Abstract
Molecular mimicry is an attractive mechanism for triggering autoimmunity. In this review, we explore the potential role of evolutionary conserved bacterial proteins in the production of autoantibodies with focus on granulomatosis with polyangiitis (GPA) and rheumatoid arthritis (RA). Seven autoantigens characterized in GPA and RA were BLASTed against a bacterial protein database. Of the seven autoantigens, proteinase 3, type II collagen, binding immunoglobulin protein, glucose-6-phosphate isomerase, α-enolase, and heterogeneous nuclear ribonuclear protein have well-conserved bacterial orthologs. Importantly, those bacterial orthologs are also found in human-associated bacteria. The wide distribution of the highly conserved stress proteins or enzymes among the members of the normal flora and common infectious microorganisms raises a new question on how cross-reactive autoantibodies are not produced during the immune response to these bacteria in most healthy people. Understanding the mechanisms that deselect auto-reactive B cell clones during the germinal center reaction to homologous foreign antigens may provide a novel strategy to treat autoimmune diseases.
Keywords: Molecular mimicry, Autoantigens, Bacterial orthologs, Granulomatosis with polyangiitis, Rheumatoid arthritis
INTRODUCTION
Autoimmune diseases are complex immune-mediated diseases that involve both genetic and environmental factors in their pathogenesis. Infectious microorganisms have long been suggested to trigger an immune response to autoantigens by providing stimuli for the breakdown of self-tolerance and also by generating cross-reactive T cells and antibodies via molecular mimicry. Molecular mimicry is a mechanism that has a proposed role in many autoimmune diseases such as acute rheumatic fever, rheumatoid arthritis, Guillain-Barré syndrome, multiple sclerosis, type 1 diabetes mellitus, and Lyme arthritis. In autoimmune diseases, the concept of molecular mimicry has often been used to describe similar structures shared by molecules from dissimilar proteins, as illustrated by the α-helical coiled-coil streptococcal M protein and cardiac myosin in rheumatic fever. However, some proteins such as heat shock proteins are evolutionally highly conserved from prokaryotes to eukaryotes. In this review, we explore the potential role of the evolutionary conserved bacterial proteins in the production of autoantibodies with focus on granulomatosis with polyangiitis (GPA) and rheumatoid arthritis (RA).
GPA AND ANTI-NEUTROPHIL CYTOPLASMIC AUTOANTIBODIES (ANCA)
GPA (Wegener's) is a type of ANCA-associated vasculitis that affects small- and medium-sized vessels in many organs. Its clinical symptoms include fever, fatigue, weight loss, nasal discharge, sinusitis, cough, dyspnea, hematuria, and proteinuria. Pathologically, GPA is characterized by multi-focal granulomatous inflammation with central necrosis and necrotizing vasculitis. In addition, the presence of an ANCA in serum is used as a diagnostic marker. There are two types of ANCAs, i.e., directed against either proteinase 3 (PR3-ANCA) or myeloperoxidase (MPO-ANCA). The ANCA antigen specificity of GPA in European patients is predominantly PR3, whereas that in Japanese patients is predominantly MPO. The pathogenesis of necrotizing vasculitis is understood to involve infiltration of neutrophils into vessel walls and their subsequent activation. ANCA is known to mediate the activation of neutrophils and degranulation via crosslinking of the Fc receptor and antigens expressed on the membrane. However, what causes the production of ANCA and granulomatous inflammation, is not known.
Interestingly, bacterial infection has been implicated in the initiation and relapse of GPA. Early observations suggested a potential role of infection in the relapse of disease. Chronic nasal carriage of Staphylococcus aureus is increased in GPA patients compared to healthy subjects (63% vs. 25%), and the carriage of S. aureus increases the risk of relapse by 7.16 fold (9). Furthermore, the addition of trimethoprim/sulfamethoxazole to maintenance treatment reduced the relapses by 60% (10). Although the underlying mechanisms for the increased relapse by S. aureus is not clear, potential roles in polyclonal activation of B cells, priming of neutrophils, and induction of anti-idiotypic antibodies to PR3-ANCA have been suggested (6). Kain et al. reported a new ANCA directed against lysosomal membrane glycoprotein 2 (LAMP-2) as a specific marker for focal necrotizing glomerulonephritis and showed that the autoantibodies to LAMP-2 can be induced by immunization with FimH, a bacterial fimbrial adhesin of Gram-negative bacteria (11). The eight amino acids of one LAMP-2 epitope (P41-49) recognized by autoantibodies have a strong homology with the FimH of several common Gram-negative species, suggesting molecular mimicry between the two proteins.
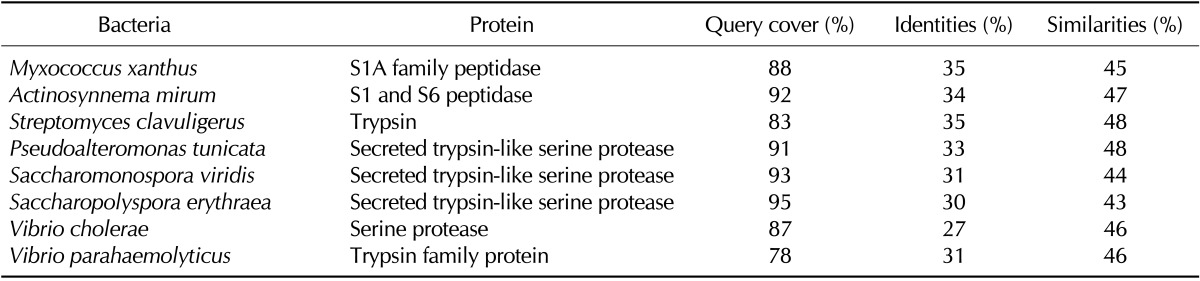
We previously demonstrated that the membrane bound PR3 on neutrophils acts as a receptor for non-opsonic phagocytosis of bacteria and that the neutralization of PR3 with ANCA reduces both binding and phagocytosis of bacteria (12). It raised the possibility that ANCA may be induced by some pathogens possessing a PR3-homologous protein to avoid host immunity. When the bacterial protein database was searched using the PR3 protein sequence as a query, hundreds of bacterial proteases with 28% to 36% identity were indeed found. Among the bacteria containing PR3-homologous proteases, only Vibrio cholerae, V. vulnificus, V. parahaemolyticus, and Saccharomonospora viridis are known to infect humans (Table I). The spores of S. viridis, a gram-negative bacterium frequently found in hot compost and hay, can be readily dispersed in air. Prolonged exposure to those spores can cause farmer's lung, bagassosis, and humidifier fever that manifest symptoms such as fever, malaise, cough, and dyspnea similar to GPA (13). Granulomas are usually formed in an attempt to segregate foreign substances that are resistant to phagocytic clearance. If S. viridis induces ANCA production via molecular mimicry, the ANCA would inhibit phagocytosis of S. viridis. Furthermore, if S. viridis survives after phagocytosis, infection with S. viridis may also contribute to the formation of granulomatous inflammation.
Table I.
Selected bacteria that contain human PR3-homologous proteins
AUTOANTIGENS CHARACTERIZED IN RHEUMATOID ARTHRITIS (RA)
A literature search revealed that Wegner et al. already proposed evolutionarily conserved antigens as stimuli to cause breakdown of tolerance and reported well-conserved bacterial orthologs for pyruvate dehydrogenase complex E2, glutamic acid decarboxylase, histidyl-tRNA synthetase, and enolase among seven major autoantigens examined (14). Therefore, we further explored the possibility that evolutionarily conserved bacterial proteins are involved in autoantibody production in RA, the most common systemic autoimmune disease.
RA is characterized by the presence of diverse autoantibodies in serum and synovial fluid. Rheumatoid factor (RF) is the first autoantibody described in RA, which is directed against the Fc portion of IgG, a major serum component (15). Together with the RF, anti-citrullinated protein antibodies are clinically important. The anti-citrullinated protein antibodies are a group of autoantibodies that recognize citrulline-containing peptides/proteins as common antigenic epitopes (16). Other antigens characterized as targets of autoantibodies in RA include type II collagen (CII), binding immunoglobulin protein (BiP), glucose-6-phosphate isomerase (G6PI), α-enolase, and heterogeneous nuclear ribonuclear protein (hnRNP) A2 (17). We searched the bacterial protein database using these human proteins as a query.
Members of the immunoglobulin superfamily have also been found in bacteria (18). Homology search with the sequence of human immunoglobulin gamma-1 heavy chain constant region did not retrieve any bacterial proteins. When the CH2 and CH3 domains, the antigenic epitopes of RF, were searched separately, however, several bacterial proteins such as the cell wall binding repeat 2 family protein and cell surface protein of Clostridium difficile were found to share 46% similarities with the CH2 over 76% of the domain.
CII is a component of both articular and hyaline cartilage. RA patients often contain high titers of anti-CII antibodies in sera and synovial fluids (19). Because CII is exclusively expressed in the cartilage, autoantibodies against CII induce joint destruction. Surprisingly, a large number of bacterial species contain collagen triple helix repeat family proteins that share a high degree of identity (32~47%) and homology (36~52%) with human CII. Among those, important human pathogens such as Clostridium difficile and Bacillus cereus (20,21) and a member of normal gut flora C. beijerinckii (22) are included (Table II).
Table II.
Selected human-associated bacteria that contain human CII-homologous proteins
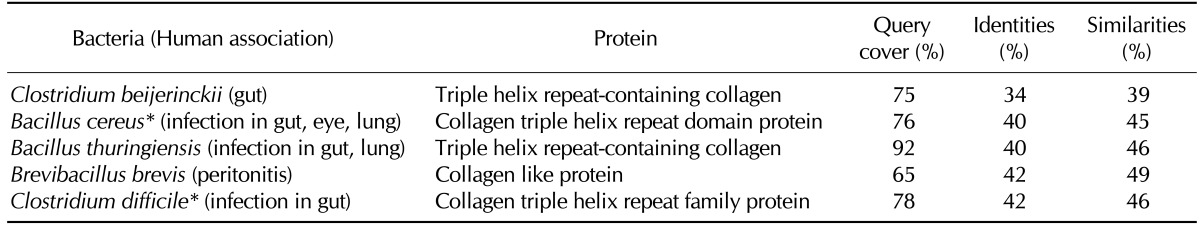
*Bacteria that have been reported to develop reactive arthritis, septic arthritis, or rheumatic symptoms.
BiP (also known as 78 kDa glucose-regulated protein or heat shock 70 kDa protein 5), is a stress protein located in the endoplasmic reticulum. Stress proteins are evolutionally highly conserved from bacteria to human. Furthermore, microbial stress proteins are highly immunogenic (23). Therefore, cellular and humoral immune responses initiated by microbial stress proteins may target cross-reactive self proteins, resulting in autoimmunity (24). Among the hundreds of bacterial species that contain a BiP-homologous stress protein DnaK, human pathogens such as Bartonella spp., Brucella spp., Borrelia spp., and Rickettsia spp. were found. In addition to the pathogenic bacteria, a number of bacteria in the human oral flora such as Prevotella spp. and Atopobium parvulum contained the BiP-homologous DnaK. Selective examples of bacterial DnaK listed in Table III have 49~54% identities and 67~70% similarities with the human BiP over 92% of the entire protein.
Table III.
Selected human-associated bacteria that contain human BiP-homologous proteins
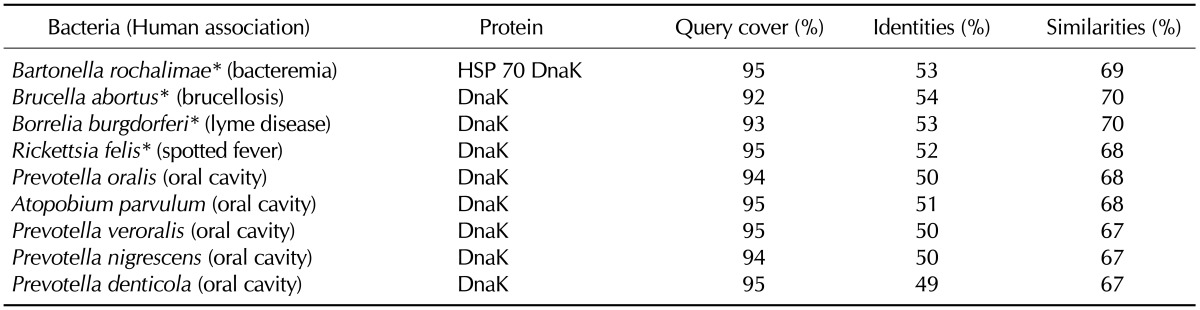
*Bacteria that have been reported to develop reactive arthritis, septic arthritis, or rheumatic symptoms.
Glucose-6-phosphate isomerase (G6PI) is a ubiquitously expressed glycolytic enzyme that is also highly conserved through evolution. Selected bacterial G6PIs for several human-associated species present even higher identities (65~68%) and similarities (78~80%) than those observed in the stress protein BiP (Table IV). The human-associated bacteria include both commensals and pathogens that colonize the respiratory tract, urinary tract, or gastrointestinal tract. Given the high degree of similarity between the human and bacterial G6PIs shown in Table IV, it can be speculated that a large body of human-associated bacteria, including those in the normal flora, may share significant homology (greater than 30%) in their G6PIs with the human protein. K/BxN mice spontaneously develop autoantibodies to G6PI and autoimmune arthritis (25). The serum autoantibody titer and autoimmune arthritis in the K/BxN mice are significantly attenuated under germ-free conditions, which are reinstated by the introduction of a single gut-residing species, asegmented filamentous bacteria (26). A homology search revealed that the G6PI of the segmented filamentous bacteria Candidatus Arthromitus sp. SFB-mouse-Japan has 40% homology with a mouse G6PI, supporting the role of this bacterium in anti-G6PI antibody production.
Table IV.
Selected human-associated bacteria that contain human G6PI-homologous proteins
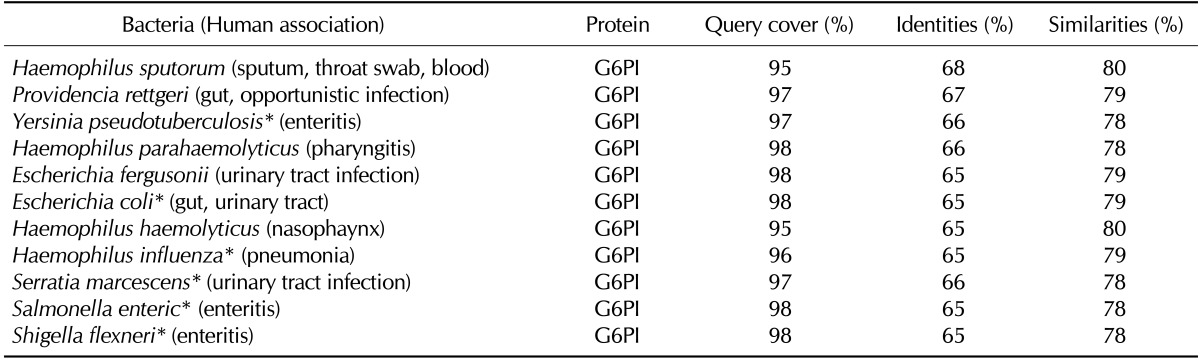
*Bacteria that have been reported to develop reactive arthritis, septic arthritis, or rheumatic symptoms.
The α-enolase is another glycolytic enzyme ubiquitously expressed in the cytosol. It is also expressed on the surface of stimulated leukocytes, and then serves as a plasminogen-binding receptor, which assists in inflammatory cell invasion (27). The similar usage of enolase and plasminogen for the invasion of host tissue has been demonstrated by several pathogens (28). Interestingly, members of the normal flora in the oral cavity and gut express the enolase that shares a high degree of identity (51~55%) and similarity (68~72%) with the human α-enolase (Table V). The enolases of several important human pathogens such as Treponema denticola, Turicella otitidis, Clostridium botulinum, Bacillus cereus, and Neisseria meningitidis also have high degrees of homology with the human α-enolase.
Table V.
Selected human-associated bacteria that contain human α-enolase-homologous proteins
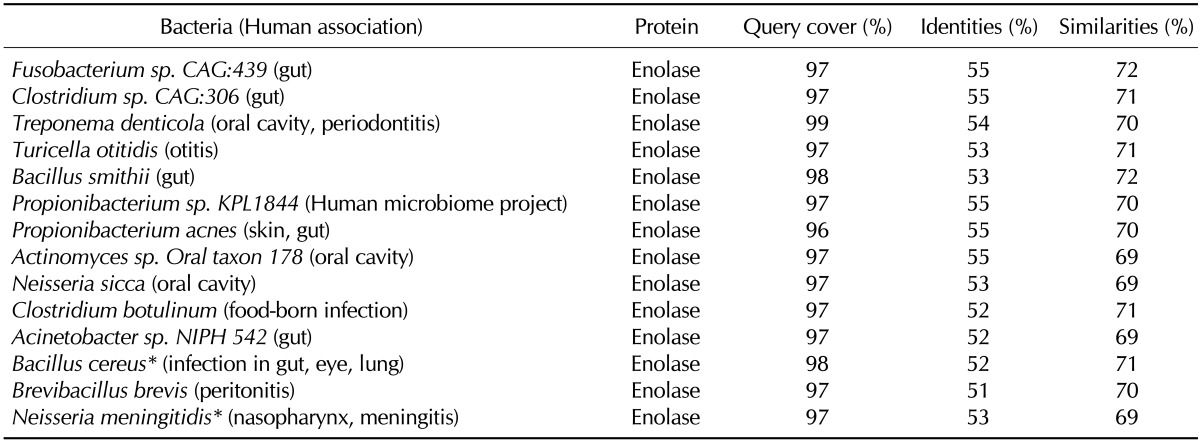
*Bacteria that have been reported to develop reactive arthritis, septic arthritis, or rheumatic symptoms.
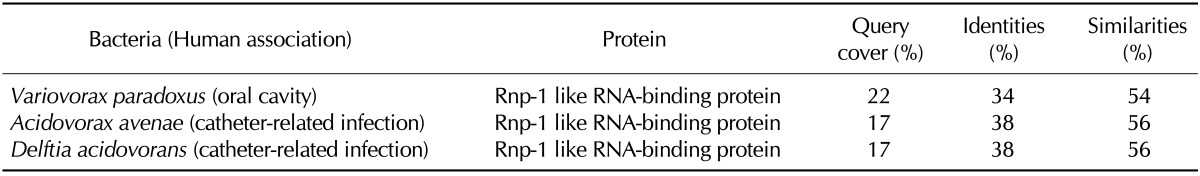
The hnRNP A2 is an abundant RNA-binding protein that is predominantly expressed inside the nucleus and involved in pre-mRNA splicing, mRNA transport, and translation (29). Although bacteria do not make mRNA, a homology search using the hnRNP A2 retrieved 61 bacterial proteins that share 27~49% identities and 51~73% similarities with the first RNA recognition motif (RRM) superfamily domain of hnRNP A2. Indeed, all retrieved bacterial proteins were RNA-binding proteins. Among them, Variovorax paradoxus is a member of the human oral flora, and Acidovorax avenae and Delftia acidovorans are involved in catheter-related infection (Table VI).
Table VI.
Selected human-associated bacteria that contain human hnRNP A2-homologus proteins
Collectively, bacterial orthologs exist for almost all autoantigens characterized in RA. It is remarkable that most of the pathogenic bacteria listed in Table II~V have been reported to develop reactive arthritis, septic arthritis, or rheumatic symptoms (30-38). Furthermore, there is evidence for the possible involvement of Clostridium spp., and Prevotella spp. in the pathogenesis of RA (39). Accumulating evidence suggests that not only infectious but also indigenous microorganisms may be involved in the initiation and perpetuation of RA (39). Increased epithelial permeability, loss of immune tolerance, and trafficking of both microbial components and activated immune cells to the joints have been suggested as underlying mechanisms for the involvement of the indigenous bacteria (39).
CONCLUSION
We explored seven autoantigens for the presence of evolutionary conserved counterparts in the bacterial protein database. Of the seven autoantigens, PR3, CII, BiP, G6PI, α-enolase, and hnRNP A2 have well conserved bacterial orthologs. Importantly, those bacterial orthologs are also found in human-associated bacteria. Although there are no bacterial orthologs for human immunoglobulin gamma-1 heavy chain constant region, proteins with an immunoglobulin superfamily domain are found in bacteria. The wide distribution of the highly conserved DnaK, G6PI, or enolase among the members of the normal flora and common infectious microorganisms rather raises the question on how cross-reactive autoantibodies are not produced during the immune response to these bacteria in most healthy people. Understanding the mechanisms that deselect auto-reactive B cell clones during the germinal center reaction to homologous foreign antigens may provide a novel strategy to treat autoimmune diseases.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare (HI13C0016), Republic of Korea.
Abbreviations
- GPA
granulomatosis with polyangiitis
- RA
rheumatoid arthritis
- ANCA
anti-neutrophil cytoplasmic autoantibodies
- PR3
proteinase 3
- MPO
myeloperoxidase
- LAMP-2
lysosomal membrane glycoprotein 2
- RF
heumatoid factor
- CII
type II collagen
- BiP
binding immunoglobulin protein
- G6PPI
glucose-6-phosphate isomerase
- hnRNP
heterogeneous nuclear ribonuclear protein
Footnotes
The authors have no financial conflict of interest.
References
- 1.Albert LJ, Inman RD. Molecular mimicry and autoimmunity. N Engl J Med. 1999;341:2068–2074. doi: 10.1056/NEJM199912303412707. [DOI] [PubMed] [Google Scholar]
- 2.Libbey JE, McCoy LL, Fujinami RS. Molecular mimicry in multiple sclerosis. Int Rev Neurobiol. 2007;79:127–147. doi: 10.1016/S0074-7742(07)79006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ang CW, Jacobs BC, Laman JD. The Guillain-Barré syndrome: a true case of molecular mimicry. Trends Immunol. 2004;25:61–66. doi: 10.1016/j.it.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme L, Kalil J, Cunningham M. Molecular mimicry in the autoimmune pathogenesis of rheumatic heart disease. Autoimmunity. 2006;39:31–39. doi: 10.1080/08916930500484674. [DOI] [PubMed] [Google Scholar]
- 5.Hu N, Westra J, Kallenberg CG. Membrane-bound proteinase 3 and its receptors: relevance for the pathogenesis of Wegener's Granulomatosis. Autoimmun Rev. 2009;8:510–514. doi: 10.1016/j.autrev.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Chen M, Kallenberg CG. The environment, geoepidemiology and ANCA-associated vasculitides. Autoimmun Rev. 2010;9:A293–A298. doi: 10.1016/j.autrev.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Watts RA, Scott DGI. ANCA vasculitis: to lump or split? Why we should study MPA and GPA separately. Rheumatology (Oxford) 2012;51:2115–2117. doi: 10.1093/rheumatology/kes230. [DOI] [PubMed] [Google Scholar]
- 8.Pinching AJ, Rees AJ, Pussell BA, Lockwood CM, Mitchison RS, Peters DK. Relapses in Wegener's granulomatosis: the role of infection. Br Med J. 1980;281:836–838. doi: 10.1136/bmj.281.6244.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stegeman CA, Tervaert JW, Sluiter WJ, Manson WL, de Jong PE, Kallenberg CG. Association of chronic nasal carriage of Staphylococcus aureus and higher relapse rates in Wegener granulomatosis. Ann Intern Med. 1994;120:12–17. doi: 10.7326/0003-4819-120-1-199401010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Stegeman CA, Tervaert JW, de Jong PE, Kallenberg CG Dutch Co-Trimoxazole Wegener Study Group. Trimethoprim-sulfamethoxazole (co-trimoxazole) for the prevention of relapses of Wegener's granulomatosis. N Engl J Med. 1996;335:16–20. doi: 10.1056/NEJM199607043350103. [DOI] [PubMed] [Google Scholar]
- 11.Kain R, Exner M, Brandes R, Ziebermayr R, Cunningham D, Alderson CA, Davidovits A, Raab I, Jahn R, Ashour O, Spitzauer S, Sunder-Plassmann G, Fukuda M, Klemm P, Rees AJ, Kerjaschki D. Molecular mimicry in pauci-immune focal necrotizing glomerulonephritis. Nat Med. 2008;14:1088–1096. doi: 10.1038/nm.1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim YC, Shin JE, Lee SH, Chung WJ, Lee YS, Choi BK, Choi Y. Membrane-bound proteinase 3 and PAR2 mediate phagocytosis of non-opsonized bacteria in human neutrophils. Mol Immunol. 2011;48:1966–1974. doi: 10.1016/j.molimm.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 13.Pati A, Sikorski J, Nolan M, Lapidus A, Copeland A, Glavina Del Rio T, Lucas S, Chen F, Tice H, Pitluck S, Cheng JF, Chertkov O, Brettin T, Han C, Detter JC, Kuske C, Bruce D, Goodwin L, Chain P, D'haeseleer P, Chen A, Palaniappan K, Ivanova N, Mavromatis K, Mikhailova N, Rohde M, Tindall BJ, Göker M, Bristow J, Eisen JA, Markowitz V, Hugenholtz P, Kyrpides NC, Klenk HP. Complete genome sequence of Saccharomonospora viridis type strain (P101) Stand Genomic Sci. 2009;1:141–149. doi: 10.4056/sigs.20263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wegner N, Wait R, Venables PJ. Evolutionarily conserved antigens in autoimmune disease: implications for an infective aetiology. Int J Biochem Cell Biol. 2009;41:390–397. doi: 10.1016/j.biocel.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 15.Artandi SE, Calame KL, Morrison SL, Bonagura VR. Monoclonal IgM rheumatoid factors bind IgG at a discontinuous epitope comprised of amino acid loops from heavy-chain constant-region domains 2 and 3. Proc Natl Acad Sci U S A. 1992;89:94–98. doi: 10.1073/pnas.89.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schellekens GA, de Jong BA, van den Hoogen FH, van de Putte LB, van Venrooij WJ. Citrulline is an essential constituent of antigenic determinants recognized by rheumatoid arthritis-specific autoantibodies. J Clin Invest. 1998;101:273–281. doi: 10.1172/JCI1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song YW, Kang EH. Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies. QJM. 2010;103:139–146. doi: 10.1093/qjmed/hcp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bateman A, Eddy SR, Chothia C. Members of the immunoglobulin superfamily in bacteria. Protein Sci. 1996;5:1939–1941. doi: 10.1002/pro.5560050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho YG, Cho ML, Min SY, Kim HY. Type II collagen autoimmunity in a mouse model of human rheumatoid arthritis. Autoimmun Rev. 2007;7:65–70. doi: 10.1016/j.autrev.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 20.Sammons JS, Localio R, Xiao R, Coffin SE, Zaoutis T. Clostridium difficile infection is associated with increased risk of death and prolonged hospitalization in children. Clin Infect Dis. 2013;57:1–8. doi: 10.1093/cid/cit155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bottone EJ. Bacillus cereus, a volatile human pathogen. Clin Microbiol Rev. 2010;23:382–398. doi: 10.1128/CMR.00073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima N, Matsuura Y. Purification and characterization of konjac glucomannan degrading enzyme from anaerobic human intestinal bacterium, Clostridium butyricum-Clostridium beijerinckii group. Biosci Biotechnol Biochem. 1997;61:1739–1742. doi: 10.1271/bbb.61.1739. [DOI] [PubMed] [Google Scholar]
- 23.Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 24.Rajaiah R, Moudgil KD. Heat-shock proteins can promote as well as regulate autoimmunity. Autoimmun Rev. 2009;8:388–393. doi: 10.1016/j.autrev.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schubert D, Maier B, Morawietz L, Krenn V, Kamradt T. Immunization with glucose-6-phosphate isomerase induces T cell-dependent peripheral polyarthritis in genetically unaltered mice. J Immunol. 2004;172:4503–4509. doi: 10.4049/jimmunol.172.7.4503. [DOI] [PubMed] [Google Scholar]
- 26.Fan LY, Zong M, Wang Q, Yang L, Sun LS, Ye Q, Ding YY, Ma JW. Diagnostic value of glucose-6-phosphate isomerase in rheumatoid arthritis. Clin Chim Acta. 2010;411:2049–2053. doi: 10.1016/j.cca.2010.08.043. [DOI] [PubMed] [Google Scholar]
- 27.Wygrecka M, Marsh LM, Morty RE, Henneke I, Guenther A, Lohmeyer J, Markart P, Preissner KT. Enolase-1 promotes plasminogen-mediated recruitment of monocytes to the acutely inflamed lung. Blood. 2009;113:5588–5598. doi: 10.1182/blood-2008-08-170837. [DOI] [PubMed] [Google Scholar]
- 28.Pancholi V, Fontan P, Jin H. Plasminogen-mediated group A streptococcal adherence to and pericellular invasion of human pharyngeal cells. Microb Pathog. 2003;35:293–303. doi: 10.1016/j.micpath.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 29.Hoffmann MH, Skriner K, Herman S, Baumann C, Steiner CW, Ospelt C, Meyer B, Gleiss A, Pfatschbache J, Niederreiter B, Tuncel J, Zanoni G, Steiner G. Nucleic acid-stimulated antigen-presenting cells trigger T cells to induce disease in a rat transfer model of inflammatory arthritis. J Autoimmun. 2011;36:288–300. doi: 10.1016/j.jaut.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 30.Prati C, Bertolini E, Toussirot E, Wendling D. Reactive arthritis due to Clostridium difficile. Joint Bone Spine. 2010;77:190–192. doi: 10.1016/j.jbspin.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Cuchacovich R, Japa S, Huang WQ, Calvo A, Vega L, Vargas RB, Singh R, Flores D, Castro I, Espinoza LR. Detection of bacterial DNA in Latin American patients with reactive arthritis by polymerase chain reaction and sequencing analysis. J Rheumatol. 2002;29:1426–1429. [PubMed] [Google Scholar]
- 32.Maggi RG, Mozayeni BR, Pultorak EL, Hegarty BC, Bradley JM, Correa M, Breitschwerdt EB. Bartonella spp. bacteremia and rheumatic symptoms in patients from Lyme disease-endemic region. Emerg Infect Dis. 2012;18:783–791. doi: 10.3201/eid1805.111366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasanoglu I, Guven T, Maras Y, Guner R, Tasyaran MA, Acikgoz ZC. Brucellosis as an aetiology of septic arthritis. Trop Doct. 2013 doi: 10.1177/0049475513512645. in press: doi: 10.1177/0049475513512645. [DOI] [PubMed] [Google Scholar]
- 34.Steere AC, Drouin EE, Glickstein LJ. Relationship between immunity to Borrelia burgdorferi outer-surface protein A (OspA) and Lyme arthritis. Clin Infect Dis. 2011;52(Suppl 3):s259–s265. doi: 10.1093/cid/ciq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhry MA, Scofield RH. Atypical rocky mountain spotted Fever with polyarticular arthritis. Am J Med Sci. 2013;346:427–429. doi: 10.1097/MAJ.0b013e318295c788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leo JC, Skurnik M. Adhesins of human pathogens from the genus Yersinia. Adv Exp Med Biol. 2011;715:1–15. doi: 10.1007/978-94-007-0940-9_1. [DOI] [PubMed] [Google Scholar]
- 37.Bonet Llorach M, Arnam Fernandez D, Valles Daunis J, Maymo Guarch J, Carbonell Abelló H. Haemophilus influenza septic arthritis in the adult. Clin Rheumatol. 1989;8:292–293. doi: 10.1007/BF02030090. [DOI] [PubMed] [Google Scholar]
- 38.Acar JF. Serratia marcescens infections. Infect Control. 1986;7:273–278. doi: 10.1017/s0195941700064201. [DOI] [PubMed] [Google Scholar]
- 39.Yeoh N, Burton JP, Suppiah P, Reid G, Stebbings S. The role of the microbiome in rheumatic diseases. Curr Rheumatol Rep. 2013;15:314. doi: 10.1007/s11926-012-0314-y. [DOI] [PubMed] [Google Scholar]


