Abstract
Natural residues of the dimeric opioid peptide Biphalin were replaced by the corresponding homo-β3 amino acids. The derivative 1 containing hβ3 Phe in place of Phe showed good μ- and δ-receptor affinities ( ) and antinociceptive activity in vivo together with an increased enzymatic stability in human plasma.
INTRODUCTION
Biphalin is a unique dimeric opioid peptide composed of two enkephalin-based tetrapeptides (Tyr-D-Ala-Gly-Phe) linked by a hydrazide bridge between the two C-terminal phenylalanine moieties. Biphalin displays a strong affinity for both μ and δ receptors, with an EC50 in the nanomolar range. When administered intracerebroventricularly (icv) it shows higher potency than morphine and etorphine in animal models of pain.1 The remarkable activity is due primarily to its dimeric structure which improves the ability to meet the topographical requirements of involved receptors.2 Furthermore, this opioid octapeptide produced less physical dependence than other opioid agonists.1a,3
A major problem concerning the use of peptides, including the opioid peptides, as drugs, is their susceptibility to enzymatic hydrolysis when administered in vivo.4 Approaches that have been explored in an effort to overcome this problem in opioid peptides include the use of D-amino acids, β-amino acids, various types of synthetic residues, and backbone cyclization.5
A well-known and useful approach is the design and synthesis of α/β hybrid peptides, where one or more native residues of the backbone sequence is replaced by the corresponding β2- or β3-amino acid analogues.6 Hybrid α/β peptides fold the backbone in a manner more similar to the natural α-peptides than full β-peptides and together with high their intrinsic metabolic stability make them good candidates for drug design. β3-amino acids have been used in the design of several classes of ligands, including platelet aggregation factors, angiotensin II, chemotactic agents, and neuropeptides, including opioid peptides.7–10
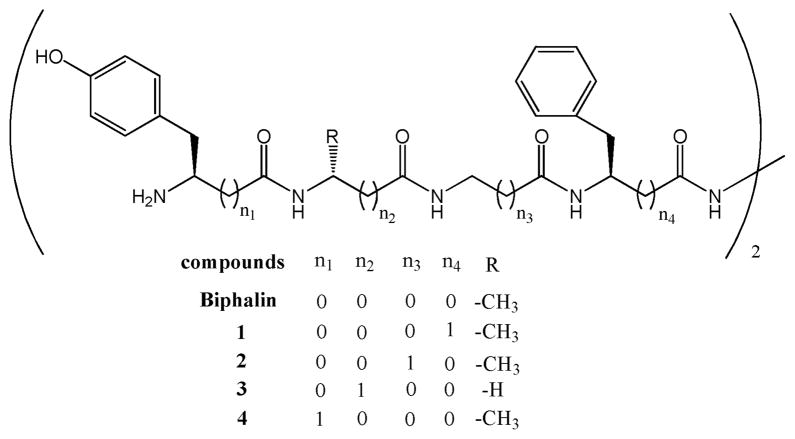
This work reports the synthesis and in vitro biological evaluation of four biphalin analogues (Figure 1) containing β3 homoamino acids (hβ3Xaa). hβ3Tyr and hβ3Phe were incorporated in place of the tyrosine1–1′ and phenylalanine4–4′ residues, respectively, and D-alanine2–2′ and glycine3–3′ were replaced with a β-alanine residue. The most active product of this series, which contains hβ3 Phe (1), was further tested in in vivo thermal models of nociception by icv and iv administrations in mice, with human plasma stability also evaluated.
Figure 1.
Structure of biphalin and analogues 1–4.
BIOLOGICAL EVALUATION
Binding Affinities at μ/δ Opioid Receptors.11
To determine the affinity to the μ-opioid (MOR) and the δ-opioid receptors (DOR) of compounds 1–4, tritiated opioid peptides DAMGO ([3H]-[D-Ala(2), N-Me-Phe-(4), Gly-ol(5)] enkephalin) and Deltorphin (selective agonists for MOR and DOR, respectively) were used. Binding IC50 and Ki values are shown in Table 1.
Table 1.
Binding Affinities and in Vitro Activities for Peptide Derivatives at δ/μ Opioid Receptors
| compd | hDORa, [3H]Deltorphinb
|
rMORa, [3H]DAMGOc
|
|
MVD (δ)
|
GPI (μ)
|
IC50 (GPI)/IC50 (MVD) | |||
|---|---|---|---|---|---|---|---|---|---|
| log IC50d | Ki (nM) | log IC50d | Ki (nM) | IC50 (nM)e,f | IC50 (nM)e,f | ||||
| 1 | −8.84 ± 0.11 | 0.72 | −8.64 ± 0.07 | 1.1 | 1.53 | 33 ± 9.4 | 50 ± 6.5 | 1.5 | |
| 2 | −4.97 ± 0.16 | 240 | −6.42 ± 0.08 | 172 | 0.72 | 1300 ± 221 | 9.2% at 1 μM | >0.75 | |
| 3 | −8.75 ± 0.11 | 450 | −6.90 ± 0.08 | 57 | 0.13 | 1800 ± 266 | 1700 ± 306 | 0.9 | |
| 4 | −8.27 ± 0.14 | 480 | −6.87 ± 0.11 | 62 | 0.13 | 710 ± 97 | 670 ± 139 | 0.9 | |
| Bphg | 2.6 | 1.4 | 0.54 | 2.7 ± 1.5 | 8.8 ± 0.3 | 3.2 | |||
Displacement of [3H]Deltorphin (μ-selective) and [3H]DAMGO (δ-selective) from rat brain membrane binding site.
Kd = 0.50 ± 0.3 nM.
Kd = 0.50 ± 0.1 nM.
The log IC50 ± standard error are expressed as logarithmic values determined from the nonlinear regression analysis of data collected from at least two independent experiments performed in duplicate. The Ki values are calculated using the Cheng and Prusoff equation to correct for the concentration of the radioligand used in the assay.
Concentration at 50% inhibition of muscle contraction in electrically stimulated isolated tissues (n = 4).
±SEM.
Biphalin was used as reference.1b Analogue 1 has a very good opioid receptor affinity, showing subnanomolar affinity at the DOR and a potent Ki value at the MOR (0.72 and 1.1 nM, respectively). Analogues 2–4 display poor affinity for opioid receptors, with a mild μ-selectivity for peptides 3 and 4 ( ). Peptide 2 showed little MOR/DOR discrimination, with Ki values higher than 100 nM at both receptors.
GPI and MVD in Vitro Bioassay.12
The biological activity was also investigated through isolated tissue-based functional assay using guinea pig ileum/longitudinal muscle myenteric plexus (GPI) and mouse vas deferens (MVD) tissues (Table 1). Biphalin was used as a reference.13 Compound 1 showed the greatest potency, with no relevant selectivity for μ- and δ-receptor (with Ki values of 33 and 50 nM, respectively, for MVD and GPI). Analogue 4 showed near identical IC50 around 700 nM in both MVD and GPI assays, while 2 and 3 had weak activity. These data are in agreement with results of binding assay.
In Vivo “Hot Plate” and “Tail-Flick” Bioassays.14
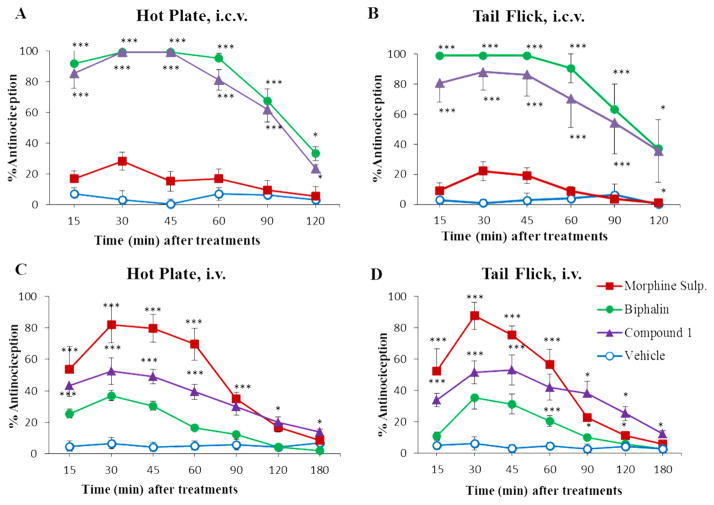
Antinociceptive properties of compound 1 were further investigated using the mouse hot plate and tail-flick assays, following both local (icv, 0.6 nmol) and systemic (iv, 3000 nmol) injections. Morphine and Biphalin were used as reference compounds (Figure 2).
Figure 2.
Antinociceptive results of hot plate and tail-flick in vivo bioassays for compound 1, Biphalin, and morphine sulfate. Compounds were injected by icv administration (A,B) at a dose of 0.6 nmol, and systemic iv administration (C,D) at a dose of 3000 nmol. The data represent the mean ± SEM. The statistical significance among groups was determined, in comparison with vehicle-treated animals with the analysis of variance (two-way ANOVA test) followed by Bonferroni’s posthoc comparisons using the statistical software SPSS. Statistical significance was P < 0.05 (*P < 0.05; ***P < 0.001).
In Vitro Metabolic Stability.15
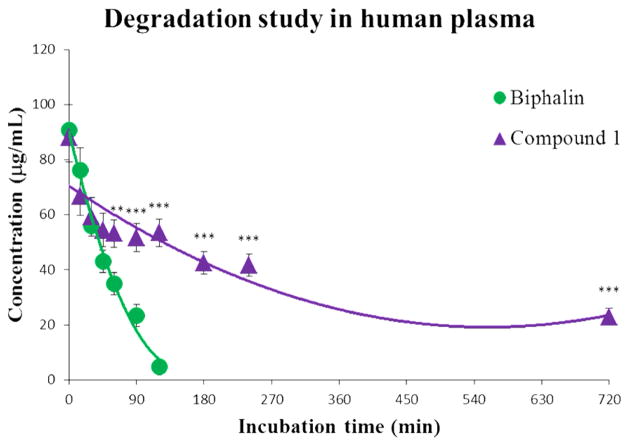
The enzymatic stability of both Biphalin and the Biphalin analogue 1 was tested by incubation in human plasma at 37 °C. Degradation curves (Figure 3) were obtained by plotting the total amount of remaining parent compound (expressed as μg/mL) versus time (expressed as minutes), demonstrating increased stability for the modified compound due to lack of recognition by degrading enzymes present in human plasma.
Figure 3.
Stability curves for Biphalin (green line) and the Biphalin derivative 1 (purple line). The samples were tested in three independent experiments (n = 3) and represent the mean ± SEM. The significance among groups was evaluated with the analysis of variance (two-way ANOVA test) followed by Bonferroni’s posthoc comparisons between compound 1 and Biphalin using the statistical software GraphPad Prism v.4. Statistical significance was P < 0.05 (**P < 0.01; ***P < 0.001); times from t0 to t45 present no statistical significance (P > 0.05).
DISCUSSION AND CONCLUSION
In this work, four new biphalin analogues were synthesized and investigated. The novel compounds were evaluated for their μ/δ receptor activity, and the results are shown in Table 1. Compound 1, containing hβ3Phe residues in position 4 and 4′, showed remarkable binding affinity, with Ki values of 0.72 and 1.1 nM, respectively, for DOR and MOR, resulting in a receptor affinity comparable with that of Biphalin. In the GPI and MVD in vitro bioassays (Table 1), compound 1 was still the most potent of the series, but all the peptides showed lower potency than Biphalin, suggesting that the activities of the compounds do not totally reflect the ability to induce a biological response.
The high affinity and the strong in vitro activity of 1, confirmed by in vivo nociception tests, may be explained by the fact that the distance between the two aromatic rings of Tyr and Phe is the same as the parent peptide, therefore the additional methylene group increases the flexibility of the compound without compromising side chain arrangements. The activity found for compounds 1–4 is compatible with previous biological studies on Biphalin analogues with non-hydrazine linkers. Those studies state that an increased distance between the two pharmacophores of Biphalin, obtained by using short diamine bridges (containing one or two methylene groups) or cyclic linkers (e.g., piperazine) is well tolerated or can even improve the in vitro affinity, and our data here confirm those conlcusions.16 On the other hand, the distance between Phe4,4′ and Tyr1,1′ side chains, as well as the reciprocal pharmacophores arrangement of the dimeric structure, are crucial for the activity. Thus, the use of β-residues in position 1,1′, 2,2′, and 3,3′ of the backbone led to an evident loss of affinity and activity (see products 2–4), whereas the introduction of the additional methylene group in position 4,4′ is well tolerated (see product 1).16b These data support the importance of D-Ala and Gly as keystructural residues, in addition to the well-known role of tyrosine. The lack of activity and affinity of compounds 2–4 is probably due to the β-residues that affect the spacing between the pharmacophoric Tyr and Phe residues. Interestingly, compounds 3 and 4 showed a significant selectivity for MOR (about 8-fold), suggesting a higher sensitivity of the DOR for modifications induced by β-residues. The antinociceptive in vivo profile of compound 1 clearly indicates that 1 is endowed with good activity, several times higher than morphine tested under the same conditions (for icv), but slightly lower than Biphalin, as expected from the MVD/GPI tests. In the hot plate test, after icv administration, the antinociceptive profile of the analogue 1 was very similar to Biphalin, producing 100% of the MPE 30 and 45 min after administration. In both in vivo models, the maximum effect is reached 15–30 min after drug injection, and no significant decrease is observed for the next 30 min.
Following iv administration (hot plate and tail-flick tests), compound 1 displayed a higher (ranging from 40 to 140% 15– 60 min after administration) and more long lasting antinociceptive effect than Biphalin, thus confirming the improved plasma stability, in full accord with in vitro stability data, reported in Figure 3 (for detailed experimental data see Supporting Information).
The improved metabolic stability paired with good antinociceptive activity confirms that Phe moiety modification16c, d is a promising strategy in the field of Biphalin analogues development.
EXPERIMENTAL SECTION
Chemistry
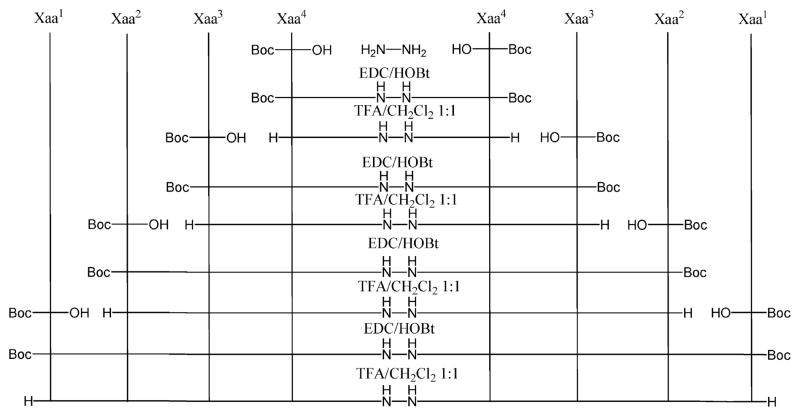
Synthesis of all new analogues was performed in solution phase using the Nα-Boc strategy. All synthesis began with hydrazine, with repeated steps of coupling/purification/deprotection of the intermediate products, until the final products were obtained as TFA salts (Scheme 1).
Scheme 1.
Synthesis of Biphalin Analogues 1–4a
a(1) Xaa4 = hβ3Phe; Xaa3 = Gly; Xaa2 = D-Ala; Xaa1 = Tyr; (2) Xaa4 = Phe; Xaa3 = βAla; Xaa2 = D-Ala; Xaa1 = Tyr; (3) Xaa4 = Phe; Xaa3 = Gly; Xaa2 = βAla; Xaa1 = Tyr; (4) Xaa4 = Phe; Xaa3 = Gly; Xaa2 = D-Ala; Xaa1 = hβ3Tyr.
All coupling reactions were performed with the standard method of HOBt/EDC/NMM in DMF.16b Deprotection of Nα-tert-butyloxycarbonyl group was performed using TFA/CH2Cl2 1:1 for 1 h, under nitrogen atmosphere. The intermediate TFA salts were used for subsequent reactions without further purification. Boc protected intermediate products were purified by silica gel column chromatography, or in the case of scarcely soluble products, the purification was performed by trituration in EtOAc.16c Final products 1–4 were purified by RP-HPLC using a Waters XBridge Prep BEH130 C18, 5.0 μm, 250 mm × 10 mm column at a flow rate of 4 mL/min on a Waters Binary pump 1525, using as eluent a linear gradient of H2O/acetonitrile 0.1% TFA ranging from 5% acetonitrile to 90% acetonitrile in 45 min. The purity of the Nα-Boc-protected products was confirmed by NMR analysis on a Varian VXR 300 MHz and mass spectrometry ESI-HRMS. The purity of all final TFA salts was confirmed by NMR analysis, ESI-HRMS, and by analytical RP-HPLC (C18-bonded 4.6 mm × 150 mm) at a flow rate of 1 mL/min, using as eluent a gradient of H2O/acetonitrile 0.1% TFA ranging from 5% acetonitrile to 95% acetonitrile in 50 min, and was found to be ≥95%. All synthetic procedure and intermediates’ characterizations are reported in the Supporting Information.
2 TFA·(Tyr-D-Ala-Gly-hβ3Phe-NH)2 (1)
Rf = 0.61 (n-Bu-OH/ AcOH/H2O 4:1:1). Rt (HPLC) = 20.24 min. 1HNMR (DMSO-d6) δ: 1.05 (6H, d, D-Ala CH3), 2.53 (4H, t, hβ3Phe hβCH2), 2.71–2.85 (4H, m, Tyr βCH2), 2.73–2.80 (4H, m, hβ3Phe βCH2), 3.53–3.61 (4H, m, Gly αCH2), 3.85 (2H, t, Tyr αCH), 4.15 (2H, m, hβ3Phe αCH), 4.34 (2H, t, D-Ala αCH), 6.65–7.01 (8H, m, Tyr Ar), 7.12– 7.27 (10H, m, hβ3Phe Ar), 7.88 (2H, d, hβ3Phe NH), 8.05 (6H, d, Tyr NH3+), 8.17 (2H, t, Gly NH), 8.52 (2H, d, D-Ala NH), 9.44 (2H, s, OH), 10.03 (2H, s, NH–NH). ESI-HRMS calcd for C52H62F6N10O14 m/z: 1165.4429 [M + H]+; found 1165.4431.
2 TFA·(Tyr-D-Ala-βAla-Phe-NH)2 (2)
Rf = 0.41 (n-Bu-OH/AcOH/ H2O 4:1:1). Rt (HPLC) = 19.26 min. 1HNMR (DMSO-d6) δ: 1.21 (6H, d, D-Ala CH3), 2.16–2.27 (4H, m, βAla CH2–CO), 2.70–2.80 (4H, m, Tyr βCH2), 2.77–2.86 (4H, m, Phe βCH2), 3.54–3.71 (4H, m, βAla CH2–N), 3.85 (2H, t, Tyr αCH), 4.38–4.49 (2H, m, D-Ala αCH), 4.52 (2H, t, Phe αCH), 6.69–6.96 (8H, m, Tyr Ar), 7.09–7.25 (10H, m, Phe Ar), 7.76 (2H, d, βAla NH), 8.04 (6H, d, Tyr NH3+), 8.20 (2H, d, Phe NH), 8.36 (2H, d, D-Ala NH), 9.36 (2H, s, OH), 10.21 (2H, s, NH–NH). ESI-HRMS calcd for C52H62F6N10O14 m/z: 1165.4429 [M + H]+; found 1165.4431.
2 TFA·(Tyr-βAla-Gly-Phe-NH)2 (3)
Rf = 0.39 (n-Bu-OH/AcOH/H2O 4:1:1). Rt (HPLC) = 19.55 min. 1HNMR (DMSO-d6) δ: 2.27– 2.32 (4H, m, βAla CH2–CO), 2.67–2.81 (4H, m, Tyr βCH2), 2.87– 2.92 (4H, m, βAla CH2–N), 2.91–2.99 (4H, m, Phe βCH2), 3.28– 3.39 (4H, m, Gly αCH2), 3.77 (2H, t, Tyr αCH), 4.24 (2H, t, Phe αCH), 6.65–6.95 (8H, m, Tyr Ar), 7.11–7.28 (10H, m, Phe Ar), 7.71 (2H, d, βAla NH), 8.07 (6H, d, Tyr NH3+), 8.16 (2H, t, Gly NH), 8.22 (2H, d, Phe NH), 9.25 (2H, s, OH), 10.19 (2H, s, NH–NH). ESI-HRMS calcd for C50H58F6N10O14 m/z: 1137.4116 [M + H]+; found 1137.4120.
2 TFA·(hβ3Tyr-D-Ala-Gly-Phe-NH)2 (4)
Rf = 0.53 (n-Bu-OH/ AcOH/H2O 4:1:1). Rt (HPLC) = 29.48 min. 1HNMR (DMSO-d6) δ: 1.12 (6H, d, D-Ala CH3), 2.61 (4H, t, hβ3Tyr βCH2), 2.78 (4H, t, hβ3Tyr hβCH2), 2.96–3.01 (4H, m, Phe βCH2), 3.37–3.49 (4H, m, Gly αCH2), 3.66–3.74 (2H, m, hβ3Tyr αCH), 4.19–4.23 (2H, m, DAla αCH), 4.60 (2H, m, Phe αCH), 6.60–6.93 (8H, m, hβ3Tyr Ar), 7.07–7.20 (10H, m, Phe Ar), 7.81 (6H, d, hβ3Tyr NH3+), 8.07 (2H, t, Gly NH), 8.21 (2H, d, Phe NH), 8.33 (2H, d, D-Ala NH), 9.38 (2H, s, OH), 10.15 (2H, s, NH–NH). ESI-HRMS calcd for C52H62F6N10O14 m/z: 1165.4429 [M + H]+; found 1165.4426.
Supplementary Material
Acknowledgments
This work was supported in part by grants from the U.S. Public Health Service, Natural Institutes of Health, DA00624 and DA13449.
ABBREVIATIONS USED
- DOR
δ-opioid receptor
- EDC
1-ethyl-(3-dimethylaminopropyl)-carbodiimide
- ESI-HRMS
electrospray ionization–high resolution mass spectrometry
- GPI
guinea pig ileum
- [3H]-DAMGO
[3H]-[D-Ala(2), N-Me-Phe-(4), Gly-ol(5)] enkephalin
- HOBt
1-hydroxybenzotriazole
- icv
intracerebroventricular
- iv
intravenous
- MOR
μ-opioid receptor
- MVD
mouse vas deferens
- NMM
N-methylmorpholine
- RP-HPLC
reversed phase high performance liquid chromatography
Footnotes
The authors declare no competing financial interest.
Synthetic procedures, characterization of intermediates, biological assays, and stability assay. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.(a) Horan PJ, Mattia A, Bilsky EJ, Weber S, Davis TP, Yamamura HI, Malatynska E, Appleyard SM, Slaninova J, Misicka A. Antinociceptive profile of Biphalin, a dimeric enkephalin analog. J Pharmacol Exp Ther. 1993;265:1446–1454. [PubMed] [Google Scholar]; (b) Lipkowski AW, Misicka A, Davis P, Stropova D, Janders J, Lachwa M, Porreca F, Yamamura HI, Hruby V. Biological activity of fragments and analogues of the potent dimeric opioid peptide, Biphalin. Bioorg Med Chem Lett. 1999;9:2763–2766. doi: 10.1016/s0960-894x(99)00464-3. [DOI] [PubMed] [Google Scholar]; (c) Silbert BS, Lipkowski AW, Cepeda MS, Szyfelbein SK, Osgood PF, Carr DB. Analgesic activity of a novel bivalent opioid peptide compared to morphine via different routes of administration. Agents Actions. 1991;3:382–387. doi: 10.1007/BF01986590. [DOI] [PubMed] [Google Scholar]
- 2.(a) Flippen-Anderson JL, Deschamps JR, George C, Hruby VJ, Misicka A, Lipkowski AW. Crystal structure of Biphalin sulfate: a multireceptor opioid peptide. J Pept Res. 2002;59:123–133. doi: 10.1034/j.1399-3011.2002.01967.x. [DOI] [PubMed] [Google Scholar]; (b) Abbruscato TJ, Thomas SA, Hruby VJ, Davis TP. Brain and spinal cord distribution of Biphalin: correlation with opioid receptor density and mechanism of CNS entry. J Neurochem. 1997;69:1236–1245. doi: 10.1046/j.1471-4159.1997.69031236.x. [DOI] [PubMed] [Google Scholar]
- 3.Yamazaki M, Suzuki T, Narita M, Lipkowski AW. The opioid peptide analogue Biphalin induces less physical dependence than morphine. Life Sci. 2001;69:1023–1028. doi: 10.1016/s0024-3205(01)01194-8. [DOI] [PubMed] [Google Scholar]
- 4.(a) Koda Y, Del Borgo M, Wessling ST, Lazarus LH, Okada Y, Toth I, Blanchfieldm JT. Synthesis and in vitro evaluation of a library of modified endomorphin 1 peptides. Bioorg Med Chem. 2008;16:6286–6296. doi: 10.1016/j.bmc.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Tömböly C, Péter A, Tóth G. In vitro quantitative study of the degradation of endomorphins. Peptides. 2002;23:1573–1580. doi: 10.1016/s0196-9781(02)00100-6. [DOI] [PubMed] [Google Scholar]
- 5.(a) Mollica A, Davis P, Ma SW, Porreca F, Lai J, Hruby VJ. Synthesis and biological activity of the first cyclic Biphalin analogues. Bioorg Med Chem Lett. 2006;16:367–372. doi: 10.1016/j.bmcl.2005.09.080. [DOI] [PubMed] [Google Scholar]; (b) Mollica A, Guardiani G, Davis P, Ma SW, Porreca F, Lai J, Mannina L, Sobolev AP, Hruby VJ. Synthesis of stable and potent δ/μ opioid peptides: analogues of H-Tyr-c[D-Cys-Gly-Phe-D-Cys]-OH by ringclosing metathesis. J Med Chem. 2007;50:3138–3142. doi: 10.1021/jm061048b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Zieleniak A, Rodziewicz-Motowidło S, Rusak L, Chung NN, Czaplewski C, Witkowska E, Schiller PW, Ciarkowski J, Izdebski J. Deltorphin analogs restricted via a urea bridge: structure and opioid activity. J Pept Sci. 2008;14:830–837. doi: 10.1007/978-0-387-73657-0_212. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Weltrowska G, Berezowska I, Lemieux C, Chung NN, Wilkes BC, Schiller PW. N-Methylated cyclic enkephalin analogues retain high opioid receptor binding affinity. Chem Biol Drug Des. 2010;75:82–88. doi: 10.1111/j.1747-0285.2009.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Hruby VJ. Designing peptide receptor agonists and antagonists. Nature Rev Drug Discovery. 2002;1:847–858. doi: 10.1038/nrd939. [DOI] [PubMed] [Google Scholar]
- 6.Seebach D, Beck AK, Bierbaum DJ. The world of β- and γ-peptides comprised of homologated proteinogenic amino acids and other components. Chem Biodiversity. 2004;1:1111–1239. doi: 10.1002/cbdv.200490087. [DOI] [PubMed] [Google Scholar]
- 7.(a) Steer DL, Lew RA, Perlmutter P, Smith AI, Aguilar MI. Design and Synthesis of Inhibitors Incorporating β-Amino Acids of Metalloendopeptidase EC 3.4.24.15. J Pept Sci. 2000;6:470–477. doi: 10.1002/1099-1387(200009)6:9<470::AID-PSC287>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]; (b) Seebach D, Abele S, Gademann K, Guichard G, Hintermann T, Jaun B, Matthews JL, Schreiber JV, Oberer L, Hommel U, Widmer H. β2- and β3-Peptides with Proteinaceous Side Chains: Synthesis and Solution Structures of Constitutional Isomers, a Novel Helical Secondary Structure and the Influence of Solvation and Hydrophobic Interactions on Folding. Helv Chim Acta. 2003;86:2098–2103. [Google Scholar]
- 8.(a) Seebach D, Matthews JL. β-Peptides: a surprise at every turn. Chem Commun. 1997;21:2015–2126. [Google Scholar]; (b) Seebach D, Abele S, Gademann K, Guichard G, Hintermann T, Jaun B, Matthews JL, Schreiber JV, Oberer L, Hommel U, Widmer H. β2- And β3-Peptides with Proteinaceous Side Chains: Synthesis and Solution Structures of Constitutional Isomers, a Novel Helical Secondary Structure and the Influence of Solvation and Hydrophobic Interactions on Folding. Helv Chim Acta. 1998;81:932–982. [Google Scholar]
- 9.(a) Stachowiak K, Khosla MC, Plucinska K, Khairallah PA, Bumpus FM. Synthesis of angiotensin II analogues by incorporating β-homotyrosine or β-homoisoleucine residues. J Med Chem. 1979;9:1128–1130. doi: 10.1021/jm00195a025. [DOI] [PubMed] [Google Scholar]; (b) Zablocki JA, Tjoeng FS, Bovy PR, Miyano M, Garland RB, Williams K, Schretzman L, Zupec ME, Rico JG, Lindmark RJ, Toth MV, McMakins DE, Adams SP, Panzer-Knodle SG, Nicholson NS, Taite BB, Salyers AK, King LW, Campion JG, Feigen LP. A novel series of orally active antiplatelet agents. Bioorg Med Chem. 1995;5:539–551. doi: 10.1016/0968-0896(95)00045-i. [DOI] [PubMed] [Google Scholar]; (c) Giordano C, Lucente G, Mollica A, Nalli M, Pagani Zecchini G, Paglialunga Paradisi M, Gavuzzo E, Mazza F, Spisani S. Hybrid α/β3-peptides with proteinogenic side chains. Monosubstituted analogues of the chemotactic tripeptide For-Met-Leu-Phe-OMe. J Pept Sci. 2004;10:510–523. doi: 10.1002/psc.562. [DOI] [PubMed] [Google Scholar]
- 10.(a) Wilczyńska D, Kosson P, Kwasiborska M, Ejchart A, Olma A. Synthesis and receptor binding of opioid peptide analogues containing β3-homo-amino acids. J Pept Sci. 2009;15:777–782. doi: 10.1002/psc.1175. [DOI] [PubMed] [Google Scholar]; (b) Bozü B, Fülöp F, Tóth GK, Tóth G, Szücs M. Synthesis and opioid binding activity of dermorphin analogues containing cyclic β-amino acids. Neuropeptides. 1997;31:367–372. doi: 10.1016/s0143-4179(97)90073-1. [DOI] [PubMed] [Google Scholar]; (c) Cardillo G, Gentilucci L, Qasem AR, Sgarzi F, Spampinato S. Endomorphin-1 analogues containing β-proline are μ-opioid receptor agonists and display enhanced enzymatic hydrolysis resistance. J Med Chem. 2002;45:2571–2578. doi: 10.1021/jm011059z. [DOI] [PubMed] [Google Scholar]
- 11.Misicka A, Lipkowski AW, Horvath R, Davis P, Kramer TH, Yamamura HI, Hruby VJ. Topographical requirements for delta opioid ligands: common structural features of dermenkephalin and deltorphin. Life Sci. 1992;51:1025–1032. doi: 10.1016/0024-3205(92)90501-f. [DOI] [PubMed] [Google Scholar]
- 12.Szekeres PG, Traynor JR. Delta opioid modulation of the binding of guanosine-5′-O-(3-[35S]thio)triphosphate to NG108-15 cell membranes: characterization of agonist and inverse agonist effects. J Pharmacol Exp Ther. 1997;283:1276–1284. [PubMed] [Google Scholar]
- 13.Yamamoto T, Nair P, Largent-Milnes TM, Jacobsen NE, Davis P, Ma SW, Yamamura HI, Vanderah TW, Porreca F, Lai J, Hruby VJ. Discovery of a potent and efficacious peptide derivative for δ/μ opioid agonist/neurokinin 1 antagonist activity with a 2′,6′-dimethyl-L-tyrosine: in vitro, in vivo, and NMR-based structural studies. J Med Chem. 2011;54:2029–2038. doi: 10.1021/jm101023r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pieretti S, Di Giannuario A, De Felice M, Perretti M, Cirino G. Stimulus-dependent specificity for annexin 1 inhibition of the inflammatory nociceptive response: the involvement of the receptor for formylated peptides. Pain. 2004;109:52–63. doi: 10.1016/j.pain.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 15.Al-Fayoumi SI, Brugos B, Arya V, Mulder E, Eppler B, Mauderli AP, Hochhaus G. Identification of stabilized dynorphin derivatives for suppressing tolerance in morphine-dependent rats. Pharm Res. 2004;21:1450–1456. doi: 10.1023/b:pham.0000036920.50291.5b. [DOI] [PubMed] [Google Scholar]
- 16.(a) Shimohigashi Y, Costa T, Chen HC, Rodbard D. Dimeric tetrapeptide enkephalins display extraordinary selectivity for the δ opiate receptor. Nature. 1982;297:333–335. doi: 10.1038/297333a0. [DOI] [PubMed] [Google Scholar]; (b) Mollica A, Davis P, Ma SW, Lai J, Porreca F, Hruby VJ. Synthesis and biological evaluation of new Biphalin analogues with non-hydrazine linkers. Bioorg Med Chem Lett. 2005;15:2471–2475. doi: 10.1016/j.bmcl.2005.03.067. [DOI] [PubMed] [Google Scholar]; (c) Mollica A, Pinnen F, Feliciani F, Stefanucci A, Lucente G, Davis P, Porreca F, Ma SW, Lai J, Hruby VJ. New potent Biphalin analogues containing p-fluoro-L-phenylalanine at the 4,4′ positions and nonhydrazine linkers. Amino Acids. 2011;40:1503–1511. doi: 10.1007/s00726-010-0760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Leone S, Chiavaroli A, Orlando G, Mollica A, Di Nisio C, Brunetti L, Vacca M. The analgesic activity of Biphalin and its analog AM 94 in rats. Eur J Pharmacol. 2012;685:70–73. doi: 10.1016/j.ejphar.2012.04.026. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.