A, Linc G, Molnár I, Molnár-Láng M (2005) Molecular cytogenetic characterization of Aegilops biuncialis and its use for the identification of 5 derived wheat-Aegilops biuncialis disomic addition lines. Genome 48: 1070–1082. [DOI] [PubMed] [Google Scholar]
- 11. Lin F, Sun Q, Xu S, Chen X, Zhang L, et al. (2009) Identification of wheat-Thinopyrum intermedium alien disomic addition lines conferring resistance to stripe rust. Canadian journal of Plant Science 89: 569–574. [Google Scholar]
- 12.Dewey DR (1984) The genomic system of classification as a guide to intergeneric hybridization with the perennial Triticeae. In: Gustafson JP (ed) Gene manipulation in plant improvement. Proceedings of 16th Stadler Genetics Symposium. New York, pp. 209–279.
- 13.Johnson D (1986) Seed and seedling relations of crested wheatgrass: a review. In: Johnson KL (ed) Crested wheatgrass: its values, problems and myths; symposium proceedings. Utah State Univ, Logan, UT, USA pp. 65–90.
- 14. Dong Y, Zhou R, Xu S, Li L, Cauderon Y, et al. (1992) Desirable characteristics in perennial Triticeae collected in China for wheat improvement. Hereditas 116: 175–178. [Google Scholar]
- 15. Smith D (1942) Intergeneric hybridization of cereals and other grasses. J Agric Res 64: 33–47. [Google Scholar]
- 16. White WJ (1940) Intergeneric crosses between Triticum and Agropyron . Scientific Agriculture 21: 198–232. [Google Scholar]
- 17. Li L, Dong Y (1990) Production and cytogenetic study of intergeneric hybrids between Triticum aestivum and Agropyron desertorum . Sci China (ser B) 34: 45–51. [Google Scholar]
- 18. Chen Q, Jahier J, Cauderon Y (1989) Production and cytogenetical studies of hybrids between Triticum aestivum L. Thell and Agropyron cristatum (L.) Gaertn. CR Acad Sci Paris Ser III 308: 425–430. [Google Scholar]
- 19. Chen Q, Jahier J, Cauderon Y (1990) Intergeneric hybrids between Triticum aestivum and three crested wheatgrasses: Agropyron mongolicum, A. michnoi, and A. desertorum . Genome 33: 663–667. [Google Scholar]
- 20. Limin A, Fowler D (1990) An interspecific hybrid and amphiploid produced from Triticum aestivum crosses with Agropyron cristatum and Agropyron desertorum . Genome 33: 581–584. [Google Scholar]
- 21. Ahmad F, Comeau A (1991) A new intergeneric hybrid between Triticum aestivum L. and Agropyron fragile (Roth) Candargy: Variation in A. fragile for Suppression of the Wheat Ph-Locus Activity. Plant Breeding 106: 275–283. [Google Scholar]
- 22. Li L, Dong Y (1991) Hybridization between Triticum aestivum L. and Agropyron michnoi Roshev. Theor Appl Genet 81: 312–316. [DOI] [PubMed] [Google Scholar]
- 23. Li L, Dong Y, Zhou R, Li X, Li P (1995) Cytogenetics and self-fertility of hybrids between Triticum aestivum L. and Agropyron cristatum (L.) Gaertn. Acta Genetica Sinica 22: 109–114. [Google Scholar]
- 24. Jauhar PP (1992) Chromosome pairing in hybrids between hexaploid bread wheat and tetraploid crested wheatgrass (Agropyron cristatum). Hereditas 116: 107–109. [Google Scholar]
- 25. Li L, Li X, Li P, Dong Y, Zhao G (1997) Establishment of wheat-Agropyron cristatum alien addition lines. I. Cytology of F3, F2 BC1, BC4, and BC3 F1progenies. Acta Genetica Sinica 24: 154–159. [Google Scholar]
- 26. Li L, Yang X, Zhou R, Li X, Dong Y (1998) Establishment of wheat-Agropyron cristatum alien addition lines II. Identification of alien chromosomes and analysis of development approaches. Acta Genetica Sinica 25: 538–544. [Google Scholar]
- 27. Wu J, Yang X, Wang H, Li H, Li L, et al. (2006) The introgression of chromosome 6P specifying for increased numbers of florets and kernels from Agropyron cristatum into wheat. Theor Appl Genet 114: 13–20. [DOI] [PubMed] [Google Scholar]
- 28.Li LH, Li XQ (2006) Descriptors and Data Standard for wheat (Triticum aestivum L.) China Agricultural Press.
- 29. Cuadrado A, Schwarzacher T, Jouve N (2000) Identification of different chromatin classes in wheat using in situ hybridization with simple sequence repeat oligonucleotides. Theor Appl Genet 101: 711–717. [Google Scholar]
- 30. Liu WH, Luan Y, Wang JC, Wang XG, Su JJ, et al. (2010) Production and identification of wheat - Agropyron cristatum (1.4P) alien translocation lines. Genome 53: 472–481. [DOI] [PubMed] [Google Scholar]
- 31. Sharp P, Chao S, Desai S, Gale M (1989) The isolation, characterization and application in the Triticeae of a set of wheat RFLP probes identifying each homoeologous chromosome arm. Theor Appl Genet 78: 342–348. [DOI] [PubMed] [Google Scholar]
- 32. Chen H, Li L, Wei X, Li S, Lei T, et al. (2005) Development, chromosome location and genetic mapping of EST-SSR markers in wheat. Chinese Science Bulletin 50: 2328–2336. [Google Scholar]
- 33. Pestsova E, Ganal M, Röder M (2000) Isolation and mapping of microsatellite markers specific for the D genome of bread wheat. Genome 43: 689–697. [PubMed] [Google Scholar]
- 34. Röder MS, Korzun V, Wendehake K, Plaschke J, Tixier MH, et al. (1998) A microsatellite map of wheat. Genetics 149: 2007–2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Somers DJ, Isaac P, Edwards K (2004) A high-density microsatellite consensus map for bread wheat (Triticum aestivum L.). Theor Appl Genet 109: 1105–1114. [DOI] [PubMed] [Google Scholar]
- 36. Yu JK, Dake TM, Singh S, Benscher D, Li W, et al. (2004) Development and mapping of EST-derived simple sequence repeat markers for hexaploid wheat. Genome 47: 805–818. [DOI] [PubMed] [Google Scholar]
- 37. Luan Y, Wang X, Liu W, Li C, Zhang J, et al. (2010) Production and identification of wheat-Agropyron cristatum 6P translocation lines. Planta 232: 501–510. [DOI] [PubMed] [Google Scholar]
- 38. Sorrells ME, La Rota M, Bermudez-Kandianis CE, Greene RA, Kantety R, et al. (2003) Comparative DNA sequence analysis of wheat and rice genomes. Genome Research 13: 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rota ML, Sorrells ME (2004) Comparative DNA sequence analysis of mapped wheat ESTs reveals the complexity of genome relationships between rice and wheat. Functional & Integrative Genomics 4: 34–46. [DOI] [PubMed] [Google Scholar]
- 40. Munkvold J, Greene R, Bermudez-Kandianis C, La Rota C, Edwards H, et al. (2004) Group 3 chromosome bin maps of wheat and their relationship to rice chromosome 1. Genetics 168: 639–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Conley E, Nduati V, Gonzalez-Hernandez J, Mesfin A, Trudeau-Spanjers M, et al. (2004) A 2600-locus chromosome bin map of wheat homoeologous group 2 reveals interstitial gene-rich islands and colinearity with rice. Genetics 168: 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Qi L, Echalier B, Friebe B, Gill BS (2003) Molecular characterization of a set of wheat deletion stocks for use in chromosome bin mapping of ESTs. Functional & Integrative Genomics 3: 39–55. [DOI] [PubMed] [Google Scholar]
- 43. Qi L, Echalier B, Chao S, Lazo G, Butler G, et al. (2004) A chromosome bin map of 16,000 expressed sequence tag loci and distribution of genes among the three genomes of polyploid wheat. Genetics 168: 701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Randhawa H, Dilbirligi M, Sidhu D, Erayman M, Sandhu D, et al. (2004) Deletion mapping of homoeologous group 6-specific wheat expressed sequence tags. Genetics 168: 677–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Potvin C, Lechowicz MJ, Tardif S (1990) The statistical analysis of ecophysiological response curves obtained from experiments involving repeated measures. Ecology: 1389–1400.
- 46. Hsiao C, Asay KH, Dewey DR (1989) Cytogenetic analysis of interspecific hybrids and amphiploids between two diploid crested wheatgrasses, Agropyron mongolicum and A. cristatum . Genome 32: 1079–1084. [Google Scholar]
- 47. Li L, Dong Y (1993) Progress in studies of Agropyron Gaertn. Acta Genetica Sinica 15: 45–48. [Google Scholar]
- 48. Stebbins Jr GL (1947) Types of Polyploids: Their Classification and Significance. Advances in Genetics 1: 403–429. [DOI] [PubMed] [Google Scholar]
- 49. Collard BCY, Mackill DJ (2008) Marker-assisted selection: an approach for precision plant breeding in the twenty-first century. Philosophical Transactions of the Royal Society B: Biological Sciences 363: 557–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Lande R, Thompson R (1990) Efficiency of marker-assisted selection in the improvement of quantitative traits. Genetics 124: 743–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Stuber CW, Polacco M, Senior ML (1999) Synergy of empirical breeding, marker-assisted selection, and genomics to increase crop yield potential. Crop Sci 39: 1571–1583. [Google Scholar]
- 52.Wang J, Liu W, Wang H, Li L, Wu J, et al.. (2011) QTL mapping of yield-related traits in the wheat germplasm 3228. Euphytica: 1–16.
- 53. Song L, Jiang L, Han H, Gao A, Yang X, et al. (2013) Efficient induction of wheat-Agropyron cristatum 6P translocation lines and GISH detection. PLoS One 8: e69501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Du W, Wang J, Pang Y, Li Y, Chen X, et al. (2013) Isolation and characterization of a Psathyrostachys huashanica Keng 6Ns chromosome addition in common wheat. PLoS One 8: e53921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Qi Z, Du P, Qian B, Zhuang L, Chen H, et al. (2010) Characterization of a wheat-Thinopyrum bessarabicum (T2JS-2BS.2BL) translocation line. Theor Appl Genet 121: 589–597. [DOI] [PubMed] [Google Scholar]
- 56. Fischer R, Rees D, Sayre K, Lu ZM, Condon A, et al. (1998) Wheat yield progress associated with higher stomatal conductance and photosynthetic rate, and cooler canopies. Crop Sci 38: 1467–1475. [Google Scholar]
- 57. Ainsworth EA, Long SP (2004) What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytologist 165: 351–372. [DOI] [PubMed] [Google Scholar]
- 58. Devos KM, Gale MD (1997) Comparative genetics in the grasses. Plant Molecular Biology 35: 3–15. [PubMed] [Google Scholar]
- 59. Devos KM, Gale MD (2000) Genome relationships: the grass model in current research. The Plant Cell 12: 637–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gale MD, Devos KM (1998) Comparative genetics in the grasses. Proceedings of the National Academy of Sciences 95: 1971–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Niu Z, Klindworth DL, Friesen TL, Chao S, Jin Y, et al. (2011) Targeted introgression of a wheat stem rust resistance gene by DNA marker-assisted chromosome engineering. Genetics 187: 1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kishii M, Yamada T, Sasakuma T, Tsujimoto H (2004) Production of wheat–Leymus racemosus chromosome addition lines. Theor Appl Genet 109: 255–260. [DOI] [PubMed] [Google Scholar]
- 63. Wang RRC, Larson SR, Jensen KB (2010) Analyses of Thinopyrum bessarabicum, T. elongatum, and T. junceum chromosomes using EST-SSR markers. Genome 53: 1083–1089. [DOI] [PubMed] [Google Scholar]
- 64.McArthur RI, Zhu X, Oliver RE, Klindworth DL, Xu SS, et al. (2012) Homoeology of Thinopyrum junceum and Elymus rectisetus chromosomes to wheat and disease resistance conferred by the Thinopyrum and Elymus chromosomes in wheat. Chromosome Research: 1–17. [DOI] [PubMed]
- 65.Hu LJ, Liu C, Zeng ZX, Li GR, Song XJ, et al. (2012) Genomic rearrangement between wheat and Thinopyrum elongatum revealed by mapped functional molecular markers. Genes & Genomics: 1–9.
- 66. Liu W, Liu W, Wu J, Gao A, Li L (2010) Analysis of genetic diversity in natural populations of Psathyrostachys huashanica Keng using microsatellite (SSR) markers. Agricultural Sciences in China 9: 463–471. [Google Scholar]
- 67. Wang Q, Xiang J, Gao A, Yang X, Liu W, et al. (2010) Analysis of chromosomal structural polymorphisms in the St, P, and Y genomes of Triticeae (Poaceae). Genome 53: 241–249. [DOI] [PubMed] [Google Scholar]
- 68. Alkhimova AG, Heslop-Harrison JS, Shchapova AI, Vershinin AV (1999) Rye chromosome variability in wheat–rye addition and substitution lines. Chromosome Research 7: 205–212. [DOI] [PubMed] [Google Scholar]
- 69. Szakács É, Molnár-Láng M (2010) Molecular cytogenetic evaluation of chromosome instability in Triticum aestivum–Secale cereale disomic addition lines. J Appl Genet 51: 149–152. [DOI] [PubMed] [Google Scholar]
- 70. Bento M, Gustafson P, Viegas W, Silva M (2010) Genome merger: from sequence rearrangements in triticale to their elimination in wheat–rye addition lines. Theor Appl Genet 121: 489–497. [DOI] [PubMed] [Google Scholar]
- 71. Tomás D, Bento M, Viegas W, Silva M (2012) Involvement of disperse repetitive sequences in wheat/rye genome adjustment. International journal of molecular sciences 13: 8549–8561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Fu S, Yang M, Fei Y, Tan F, Ren Z, et al. (2013) Alterations and abnormal mitosis of wheat chromosomes induced by wheat-rye monosomic addition lines. PLoS One 8: e70483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liu C, Atkinson M, Chinoy C, Devos K, Gale M (1992) Nonhomoeologous translocations between group 4, 5 and 7 chromosomes within wheat and rye. Theor Appl Genet 83: 305–312. [DOI] [PubMed] [Google Scholar]
- 74. Zhao R, Wang H, Xiao J, Bie T, Cheng S, et al. (2013) Induction of 4VS chromosome recombinants using the CS ph1b mutant and mapping of the wheat yellow mosaic virus resistance gene from Haynaldia villosa. Theor Appl Genet 126: 2921–2930. [DOI] [PubMed] [Google Scholar]