Abstract
We report here the design, synthesis, and in vitro characterization of new opioid peptides featuring a 4-anilidopiperidine moiety. Despite the fact that the chemical structures of fentanyl surrogates have been found suboptimal per se for the opioid activity, the corresponding conjugates with opioid peptides displayed potent opioid activity. These studies shed an instructive light on the strategies and potential therapeutic values of anchoring the 4-anilidopiperidine scaffold to different classes of opioid peptides.
Keywords: Opioid peptide, Dynorphine analog, Bivalent ligand, Fentanyl, Analgesic
The increasing need for effective pain management prompts the invention of new strategies and pharmacological tools. Among the three opioid receptor types (μ, δ, and κ, the μ-opioid receptor is considered to be essential for efficient pain suppression. However, μ-opioids do not provide adequate treatment of chronic pain since their long-term use results in multiple side effects (e.g., 1). Close examination of the opioid system conferred that a μ–δ opioid receptor heterodimer represents the fundamental signaling unit responsible for opioid tolerance and dependence.2,3 During the last two decades, our laboratory has carried out extensive research on the synthesis of novel opioid analogs. Within the frame of our works on bivalent opioid ligands there seemed a gap between small-molecule and peptide-based opioids. We have sought to bridge this gap by incorporating the 4-ANDP4 scaffolds into opioid peptides, and also to optimize their physicochemical properties.5–7 Previously, research efforts to produce bivalent opioid peptides resulted in analogs with exceptional properties. One representative example is biphalin.8 Despite numerous advantages such as low toxicity, high activity and specificity, opioid peptides are still not being used as pain-relieving agents in general anesthetic practice because of main drawbacks such as poor bioavailability after systemic (subcutaneous or oral) administration, limited ability to cross the blood–brain barrier, and rapid degradation in vivo by peptidases. Although the fact that fentanyl9–12 is a bioavailable drug does not directly suggest that its addition to a peptide structure will yield a bioavailable hybrid, we hypothesized that its incorporation into opioid peptides may positively impact overall bioavailability of the latter.
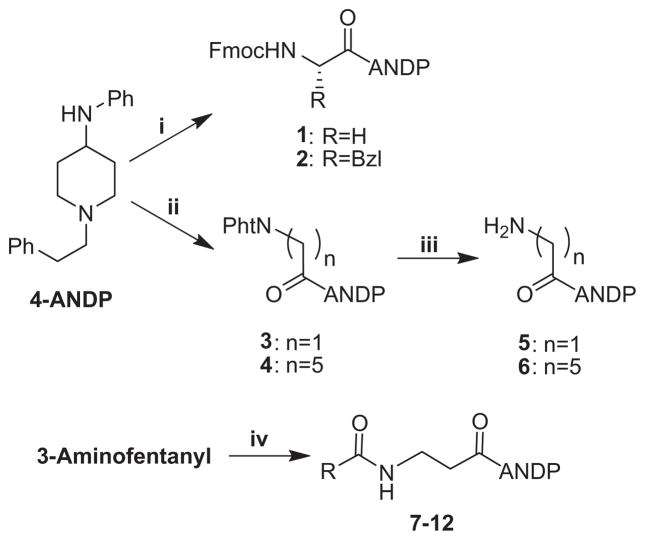
A careful analysis of the literature revealed that in the series of fentanyl analogs there was no general consensus regarding the effect of substitution in the propionyl part of the molecule. We thus set out to prepare the desired compound by using Fmoc- and Pht-protected amino acid chlorides (Scheme 1). This allowed us to synthesize 3-aminofentanyl and analogs 1–6. The synthesis, NMR characterization and binding affinity at the μ and δ opioid receptors of compounds 7–12 have been reported previously by us,5 but no results of GPI and MVD assays were provided for this series. In this report, we provide additional biological data for compounds 7–12. The effects of having the amide substituents in the propionyl moiety are summarized in Table 1. It is evident from the results presented in Table 1 that all of these analogs displayed low affinity at the μ and δ opioid receptors and low activity in MVD and GPI assays. For the major part, the chemical nature of these fentanyl surrogates may not be optimal for the opioid activity in terms of ionic and hydrophobic interactions.
Scheme 1.
Attachment of Fmoc and phthaloyl amino acids to 4-anilino-1-phenethyl-piperidine. Reagents and conditions: (i) Fmoc-AA-Cl (AA: Gly, Phe), DCM/10% NaHCO3 in water, 0 °C (83% and 49%, respectively); (ii) Pht-Gly-Cl, TEA or DIPEA, DCM, 0 °C; (iii) N2H4·nH2O, ethanol, reflux; (iv) see Ref. 5, R:Me (7), CF3 (8), Et (9), Ph (10), –NHEt (11), –CH(Bzl)(NHCOCH3) (12).
Table 1.
Bioactivities of the 4-anilidopiperidine analogs
| No. | hDORa [3H]DPDPEb Kie (μM) |
rMORa [3H]DAMGOc Kie (μM) |
IC50d (μM)
|
|
|---|---|---|---|---|
| MVD (δ) | GPI (μ) | |||
| 1f | 1.0 | 1.0 | n.d. | n.d. |
| 2f | No response | 3.1 | n.d. | n.d. |
| 3 | 13 | 2.1 | 26.4% | 11% |
| 3f | 36 | 3.2 | 14.2% | 5.9 ± 0.89g |
| 7 | 8.4 | 1.0 | 7.5% | 5.2 ± 0.21h |
| 8 | 12 | 0.15 | 4.1% | 3.0 ± 0.89h |
| 9 | 0.45 | 0.45 | 4.1% | 1.4 ± 0.48h |
| 10 | 4.6 | 7.6 | 2.9% | 3.9 ± 0.22 |
| 11 | 6.6 | 0.030 | 26.1% | 1.3 ± 0.3 |
| 12 | 7.6 | 2.7 | 6.2% | 5.6 ± 0.56h |
| Fentanyl | 0.25 | 0.0033 | 0.0094 | 0.0034 |
n.d. not determined.
Competition analyses were carried out using membrane preparations from transfected HN9.10 cells that constitutively expressed the respective receptor types.
Kd = 0.50 ± 0.1 nM.
Kd = 0.85 ± 0.2 nM.
Concentration at 50% inhibition of muscle concentration at electrically stimulated isolated tissues; these values represent the mean of four tissues within 95% confidence limit.
Competition against radiolabeled ligand, data collected from at least 2 independent experiments.
HCl salt.
Naloxone sensitive.
Naloxone insensitive.
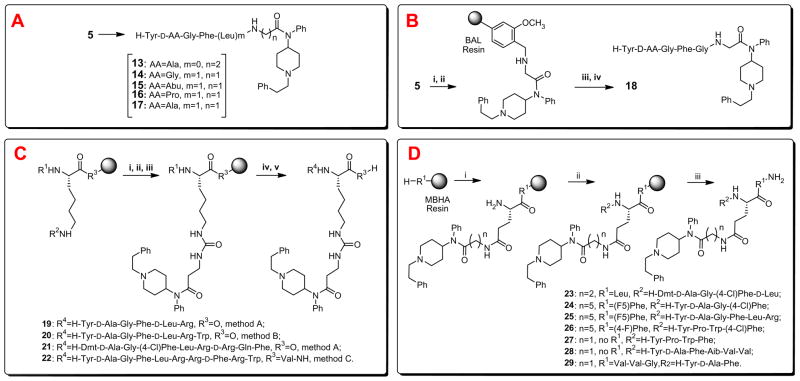
Although the opioid activities of the fentanyl surrogates 7–12 have turned out to be suboptimal for our original goal, there were several reasons to further investigate incorporation of these motifs into opioid peptides. A major reason for the continued interest in this direction is the fact that even a subtle structural and conformational change of an opioid ligand can greatly influence its overall activity profile, physicochemical properties, and biological efficacy.13 Previously, we showed the synthesis and in vitro data for the peptide analog 13, which was based on a part of fentanyl structure and ‘one arm’ (the core opioid sequence, H-Tyr-D-Ala-Gly-Phe) of biphalin.5 In view of the promising bioactivity data of 13, we then considered replacements for β-alanine for the next series of analogs (Scheme 2, block A). Although the desired linear isomers could be obtained in a convenient fashion by the standard step-by-step solution phase peptide synthesis, it was expected to be more expedient and efficient to prepare such analogs by applying solid-phase chemistry. Our attempts to employ FMPB-AM resin for the synthesis of novel analogs using the reductive amination technique14 (Scheme 2, block B) resulted in limited success—although the desired peptide 18 was obtained in the purity exceeding 96%, the yield of the target peptide was rather poor (10%). Our next alternative methods for making novel peptide conjugates by the solid phase methods included the use of N- and C-side chain substitutions. N-side chain modification of a Lys residue was performed on the resin after assembly of the first amino acid by removal of the Fmoc or Alloc group followed by treatment with p-nitrochloroformate and then with 3-aminofentanyl (Scheme 2, block C). This series also included analogs in which 3-aminofentanyl is linked to a dynorphin A structure.15–17 Furthermore, the Tyr and Phe residues in positions 1 and 4 were replaced by a Dmt and Phe(4-Cl), respectively, in 21 to facilitate opioid receptor recognition and enhance metabolic stability. C-side chain modification of the Glu residue was performed using a combination of Nα-Fmoc and Nα-Boc strategies (Scheme 2, block D).
Scheme 2.
Our strategies to introduce 4-anilidopiperidine functionality to opioid ligands. Reagents and conditions: (A) stepwise solution phase peptide synthesis, analogs 13–17; (B) reductive alkylation on solid support using (i) 4-(4-formyl-3-methoxy-phenoxy)butyryl AM (BAL) resin, NaBH(OAc)3, DMF/DCM (1/3), (ii) Fmoc-AA-OH, HOBt, HBTU, DIPEA in DMF, (iii) 50% Piperidine in DMF, and (iv) TFA/i-PrSiH3/H2O (90/5/5); (C) N-side chain modification by method A: Merrifield resin (R3=O, R1 = Boc, R2 = Fmoc), (i) 50% Piperidine in DMF, (ii) p-nitrochloroformate, DIPEA, DCM, 1 h, (iii) 2–3 equiv of 3-aminofentanyl, microwave in a closed vessel for 1 h at 80 °C, (iv) 50% TFA in DCM, (v) SPPS followed by TFMSA cleavage; method B: 2-chlorotrityl resin (R3=O, R1 = Fmoc, R2 = Alloc), (i) Pd(PPh3)4, PhSiH3 in DCM, (ii) p-nitrochloroformate, DIPEA, DCM, 1 h, (iii) 2–3 equiv of 3-aminofentanyl, microwave in a closed vessel for 1 h at 80 °C, (iv) 50% piperidine in DMF, (v) SPPS followed by TFA cleavage; method C: MBHA resin (R3 = Val-NH, R1 = Fmoc, R2 = Boc), (i) 50% TFA in DCM, (ii) p-nitrochloroformate, DIPEA, DCM, 1 h, (iii) 2–3 equiv of 3-aminofentanyl, microwave in a closed vessel for 1 h at 80 °C, (iv) 50% piperidine in DMF, (v) SPPS followed by TFMSA cleavage; (D) C-side chain modification using (i) Fmoc-Glu(Boc)-OH, HOBt, DIC, DMF→50% TFA in DCM→5% DIEA in DCM→amine (1, 5, 3-aminofentanyl), HOBt, DIC, microwave in DMF for 5 min at 80 °C→25% piperidine in DMF, (ii) solid phase peptide synthesis, and (iii) TFMSA cleavage.
The target compounds were tested for their affinity and potency in vitro (Table 2). Concerning the functionality of these ligands, no opioid antagonist activity was observed in the MVD or GPI assays. Compared to biphalin, analogs 13–18 represent peptide ligands with higher clogP values (about two- to fourfold) due to the hydrophobic character of the fentanyl moiety. As shown in Table 2, the binding assays reveal that these compounds possess μ receptor selectivity with binding affinities ranging from 0.09 nM for 14 to 260 nM for 16. In the functional assays, the selectivity of 17 was enhanced almost threefold for the δ receptor over the μ receptor as compared to biphalin and the previously synthesized 13. However, in this series, despite a slight increase in the μ receptor selectivity and binding affinity, and the overall lipophilicity, there was a moderate loss of μ bioactivity in the GPI functional assays. In contrast, ‘branched’ analogs 23 and 24, in which the 4-ANDP fragment is attached to the side chain of the Glu residue, showed potency comparable to that observed with biphalin. These observations suggested that the branched peptide ligands could be better accommodated in the opioid binding domain as opposed to the case of the linear core analogs.
Table 2.
Bioactivities of the designed peptide analogs
| No. | hDORa [3H]DPDPEb |
rMORa [3H]DAMGOc |
[35S]GTP-γ-S binding
|
IC50e (nM) | ||||
|---|---|---|---|---|---|---|---|---|
| hDORd | rMORd | |||||||
|
|
|
|
||||||
| Ki (nM)f | Ki(nM)f | EC50g (nM) | Emaxh (%) | EC50 (nM)g | Emax(%)h | MVD (δ) | GPI/LMMP (μ) | |
| 13i | 1.1 | 0.90 | 60 | 140 | 60 | 140 | 33 ± 6 | 110 ± 50 |
| 13j | 0.99 | 0.97 | 60 | 140 | 69 | 140 | 35 ± 6 | 42 ± 16 |
| 14 | 12 | 0.09 | 37 | 49 | 45 | 43 | 180 ± 20 | 950 ± 130 |
| 15 | 2.8 | 0.43 | 5.8 | 22 | 35 | 77 | 22 ± 5 | 97 ± 18 |
| 16 | 630 | 260 | n.r. | n.r. | 7500 ± 700 | 580 ± 150 | ||
| 17 | 27 | 13 | 5.9 | 64 | 0.59 | 13 | 9.5 ± 2.9 | 86 ± 16 |
| 18k | 4.0 | 0.73 | 46 | 120 | 9.1 | 50 | 360 ± 170 | 150 ± 40 |
| 19 | 11 | 0.35 | 2.2 | 84 | 2.8 | 31 | 8.6 ± 1.7 | 98 ± 24 |
| 20 | 0.26 | 0.50 | 0.22 | 19 | 1.5 | 59 | 16 ± 4 | 120 ± 20 |
| 21 | 1.8 | 0.15 | 0.34 | 10 | 0.20 | 32 | 3.2 ± 1.3 | 3.8 ± 0.8 |
| 22 | 4.6 | 0.54 | 8.3 | 7 | 1.4 | 31 | 27 ± 8.1 | 7.7 ± 2.1 |
| 23 | 0.35 | 0.17 | 170 | 11 | 380 | 45 | 40 ± 5 | 12 ± 2 |
| 24 | 0.51 | 0.28 | 1.1 | 17 | 2.2 | 68 | 8.8 ± 3.0 | 67 ± 15 |
| 25 | 0.63 | 1.5 | 2.8 | 27 | 12 | 29 | 12 ± 2 | 130 ± 60 |
| 26 | 84 | 64 | n.r. | 56. | 21 | 510 ± 130 | 480 ± 910 | |
| 27 | 590 | 130 | n.r. | n.r. | 1000 ± 200 | 2100 ± 30 | ||
| 28 | 0.28 | 9000 | 1.7 | 59 | n.r.l | 6.7 ± 3.9 | 49 ± 16 | |
| 29 | 24 | 1.5 | 90 | 33 | 220 | 21 | 120 ± 20 | 1900 ± 40 |
| Biphalin | 2.6 ± 0.4 | 1.4 ± 0.2 | 2.5 ± 0.5 | 27 ± 4 | 6.0 ± 0.2 | 25 ± 5 | 27 ± 2 | 8.8 ± 0.3 |
| Endomorphin-1 | 5100 ± 660 | 4.6 ± 0.2 | 26 ± 3 | 11 ± 1 | ||||
| Deltorphin II | 1.3 | 500 ± 40 | 0.37 ± 0.04 | >3000 | ||||
| Dyn A-(1-13)-OH | 3.8 ± 0.4 | 4.0 ± 0.4 | 6.3 ± 0.9 | 1.2 ± 0.1 | ||||
n.r. no response.
Competition analyses were carried out using membrane preparations from transfected HN9.10 cells that constitutively expressed the respective receptor types.
Kd = 0.50 ± 0.1 nM.
Kd = 0.85 ± 0.2 nM.
Expressed from CHO cell.
Concentration at 50% inhibition of muscle concentration at electrically stimulated isolated tissues; these values represent the mean of four tissues within 95% confidence limit.
Competition against radiolabeled ligand, data collected from at least 2 independent experiments in duplicate.
Anti-logarithmic value of the respective EC50.
Net total bound/basal binding × 100 ± SEM.
Compound 13 is a hydrochloride salt of 13.
Effect is not reversed by naloxone.
Tested in the free base form.
Tested as a trifluoroacetate salt.
In the endomorphin series, peptides 26 and 27 were very different in selectivity and potency from the parent opioid peptide, EM-1.18 Although 26 and 27 gained moderate binding affinity of 64 and 130 nM, respectively, at the δ opioid receptor, both analogs showed a 14- to 28-fold reduction of the affinity for the μ receptor and concomitant reduction in μ/δ potency. Interestingly, the piperidinyl analog 26, which has a longer spacer between the tetrapeptide and the 4-ANDP moiety, was slightly more potent than the analogous 27. Because in both cases the anchoring strategy had an adverse affect on affinity and potency, it seems that the 4-ANDP structure disrupts key interactions within the binding site of endomorphins.
Introduction of the 4-ANDP scaffold into the deltorphin peptide sequence19,20 (ligands 28 and 29) resulted in significant changes in binding affinities at the μ and δ opioid receptors. The incorporation of the 4-ANDP through the side chain of the Glu acid residue at a distance from the opioid message sequence afforded compound 28 with a subnanomolar binding affinity (Ki = 0.28 nM) for δ opioid receptors. As shown in the functional assays, 28 is potent at both δ and μ receptors with IC50 = 6.7 ± 3.9 and 49 ± 16 nM, respectively. In sharp contrast, the deltorphin analog 29 with the 4-ANDP closely adjacent to the opioid message region showed a reversed selectivity compared to deltorphin II and 28. In the binding assay, ligand 29 displayed a 16-fold higher selectivity for μ over the δ opioid receptors. However, 29 was 18- and 39-fold less potent at δ-receptors and μ-receptors, respectively, than the related compound 28 in the functional assays. In line with the previous observations, these results indicate that in addition to endowing molecules with higher lipophilicity, incorporation of the 4-ANDP structure confers μ opioid receptor selectivity. However, in the case of deltorphins, a synergistic or potentiating antinociceptive effect of anchoring the 4-ANDP structure is questionable.
In the dynorphin A series, potent opioid agonist activities for both receptors were observed in several ligands with binding affinities in the nanomolar and subnanomolar ranges. In this category, the introduction of 4-ANDP structure increased the lipophilicity of the parent peptide. The binding affinity of 21 at the μ receptor was subnanomolar (Ki = 0.15 nM), and nanomolar at the δ receptor (Ki = 1.8 nM), which represents almost a 13-fold improvement in selectivity for μ-opioid receptors as compared to Dyn A-(1-13). In the functional assays, a reversed trend was observed: analog 21 was about twofold more potent than the parent peptide in the MVD (IC50 = 3.2 ± 1.3 nM) assay, but threefold less potent in the GPI (IC50 = 3.8 ± 0.8 nM) assay. In the [35S]GTP-γ-S assays this compound had a EC50 value of 0.34 at the μ, and 0.2 nM at the δ opioid receptors. How much of an improvement both in affinity and bioactivity was introduced by the 4-ANDP attachment alone in this case, however, is a question that remains to be answered. In terms of useful opioid design, this result is more likely to be attributed to the introduction of the Dmt residue, as observed in many cases. A similar trend in affinity was observed for analogs 19 and 22. Analog 22 presents a case where a correlation in selectivity is maintained between binding affinities and bioactivities at the μ and δ opioid receptors. Similarly to 19, ligand 22 showed a reasonable ninefold selectivity for the μ receptor (Ki = 0.54 nM) over the δ receptor (Ki = 4.6 nM) in binding assays, albeit somewhat lower than that observed for 19 (31-fold selectivity for the μ receptor). Analog 22 was almost 13-fold more potent than the corresponding 19 in the GPI functional assay, although it also showed a moderate threefold loss of potency in the MVD assay. Ligands 20 and 25 were endowed with subnanomolar affinities of 0.26 and 0.63 nM, respectively, and selectivity (to twofold in both cases) for δ opioid receptors. Noticeably, most of the dynorphin A ligands displayed subnanomolar affinities at the μ opioid receptor. Moreover, compounds from this series also maintained good opioid potency with the 4-ANDP modification. This is in agreement with the earlier model for the dynorphin A binding pocket,21 and is also consistent with previous results, which showed that introduction of lipophilic residues into dynorphin A structure resulted in more potent analogs.22 It might be well to point out that although the high molecular weights and PSA values of the dynorphin A analogs are prohibitive of their CNS exposure, the presence of multiple positively charged residues in their structures suggests the possibility of their transport across biological membranes via the adsorptive-mediated endocytosis by binding to negatively charged sites on the surface of brain capillary endothelial cells.23
In conclusion, binding assays showed that incorporation of the 4-anilidopiperidine moiety strongly impacts both affinity and functional activity of the opioid peptides. We demonstrated that anchoring the 4-ANDP structure to short ekephalin-related or dynorphin A peptide structures represents a useful tactical approach for further enhancement of opioid potency. Attachment of the 4-ANDP moiety to the short ekephalin-related analogs appears to be a logical choice, considering the relatively small size, more lipophilic nature and high potency at both μ and δ opioid receptors. In case where the fentanyl moiety was conjugated to the dynorphin A, a superior opioid activity profile was reached to dynorphin A itself. The present findings suggest, however, that due to very weak opioid activity offered by the 4-ANDP conjugates, other similar sized, lipophilic adducts might be equally effective and easier to incorporate for the same purpose. Taken together, the results of these studies shed an instructive light on the development of mixed classes of opioid agonists as analgesic drug candidates.
Supplementary Material
Acknowledgments
The work was supported by Grants from the USDHS, National Institute on Drug Abuse (DA-12394 and DA-06284).
Abbreviations
- Aib
aminoisobutyric acid
- 4-ANDP
4-anilidopiperidine
- FMPB-AM
4-(4-formyl-3-methoxyphenoxy)butyrylaminomethyl
- clogP
calculated octanol–water partition coefficient
- DAMGO
[D-Ala2, MePhe4, Gly-ol5]enkephalin
- DCM
dichloromethane
- DIC
1,3-diisopropylcarbodiimide
- DIPEA
N,N-diisopropylethylamine
- DMF
N,N-dimethylformamide
- Dmt
2′,6′-dimethyl-(S)-tyrosine
- DPDPE
[D-Pen2, D-Pen5] enkephalin
- EM-1
endomorphin-1
- F5
pentafluoro
- Fmoc
9-(fluorenylmethoxy)carbonyl
- GPI
guineapigileum
- HBTU
2-(1H-benzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate
- HOBt
1-hydroxybenzotriazole
- MBHA
4-methylbenzhydrylamine
- MVD
mouse vasdeferens
- NaBH(OAc)3
sodiumtriacetoxyborohydride
- Pht
phthaloyl
- PSA
polar surface area
- TEA
triethylamine
- TFA
trifluoroacetic acid
- TFMSA
trifluoromethane sulfonic acid
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2013.03.065.
References and notes
- 1.Ye Y, Dang D, Viet CT, Dolan JC, Schmidt BL. J Pain. 2012;13:524. doi: 10.1016/j.jpain.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morse M, Tran E, Sun H, Levenson R, Fang Y. PLoS ONE. 2011;6:e25643. doi: 10.1371/journal.pone.0025643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Décaillot FM, Rozenfeld R, Gupta A, Devi LA. Proc Natl Acad Sci USA. 2008;105:16045. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stanley TH. J Pain Symptom Manage. 1992;7:S3. doi: 10.1016/0885-3924(92)90047-l. [DOI] [PubMed] [Google Scholar]
- 5.Petrov RR, Vardanyan RS, Lee YS, Ma SW, Davis P, Begay LJ, Lai JY, Porreca F, Hruby VJ. Bioorg Med Chem Lett. 2006;16:4946. doi: 10.1016/j.bmcl.2006.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee YS, Petrov R, Park CK, Ma SW, Davis P, Lai J, Porreca F, Vardanyan R, Hruby VJ. J Med Chem. 2007;50:5528. doi: 10.1021/jm061465o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YS, Kulkarani V, Cowell SM, Ma SW, Davis P, Hanlon KE, Vanderah TW, Lai J, Porreca F, Vardanyan R, Hruby VJ. J Med Chem. 2010;54:382. doi: 10.1021/jm100982d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horan PJ, Mattia A, Bilsky EJ, Weber S, Davis TP, Yamamura HI, Malatynska E, Appleyard SM, Slaninova J, Misicka A, Lipkowski AW, Hruby VJ, Porreca F. J Pharmacol Exp Ther. 1993;265:1446. [PubMed] [Google Scholar]
- 9.Wade CL, Schuster DJ, Domingo KM, Kitto KF, Fairbanks CA. Eur J Pharmacol. 2008;587:135. doi: 10.1016/j.ejphar.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wheeler M, Birmingham PK, Lugo RA, Heffner CL, Coté CJ. Anesth Analg. 2004;99:1347. doi: 10.1213/01.ANE.0000132777.00967.A3. [DOI] [PubMed] [Google Scholar]
- 11.Tamura M, Nakamura K, Kitamura R, Kitagawa S, Mori N, Ueda Y. Eur J Anaesthesiol. 2003;20:482. doi: 10.1017/s0265021503000772. [DOI] [PubMed] [Google Scholar]
- 12.Colpaert FC, Tarayre JP, Alliaga M, Bruins Slot LA, Attal N, Koek W. Pain. 2001;91:33. doi: 10.1016/s0304-3959(00)00413-9. [DOI] [PubMed] [Google Scholar]
- 13.Liu WX, Wang R. Med Res Rev. 2012;32:536. doi: 10.1002/med.20222. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Nair P, Jacobsen NE, Vagner J, Kulkarni V, Davis P, Ma SW, Navratilova E, Yamamura HI, Vanderah TW, Porreca F, Lai J, Hruby VJ. J Med Chem. 2009;52:5164. doi: 10.1021/jm900473p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Snyder KR, Story SC, Heidt ME, Murray TF, DeLander GE, Aldrich JV. J Med Chem. 1992;35:4330. doi: 10.1021/jm00101a010. [DOI] [PubMed] [Google Scholar]
- 16.Schäfer M, Brack A, Stein C. CNS Drugs. 2000;13:161. [Google Scholar]
- 17.Chen H, Yang Y, Weng J. Sci China, Ser B. 2009;52:338. [Google Scholar]
- 18.Liu HM, Liu XF, Yao JL, Wang CL, Yu Y, Wang R. J Pharmacol Exp Ther. 2006;319:308. doi: 10.1124/jpet.106.106484. [DOI] [PubMed] [Google Scholar]
- 19.Erspamer V, Melchiorri P, Falconieri-Erspamer G, Negri L, Corsi R, Severini C, Barra D, Simmaco M, Kreil G. Proc Natl Acad Sci USA. 1989;86:5188. doi: 10.1073/pnas.86.13.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emmerson PJ, Liu MR, Woods JH, Medzihradsky FJ. J Pharmacol Exp Ther. 1994;271:1630. [PubMed] [Google Scholar]
- 21.Chavkin C, Goldstein A. Proc Natl Acad Sci USA. 1981;78:6543. doi: 10.1073/pnas.78.10.6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lung FDT, Meyer JP, Lou BS, Xiang L, Li G, Davis P, De Leon IA, Yamamura HI, Porreca F, Hruby VJ. J Med Chem. 1996;39:2456. doi: 10.1021/jm950655o. [DOI] [PubMed] [Google Scholar]
- 23.Deguchi Y, Naito Y, Ohtsuki S, Miyakawa Y, Morimoto K, Hosoya KI, Sakurada S, Terasaki T. J Pharmacol Exp Ther. 2004;310:177. doi: 10.1124/jpet.103.064006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.