Abstract
Ischemic retinopathies are clinically well-defined chronic microvascular complications characterized by gradually progressive alterations in the retinal microvasculature and a compensatory aberrant neovascularization of the eye. The subsequent metabolic deficiencies result in structural and functional alterations in the retina which is highly susceptible to injurious stimuli such as diabetes, trauma, hyperoxia, inflammation, aging and dysplipidemia. Emerging evidence indicates that an effective therapy may require targeting multiple components of the angiogenic pathway. Conceptually, mircoRNA (miRNA)-based therapy provides the rationale basis for an effective antiangiogenic treatment. miRNAs are an evolutionarily conserved family of short RNAs, each regulating the expression of multiple protein-coding genes. The activity of specific miRNAs is important for vascular cell signaling and blood vessel formation and function. Recently, important progress has been made in mapping the miRNA-gene target network and miRNA-mediated gene expression control. Here we highlight the latest findings on angiogenic and antiangiogenic miRNAs and their targets as well as potential implications in ocular neovascular diseases. Emphasis is placed on how specific vascular-enriched miRNAs regulate cell responses to various cues by targeting several factors, receptors and/or signaling molecules in order to maintain either vascular function or dysfunction. Further improvement of our knowledge in not only miRNA specificity, turnover, and transport but also how miRNA sequences and functions can be altered will enhance the therapeutic utility of such molecules.
Keywords: MircoRNA, Angiogenesis, Retinal neovascularization, Vascular endothelial growth factor, Ischemia, Endothelial cell
Core tip: This review examines the critical regulatory role of microRNAs (miRNAs) in the process of normal and pathological angiogenesis and the prospects that they provide for the development of new treatments. miRNAs are both upstream and downstream of multiple growth factors in regulating endothelial proliferation, migration, and vascular patterning, processes critical for normal and abnormal formation of blood vessels. Emphasis in this review is placed on how specific vascular-enriched miRNAs regulate cell responses to various cues by targeting several factors, receptors and/or signaling molecules in order to maintain either vascular function or dysfunction.
INTRODUCTION
Angiogenesis is the generation of new blood vessels from pre-existing ones, a process initiated by branching “decisions” of endothelial cells (ECs) to undergo proliferation, guided migration, tubulogenesis, vessel fusion and pruning. Physiological angiogenesis is crucial in maintaining normal vascular growth and homeostasis from embryogenesis to postnatal life, especially in instances of fetal development, wound healing, transplantation, post-ischemic tissue repair and the menstrual cycle[1-4]. However, excessive angiogenesis is a commonly occurring pathogenic condition in more than 30 diseases, including eye diseases, cancer, rheumatoid arthritis, atherosclerosis, diabetic nephropathy, pathologic obesity, asthma, cystic fibrosis, inflammatory bowel disease, psoriasis, endometriosis, vasculitis and vascular malformations. In particular, the vascular beds supplying the retina often sustains injury as a result of underlying diseases such as diabetes, trauma, hyperoxia, aging, dyslipidemia, or the interaction of genetic predisposition, environmental insults and age. The high metabolic and oxygen demands make the retina highly susceptible to these injurious stimuli which lead to an arrest of vascular development, vaso-obliteration and/or vascular occlusion. The subsequent vascular pathological response observed, especially in intraocular vascular diseases, generates disorganized, leaky, and tortuous vessels that leak into the interface between the vitreous and the retinal tissue, attracting fibroglial elements causing severe hemorrhage, retinal detachment, and vision loss. These are the characteristic features of neovascular and fibrovascular diseases of the eye such as retinopathy of prematurity and proliferative diabetic retinopathy. The exudative or “wet” form of age-related macular degeneration (AMD) which largely affects choroidal vessels and cause blindness in elderly populations is characterized by the overgrowth of the choriocapillaris that invade the Bruch’s membrane and grow into subretinal spaces[5,6].
GROWTH FACTOR EXPRESSION AS A DETERMINANT FACTOR OF NORMAL AND PATHOLOGICAL ANGIOGENESIS IN THE RETINA
The formation of an aberrant and dysfunctional vasculature is commonly initiated by the uncontrolled expression or, lack thereof of growth factors including vascular endothelial growth factor (VEGF), Notch and Wnt signaling components, bone morphogenic protein, thrombospondins and insulin-like growth factors (IGFs)[7-11]. In particular, VEGF, a highly specific mitogen for ECs, is a major determinant of normal and pathological formation of the retinal vasculature[12]. Loss of VEGF attenuates blood vessel formation in mice embryos leading to early embryonic lethality and causes defective vascularization in adults[13-16]. Conversely, high expression of VEGF is common in avascular peripheral hypoxic regions of the retina compared to already vascularized areas[17]. Under conditions of oxygen deprivation, hypoxia-inducible factor 1α (HIF-1α) is activated and binds to its responsive elements in the promoter region of VEGF and other hypoxia-responsive genes, causing their upregulation and subsequent abnormal vessel growth[18]. Anti-VEGF treatments have been useful in reducing neovascularization of the eye. However, not all patients have achieved an optimal response. Safety data from several studies identified ocular and systemic adverse events including subretinal fibrosis, endophthalmatis, traumatic cataract, non-ocular hemorrhage, etc. Additionally, the use of anti-VEGF treatments, in the case of AMD in diabetic patients, interfered with myocardial revascularization and, in some cases, worsened the pathology in the diabetic eyes as a result of VEGF-dependent loss of neurotrophic and vasculotropic factors[19].
There are numerous other factors that contribute to neovascular growth. The erythropoietin (Epo) and VEGF genes, for instance, exhibit a similar expression pattern during both physiological and pathological vessel growth and inhibition of Epo suppressed retinal neovascularization both in vivo and in vitro[20,21]. Other factors such as basic fibroblast growth factor (bFGF), platelet derived growth factor (PDGF), transforming growth factor alpha, interleukin 8 (IL-8), connective tissue growth factor (CTGF), pigment epithelium-derived factor, IGF-I, and matrix metalloproteinase (MMP)-2 were similarly implicated in the neovascular response and are considered as potential therapeutic targets. In addition, inflammation-mediated cyclooxygenase-2 (COX-2) can modulate angiogenesis via its interaction with VEGF[22] and important pro-angiogenic and neovascular functions have been associated with the activation of the renin-angiotensin system, ephrins, tyrosine kinase receptors and ligands (e.g., tie/angiopoietin receptors). Together, all these factors form a well-coordinated and functional network of molecules affecting the process of normal and pathological angiogenesis. Emerging evidence indicates that antiangiogenic therapy may require therapeutic approaches that target multiple components of the angiogenic pathway[23-26]. Conceptually, microRNA-based approaches may potentially provide the rationale basis for such approaches.
MICRORNA BIOGENESIS AND FUNCTION IN THE MODULATION OF GENE EXPRESSION
Key events in gene regulation depend on specific small non-coding RNA-guided posttranscriptional regulators, commonly referred to as miRNAs that target a “mixture” of diverse growth and differentiation factor mRNAs encoding networks[27]. MicroRNAs are a relatively abundant class of gene expression regulators that function as “micromanagers” of gene expression[28]. These are short non-coding RNAs (18-25 nucleotides) which work post-transcriptionally to negatively regulate gene expression through translational inhibition or degeneration of mRNAs. They might act as on-off switches to eliminate mRNAs that should not be expressed in a particular cell type or at a particular moment. MicroRNAs can also act to fine tune mRNA abundance and adjust the levels of their mRNA targets within a physiological range in response to environmental cues. A single miRNA has the capacity to target multiple target mRNAs, which can themselves be targeted by numerous other miRNAs. To date, 1186 mouse miRNA and 1872 human miRNA sequences have been noted on the miRBase database and may control at least 30% of all the protein-coding genes[29].
Since the discovery of miRNAs, their biogenesis has been thoroughly examined and it is now known that both miRNAs and small interfering (si) RNAs share the same cellular machinery[4,30]. Most miRNA genes are transcribed by RNA polymerase II, which is usually responsible for the transcription of protein coding genes, to yield several kilobase-long primary miRNA (pri-miRNA) transcripts (Figure 1). Pri-miRNAs have characteristic loop stem (or hairpin) morphology and contain the mature miRNA sequence in the stem portion near the loop. The microprocessor, containing the endonuclease Drosha, cleaves the pri-miRNA into shorter pre-miRNAs that are transported to the cytoplasm by exportin-5. Once in the cytoplasmic compartment, pre-miRNAs undergo the final steps towards maturation. The first step involves “dicing” of the loop portion of the molecule by another endonuclease, Dicer and the transactivation response RNA binding protein (TRBP). A miRNA-miRNA duplex that is unwound is released together with the single-stranded mature miRNA. The latter is then passed to Argonaute to from a functionally mature, approximately 22 nucleotide miRNA. The 2-8-bp “seed” region in the 5’ end of miRNAs binds to target 3’UTR of mRNA sequences and inhibits translation if base-pairing is imperfect or initiates mRNA cleavage if base-pairing is perfect.
Figure 1.

Schematic representation of microRNA (miRNA) biogenesis. miRNA genes are transcribed into large pre-miRNA (capital R) that are cleaved by a protein complex containing the endonuclease Drosha into shorter pre-miRNAs. The latter are then transported to the cytoplasm by exportin-5. A complex containing the endonuclease, Dicer, then cleaves the loop portion of the pre-miRNA (capital R) to form a short duplex molecule that is unwound, and the single-stranded mature mirNA is then passed to Argonaute to from a functional mature, approximately 22 nucleotide, miRNA that inhibit translation after base-pairing with the 3’ UTR of the miRNA (capital R) target.
REGULATION OF ANGIOGENESIS BY MICRORNAS
The first studies of the functional significance of the miRNA pathway in angiogenesis were performed using conditional deletion of Dicer alleles, as complete loss of Dicer resulted in a significant reduction of the mature miRNA profile and early embryonic lethality[31,32]. Yang et al[32] have shown that mice with Dicer gene deletion lack adequate blood vessel formation in embryos and yolk sacs and die between 12.5 and 14.5 d post-gestation, thus implicating Dicer-dependent miRNA genesis in the regulation of blood vessel formation. Defects in these mice were due to dysregulation of VEGF and its receptors, KDR and FLT-1, along with Tie-1, an angiopoietin-2 receptor[32]. Similarly, silencing of Dicer or Drosha in (ECs) using siRNA significantly inhibited capillary sprouting and altered expression patterns of Tie-2, VEGF receptor 2 (VEGFR2/KDR), Tie-1, endothelial nitric oxide synthase (eNOS) and IL-8 in vitro[33,34]. Another study by Otsuka et al[35] showed that in female Dicer hypomorphic mice, infertility ensued from lack of angiogenesis in the ovaries. Further analysis revealed that impaired angiogenesis resulted from the absence of two pro-angiogenic miRNAs, miR17-5p and let-7b, which target anti-angiogenic factors[35]. Additionally, nude mice subcutaneously injected with siRNA-transfected ECs showed reduced angiogenic sprouting of transplanted cells[33]. In two EC-specific Dicer knock-out mouse models generated by Suarez et al[34], postnatal angiogenesis significantly decreased in response to multiple stimuli. In this study, transfection of cells with miR-18a, miR-17-5p, and miR-20a (collectively forming the miR-17-92 cluster) restored normal angiogenesis in Dicer knockout mice[34]. Taken together, these studies established a role of Dicer-dependent miRNA biogenesis in the control of angiogenesis in vitro and in vivo.
MICRORNA SIGNATURE IN NORMAL AND PATHOLOGICAL ANGIOGENESIS
Recent studies have examined miRNA expression profiles and patterns during retinal angiogenesis[8,36-38]. More than 250 miRNAs have been enumerated in the retina and new information on the regulation and mode of action of those miRNAs is progressively emerging[38]. Specific functions have been attributed to individual angiogenic miRNAs, although the challenge still remains in validating their protein targets[23,36,39-46]. Similarly, differential expression of miRNAs during retinal neovascularization has been studied in the mouse model of oxygen-induced retinopathy (OIR). In this model, seven miRNAs were upregulated, including miR-451, -424, -146, -214, -199a, -181 and -106a, when compared to control retinas, while miR-31, -150 and -184 were downregulated. However, this study provided only an exhaustive list of potentially key angiogenic miRNAs whose expression patterns, localization, and actual targets remain unclear.
Greater insights on angiogenic and antiangiogenic miRNA expression and function have been obtained from in vitro studies and other in vivo models of pathological angiogenesis. Poliseno et al[47] have performed the first large-scale analysis of miRNA expression in human umbilical vein endothelial cells using miRNA arrays. Twenty seven highly expressed miRNAs were identified, 15 of which were predicted to regulate the expression of receptors for angiogenic factors (e.g., Flt-1, Nrp-2, FGF-R, c-Met, c-Kit). Additional studies from other groups have identified a total of 200 miRNAs that are expressed in ECs[4,24]. Overall 28 endothelial-specific miRNAs were highly expressed in 5 out of 8 of the profiling studies including miR-221/222, miR-21, the let-7 family, miR-126, miR-17-92 cluster, and the miR 23-27-24 cluster[4,24,25]. Angiogenic factors and receptors are putative targets of those miRNAs[1,7,48,49]. However, it should be noted that both abundantly expressed miRNAs as well as the rarely expressed ones play important regulatory roles and the exact in vivo relevance of all miRNAs expressed in ECs remains to be determined. Since angiogenesis involves complex and intertwined pathways, we have classified the currently known endothelial-specific miRNAs based on the context/conditions of their expression (Figure 2).
Figure 2.
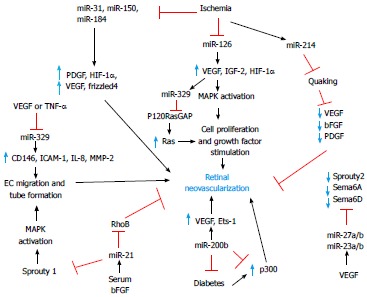
Overview of major angiogenic and antiangiogenic miRNAs and their targets in promoting or suppressing retinal neovascularization. VEGF: Vascular endothelial growth factor; IGFs: Insulin-like growth factors; HIF-1α: Hypoxia-inducible factor 1α; bFGF: Basic fibroblast growth factor; PDGF: Platelet derived growth factor; IL-8: Interleukin 8; MMP: Matrix metalloproteinase; ICAM-1: Intercellular adhesion molecule-1; TNF-α: Tumour necrosis factor-alpha; MAPK: Mitogen-activated protein kinase.
Hypoxia-sensitive miRNAs
Microarray-based expression profiling revealed that specific miRNAs are induced under hypoxic conditions and target angiogenic factors produced by ECs[50]. In particular, miR-15b, -16, -20a and -20b were shown to be upregulated under hypoxic conditions and target VEGF[50]. Additionally, miR-15b and miR-16 are predicted to be putative regulatory miRNAs of uPAR, COX2, and c-MET, which themselves are induced in response to hypoxic conditions[50]. Upregulation of these miRNAs is p53- and HIF-1α-dependent. Other microarray-based expression profiles have also revealed a set of hypoxia-induced miRNAs which are also over-expressed in tumors[51]. In particular, miR-210 is hypoxia-induced in all cell types tested[41,52]. In ECs subjected to hypoxia, miR-210 regulates the tyrosine kinase receptor ephrin-A3 that contributes to vascular remodeling. miR-210 promotes the formation of capillary-like structures in cultured ECs but, under hypoxic conditions, it decreases ECs tube formation and migration[52,53]. miR-100 is another hypoxia-sensitive miRNA that was shown to be significantly down-regulated after hind-limb ischemia[54]. Under these conditions, miR-100 repressed the expression of an angiogenic serine/threonine protein kinase targeted by rapamycin[55]. Furthermore, Shen et al[39] reported a dramatic increase in the expression of miR-106a, -146, -181, -199a, -214, -424 and -451 in a model of retinal ischemia suggesting their potential roles in the pathogenesis of neovascular diseases of the eye. Similarly, the hypoxia-induced miR-424 and miR-200 target the protein complex that stabilizes HIF-α and promote angiogenesis[56,57].
Growth factor-sensitive miRNAs
The effects of several angiogenic factors are mediated by miRNAs such as miR-155, -191, -21, -18a, -130a, -17-5p, -20a, -296, -101, -125b and -132[58]. In particular, serum, VEGF, and bFGF increased the expression of miR-130a, which enhances angiogenesis by downregulating the expression of anti-angiogenic homeobox proteins such as growth arrest-specific homeobox and Homeobox protein Hox-A5[52,59]. In the presence of VEGF or epidermal growth factor (EGF), the levels of miR-296 were significantly up-regulated in primary human brain microvascular ECs[60]. miR-296 was also found to be up-regulated in tumors and targets the hepatocyte growth factor-regulated tyrosine kinase substrate that inhibits degradation of key angiogenic growth factor receptors such as VEGF receptor 2 and PDGF receptor β[60]. Conversely, miR-101 was found to be down-regulated by VEGF which then allows the expression of histone-methyltransferase enhancer of zest homolog 2, increasing methylation of histone H3 and enhancing a pro-angiogenic response[61]. miR-125b was induced transiently by VEGF and negatively regulates vascular endothelial (VE)-cadherin to suppress tube formation in vitro and tumor growth in vivo[62]. A prolonged over-expression of miR-125b in vivo resulted in blood vessel regression. Transforming growth factor β (TGF-β) which is best known for its profibrotic activities, is a potent inducer of VEGF gene expression in retinal pigment epithelial cells[63]. However, numerous miRNAs have also been found to regulate and participate in TGF-β-induced VEGF expression[64]. Such effect is mediated by miR-29a which targets the phosphatase and tensin homolog (PTEN) gene, leading to the activation of the protein kinase B pathway, increased VEGF expression and angiogenesis[64]. Similarly, miR-132, which is undetectable in normal endothelium, was shown to be induced by VEGF and FGF in ECs and trigger neovascularization in the retina[65,66].
Inflammation and cytokines
Inflammation typically has beneficial effects on an acute basis, but it produces undesirable effects if persisting chronically. Angiogenesis sustains inflammation by providing oxygen and nutrients for inflammatory cells which, in turn, stimulates pathological angiogenesis[67]. The increased expression of many inflammatory proteins such as IL-1, IL-3 and tumour necrosis factor-alpha (TNF-α), is regulated at the level of gene transcription through the activation of proinflammatory transcription factors, including nuclear factor-kappa-B (NF-κB). In retinal ECs of diabetic rats, the expression of miR-146, -155, -132 and -21 up-regulates NF-κB gene expression and activity[67]. In contrast, miR-146 negatively regulates IL-1 receptor-associated kinase 1 and TNF receptor-associated factor 6 which are themselves induced following NF-κB activation[52,67]. Thus, targeting miR-146 may have an anti-inflammatory potential.
Meanwhile, T cell derived cytokine IL-3, a pro-inflammatory and a pro-angiogenic cytokine, was reported to down-regulate the expression of miR-296, miR-126, and -miR-221/222 in ECs[68]. The miR-222 exhibited anti-angiogenic effects by negatively regulating STAT5A in a mouse model of retinal neovascularization[68]. miR-126 has been portrayed as an anti-inflammatory molecule because it suppresses TNF-α mediated vascular cell adhesion molecule 1 (VCAM-1) expression and leukocyte interactions with ECs[26,31,52].
Reactive oxygen species as inducers of EC senescence
There is considerable evidence that increased production of reactive oxygen species (ROS) in the retina affects retinal vessel formation, although the mechanisms by which this occurs are not fully understood[69]. ROS such as superoxide anions such as H2O2 inhibit EC growth and increased cell death which are commonly associated with vaso-obliteration preceding ischemia[70]. Over-expression of miR-23a from the miR-23-27-24 cluster inhibits H2O2-induced apoptosis in retinal pigment epithelial cells from AMD patients via the repression of Fas, an activator of the apoptotic pathway[71]. Similarly, the miR-200c is up-regulated in ECs by oxidative stress and affects EC proliferation and death by inhibiting ZEB1[72].
The miRNA profiling of aging human primacy ECs revealed that miR-17,-21,-216,-217,-31b, and-181a/b are highly expressed[73]. In particular, miR-217 is progressively expressed in response to EC stimulation by ROS and targets Sirt1 (silent information regulator 1) that regulates angiogenic gene expression via deacetylation of histones[31,73]. Inhibition of miR-217 in ECs reduced senescence and enhanced angiogenesis[73]. Likewise, miR-34a targets Sirt1 and impairs angiogenesis which leads to the onset of senescence[31,74].
OTHER MIRNAS WITH POTENTIALLY IMPORTANT ANGIOGENIC FUNCTIONS IN THE RETINA
Other miRNAs with potentially important angiogenic functions in the retina were shown in Figure 2.
miR-221/222
miR-221 and miR-222 are two paralogue miRNAs located in close proximity to one another on Xp11.3 chromosome[26,47]. Over-expression of miR-221/222 reduced EC growth in vitro by targeting the c-Kit receptor, a tyrosine kinase receptor for stem cell factor which regulates EC migration, and survival as well as tube formation[47,52]. EC transfection with miR-221/222 inhibits tube formation, migration, and wound healing[47,52]. Conversely, miR-221/222 positively regulates proliferation and migration of cultured vascular smooth muscle cells, suggesting a cell type-specific function[68,75]. The proangiogenic effects of miR-221/222 in smooth muscle cells are p27 and p57-dependent. A recent study in zebra fish showed that miR-221 deficiency resulted in drastic developmental vascular defects which underscore an important function of miR-221/222 in angiogenesis[11]. In the latter study, miR-221 acts autonomously on the VEGF-C/Flt4 signaling pathway, altering endothelial tip and stalk cell phenotypes[11]. miR-221 promotes tip cell migration and proliferation by negatively regulating cyclin dependent kinase inhibitor 1b and phosphoinositide-3-kinase regulatory subunit 1[11]. The discrepancy between the in vitro and in vivo activities of miR-221/222 may be due to a differential effect on the mature and non-mature circulatory system. Further studies are needed to ascertain the regulation and function of miR-221/222 in developmental and pathological angiogenesis in the retina.
miR-17-92 cluster
The miR-17-92 cluster is a polycistronic miRNA gene located in intron 3 of chromosome 13 in humans, and contains six mature miRNAs, miR-17, -18a, -19a, -19b-1, -20a and -92a[3,4,26]. This cluster is highly expressed in ECs and tumor cells and is strongly up-regulated by ischemia[28,31,52,76]. Ectopic expression of the miR-17-92 cluster partially rescued the angiogenic phenotype of Dicer-deficient ECs[58]. Similarly, restoration of miR-17 in combination with let-7b in Dicer knockout mice also partially normalized corpus luteum angiogenesis by targeting the tissue inhibitor metalloproteinase-1, an anti-angiogenic factor[35]. The pro-angiogenic function of this cluster is due to the inhibition of the anti-angiogenic molecules thrombospondin-1 and CTGF by miR-18 and miR-19, respectively[58]. However, the function of this miRNA cluster in retinal angiogenesis remains to be elucidated.
miR-126
miR-126 is the best characterized EC-specific miRNA and is known to be highly conserved among species[1,26]. It is encoded by intron 7 of the EGF-like domain 7. miR-126 enhanced VEGF signaling by directly targeting the 3’UTR of Sprouty-related EVH1 domain containing protein-1 and phosphoinositol-3-kinase regulatory subunit 2[1,7,26,31,49]. Thus, miR-126 promotes angiogenesis by targeting negative regulators of the angiogenic pathway. miR-126 affects cell migration, reorganization of the cytoskeleton, capillary network stability, and cell survival in vitro[7]. It also altered developmental angiogenesis and vascular integrity. Fifty percent of miR-126 null mice died as a result of severe systemic edema, ruptured blood vessels and multifocal hemorrhages[49]. Vascularization of the retina was shown to be severely impaired in mice that survived the miR-126 deletion[49]. An intravitreal injection of miR-126 in the retina reduced the levels of VEGF, IGF-2, and HIF-1α[77]. Additionally, miR-126 exhibited tumor suppressor functions in lung cancer cells by negatively regulating VEGF both in vivo and in vitro[26,78]. Hence, strategies to modulate miR-126 levels may hold a great therapeutic value against retinal neovascular diseases.
miR-200b
The miR-200 family is up-regulated by stimuli such as TGF-β1 and PDGF and suppresses growth of human microvascular ECs[57]. Hypoxia inhibits miR-200b expression, prompting an elevated Ets-1 gene expression and its downstream target genes such as MMP1 and VEGFR2[57]. Intravitreal injection of miR-200b mimicked reduced elevated levels of VEGF and prevented angiogenesis in a model of diabetic retinopathy[79]. Thus, the regulation of miR-200b in retinal neovascular diseases may prevent the aberrant expression of critical factors associated with pathological angiogenesis.
miR-214
miR-214 is located on a non-coding intronic Dynamin-3 gene sequence and its expression is controlled by the transcription factor Twist-1. HIF-1α mediates Twist-1 transcription, which then allows miR-214 expression[80]. Concordantly, miR-214 was shown to be up-regulated in ischemic conditions when HIF-1α was stabilized[80]. A recent study has shown that miR-214 directly targets Quaking (QKI) and regulates the expression and secretion of angiogenic growth factors such as VEGF, bFGF and PDGF[81]. Quaking plays an essential role in vascular development[82]. In vivo silencing of miR-214 enhanced the formation of blood vessels on Matrigel plugs and increased the secretion of pro-angiogenic growth factors[81]. Additionally, miR-214 is substantially increased in the mouse model of OIR[39]. Inhibition of miR-214 enhanced normalization of the vascularization of the retina through the expression of QKI, suggesting that miR-214 may function directly to either block pathological neovascularization or prevent hyperoxia-induced vasoobliteration[81].
miR-329
miR-329 targets the important pro-angiogenic gene, CD146, and inhibits angiogenesis in vitro and in vivo[83]. CD146 is an adhesion molecule and an endothelial biomarker which actively participates in the angiogenic process[83,84]. CD146 functions as a co-receptor for VEGFR2 and activates the p38/IκB kinase/NF-κB signaling pathway leading to increased EC migration and tube formation. A study by Wang et al[83] has shown that exposure of ECs to VEGF represses endogenous miR-329 expression, resulting in the simultaneous up-regulation of CD146 and treatment with miR-329 significantly reduced retinal neovascularization. miR-329 is thought to inhibit the expression of many downstream pro-angiogenic genes including intercellular adhesion molecule-1 (ICAM-1), IL-8, and MMP-2, among others. Thus, miR-329 serves as a potential therapeutic target in pathological retinal angiogenesis.
miR-21
miR-21 is located on chromosome 17q23.2 within the protein-coding region of the transmembrane protein 49[85]. miR-21 promotes angiogenesis by inhibiting phosphate and tensin homolog deleted on chromosome 10 (PTEN), a potent negative regulator of the phosphatidyl inositol-3 kinase/AKT signaling pathway. By blocking Akt signaling, PTEN decreases both eNOS activity and VCAM-1 expression[31,86,87]. In tumor cells, overexpression of miR-21 significantly increased the levels of HIF-α and VEGF. In primary bovine retinal microvascular ECs, inhibition of miR-21 drastically reduced proliferation, migration, and tube-forming capacity reinforcing the important pro-angiogenic role of miR-21 in the retinal microvasculature[88].
miR-23-27-24 cluster
The miR-23-27-24 cluster is highly enriched in ECs and is well conserved between rodent and humans[40]. There are two paralogs of the clusters: an intergenic miR-23a-27a-24-2 cluster and an intronic miR-23b-27b-24-1 cluster on vertebrate chromosomes 8 and 13 respectively[40]. miR-27a/b and miR-23a/b mediate proper capillary formation in response to VEGF in vitro[40]. miR-27a/b and miR-23a/b repress anti-angiogenic gene expression such as SPROUTY2, SEMA6A and SEMA6D[40]. These anti-angiogenic genes inhibit the mitogen-activated protein kinase pathway and VEGF pathway[40]. Additionally, miR-23a/b and miR-27a/b also promote choroidal neovascularization (CNV)[40]. Silencing of miR-23a/b and miR-27a/b suppressed CNV in mice[40]. Thus, targeting the miR-23-27-24 cluster may have beneficial therapeutic applications in the treatment of AMD.
miR-132
miR-132 is highly up-regulated in human embryonic stem cells and tumors whereas it was undetectable in a normal endothelium[26,65]. However, stimulation of ECs by growth factors increased the levels of miR-132 which then activates quiescent endothelium by suppressing p120RasGAP[26,65,66]. Suppression of p120RasGAP led to the activation of Ras which then increases VEGF-mediated phosphorylation of mitogen-activated protein kinase extracellular related protein kinase kinase-1[65]. Ectopic expression of miR-132 was sufficient to induce EC proliferation in vitro and its inhibition significantly reduced growth factor-mediated angiogenesis in vivo and in vitro[65]. Additionally, inhibition of miR-132 also greatly decreased retinal neovascularization in mice[65]. Thus, early detection and modulation of this miRNA may inhibit the onset of neovascularization.
CONCLUSION
Treatment and management of neovascular diseases rely mainly on pharmacotherapy and/or surgical procedures. However, these treatments are seldom efficacious and they often are plagued by unwanted side effects and/or insurmountable complications. The use of miRNAs that specifically target a set of angiogenic genes appears to be a viable alternative approach. Currently, there are numerous ongoing clinical trials designed to test the efficacy and effectiveness of such approach in the treatment of various disorders (e.g., atherosclerosis, cancer, inflammatory diseases) and the preliminary results are promising[89-91]. Neovascular diseases including those of the eye will likely test/use such approach in a near future as our understanding of miRNA regulation and the molecular mechanisms underpinning their functions increases every day.
MicroRNAs are also increasingly considered as potential diagnostic markers of disease stages. Indeed, miRNAs have been discovered in a wide variety of extracellular body fluids such as saliva, serum, plasma, milk, and urine as nuclease resistant entities[24,31,92]. These extracellular circulating miRNAs enable cell-to-cell communication and also provide insight into the physiological states or progression of pathological diseases within the secreting cells[92-94]. miRNAs are thought to be secreted from cells in three possible ways: (1) via passive leakage from cells resulting from injury, inflammation, apoptosis or necrosis; (2) via an active secretion method in membrane-bound vesicles such as exosomes, shedding vesicles and apoptotic bodies; and (3) via an active secretion method of protein-miRNA complexes[92]. Exosomes are 30 nm-100 nm vesicles, arising from multivesicular bodies and their release is mediated by the enzyme sphingomyelinase-2[31,92-95]. Shedding vesicles, arising from the plasma membrane, is facilitated via a ligand-receptor method. Further insight into the exosomal miRNA formation and circulation may not only validate their prognostic potential in the slowly developing neovascular diseases of the eye but, will also help design optimal delivery systems of miRNAs in vivo.
Footnotes
P- Reviewers: Chai JY, Zou ZM S- Editor: Gou SX L- Editor: A E- Editor: Wu HL
Supported by A Grant from the National Eye Institute of the National Institutes of Health EY022091-01 to Chaqour B
References
- 1.Suárez Y, Sessa WC. MicroRNAs as novel regulators of angiogenesis. Circ Res. 2009;104:442–454. doi: 10.1161/CIRCRESAHA.108.191270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saint-Geniez M, D’Amore PA. Development and pathology of the hyaloid, choroidal and retinal vasculature. Int J Dev Biol. 2004;48:1045–1058. doi: 10.1387/ijdb.041895ms. [DOI] [PubMed] [Google Scholar]
- 3.Fish JE, Srivastava D. MicroRNAs: opening a new vein in angiogenesis research. Sci Signal. 2009;2:52; pe1. doi: 10.1126/scisignal.252pe1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heusschen R, van Gink M, Griffioen AW, Thijssen VL. MicroRNAs in the tumor endothelium: novel controls on the angioregulatory switchboard. Biochim Biophys Acta. 2010;1805:87–96. doi: 10.1016/j.bbcan.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Campochiaro PA. Retinal and choroidal neovascularization. J Cell Physiol. 2000;184:301–310. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 6.Grossniklaus HE, Kang SJ, Berglin L. Animal models of choroidal and retinal neovascularization. Prog Retin Eye Res. 2010;29:500–519. doi: 10.1016/j.preteyeres.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fish JE, Santoro MM, Morton SU, Yu S, Yeh RF, Wythe JD, Ivey KN, Bruneau BG, Stainier DY, Srivastava D. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karali M, Peluso I, Gennarino VA, Bilio M, Verde R, Lago G, Dollé P, Banfi S. miRNeye: a microRNA expression atlas of the mouse eye. BMC Genomics. 2010;11:715. doi: 10.1186/1471-2164-11-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang LT, Lawson ND, Fish JE. MicroRNA control of vascular endothelial growth factor signaling output during vascular development. Arterioscler Thromb Vasc Biol. 2013;33:193–200. doi: 10.1161/ATVBAHA.112.300142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bouïs D, Kusumanto Y, Meijer C, Mulder NH, Hospers GA. A review on pro- and anti-angiogenic factors as targets of clinical intervention. Pharmacol Res. 2006;53:89–103. doi: 10.1016/j.phrs.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Nicoli S, Knyphausen CP, Zhu LJ, Lakshmanan A, Lawson ND. miR-221 is required for endothelial tip cell behaviors during vascular development. Dev Cell. 2012;22:418–429. doi: 10.1016/j.devcel.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 13.Uemura A, Kusuhara S, Katsuta H, Nishikawa S. Angiogenesis in the mouse retina: a model system for experimental manipulation. Exp Cell Res. 2006;312:676–683. doi: 10.1016/j.yexcr.2005.10.030. [DOI] [PubMed] [Google Scholar]
- 14.Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 15.Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 16.Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fruttiger M. Development of the retinal vasculature. Angiogenesis. 2007;10:77–88. doi: 10.1007/s10456-007-9065-1. [DOI] [PubMed] [Google Scholar]
- 18.Yamakawa M, Liu LX, Date T, Belanger AJ, Vincent KA, Akita GY, Kuriyama T, Cheng SH, Gregory RJ, Jiang C. Hypoxia-inducible factor-1 mediates activation of cultured vascular endothelial cells by inducing multiple angiogenic factors. Circ Res. 2003;93:664–673. doi: 10.1161/01.RES.0000093984.48643.D7. [DOI] [PubMed] [Google Scholar]
- 19.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438:960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe D. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. Nihon Ganka Gakkai Zasshi. 2007;111:892–898. [PubMed] [Google Scholar]
- 21.Watanabe D, Suzuma K, Matsui S, Kurimoto M, Kiryu J, Kita M, Suzuma I, Ohashi H, Ojima T, Murakami T, et al. Erythropoietin as a retinal angiogenic factor in proliferative diabetic retinopathy. N Engl J Med. 2005;353:782–792. doi: 10.1056/NEJMoa041773. [DOI] [PubMed] [Google Scholar]
- 22.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, Koster KM, He Y, Zhou Q. miRNAs as potential therapeutic targets for age-related macular degeneration. Future Med Chem. 2012;4:277–287. doi: 10.4155/fmc.11.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Landskroner-Eiger S, Moneke I, Sessa WC. miRNAs as modulators of angiogenesis. Cold Spring Harb Perspect Med. 2013;3:a006643. doi: 10.1101/cshperspect.a006643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caporali A, Emanueli C. MicroRNA regulation in angiogenesis. Vascul Pharmacol. 2011;55:79–86. doi: 10.1016/j.vph.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann D, Thum T. MicroRNAs and vascular (dys)function. Vascul Pharmacol. 2011;55:92–105. doi: 10.1016/j.vph.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Roy S, Sen CK. miRNA in wound inflammation and angiogenesis. Microcirculation. 2012;19:224–232. doi: 10.1111/j.1549-8719.2011.00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuehbacher A, Urbich C, Dimmeler S. Targeting microRNA expression to regulate angiogenesis. Trends Pharmacol Sci. 2008;29:12–15. doi: 10.1016/j.tips.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Ullah S, John P, Bhatti A. MicroRNAs with a role in gene regulation and in human diseases. Mol Biol Rep. 2014;41:225–232. doi: 10.1007/s11033-013-2855-1. [DOI] [PubMed] [Google Scholar]
- 30.Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nat Genet. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- 31.Yamakuchi M. MicroRNAs in Vascular Biology. Int J Vasc Med. 2012;2012:794898. doi: 10.1155/2012/794898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang WJ, Yang DD, Na S, Sandusky GE, Zhang Q, Zhao G. Dicer is required for embryonic angiogenesis during mouse development. J Biol Chem. 2005;280:9330–9335. doi: 10.1074/jbc.M413394200. [DOI] [PubMed] [Google Scholar]
- 33.Kuehbacher A, Urbich C, Zeiher AM, Dimmeler S. Role of Dicer and Drosha for endothelial microRNA expression and angiogenesis. Circ Res. 2007;101:59–68. doi: 10.1161/CIRCRESAHA.107.153916. [DOI] [PubMed] [Google Scholar]
- 34.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 35.Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest. 2008;118:1944–1954. doi: 10.1172/JCI33680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiorano NA, Hindges R. Non-Coding RNAs in Retinal Development. Int J Mol Sci. 2012;13:558–578. doi: 10.3390/ijms13010558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu S. microRNA expression in the eyes and their significance in relation to functions. Prog Retin Eye Res. 2009;28:87–116. doi: 10.1016/j.preteyeres.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Sundermeier TR, Palczewski K. The physiological impact of microRNA gene regulation in the retina. Cell Mol Life Sci. 2012;69:2739–2750. doi: 10.1007/s00018-012-0976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen J, Yang X, Xie B, Chen Y, Swaim M, Hackett SF, Campochiaro PA. MicroRNAs regulate ocular neovascularization. Mol Ther. 2008;16:1208–1216. doi: 10.1038/mt.2008.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou Q, Gallagher R, Ufret-Vincenty R, Li X, Olson EN, Wang S. Regulation of angiogenesis and choroidal neovascularization by members of microRNA-23~27~24 clusters. Proc Natl Acad Sci USA. 2011;108:8287–8292. doi: 10.1073/pnas.1105254108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Decembrini S, Bressan D, Vignali R, Pitto L, Mariotti S, Rainaldi G, Wang X, Evangelista M, Barsacchi G, Cremisi F. MicroRNAs couple cell fate and developmental timing in retina. Proc Natl Acad Sci USA. 2009;106:21179–21184. doi: 10.1073/pnas.0909167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Conte I, Carrella S, Avellino R, Karali M, Marco-Ferreres R, Bovolenta P, Banfi S. miR-204 is required for lens and retinal development via Meis2 targeting. Proc Natl Acad Sci USA. 2010;107:15491–15496. doi: 10.1073/pnas.0914785107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lumayag S, Haldin CE, Corbett NJ, Wahlin KJ, Cowan C, Turturro S, Larsen PE, Kovacs B, Witmer PD, Valle D, et al. Inactivation of the microRNA-183/96/182 cluster results in syndromic retinal degeneration. Proc Natl Acad Sci USA. 2013;110:E507–E516. doi: 10.1073/pnas.1212655110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.La Torre A, Georgi S, Reh TA. Conserved microRNA pathway regulates developmental timing of retinal neurogenesis. Proc Natl Acad Sci USA. 2013;110:E2362–E2370. doi: 10.1073/pnas.1301837110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arora A, Guduric-Fuchs J, Harwood L, Dellett M, Cogliati T, Simpson DA. Prediction of microRNAs affecting mRNA expression during retinal development. BMC Dev Biol. 2010;10:1. doi: 10.1186/1471-213X-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu D, Krueger J, Le Noble F. The role of blood flow and microRNAs in blood vessel development. Int J Dev Biol. 2011;55:419–429. doi: 10.1387/ijdb.103220dl. [DOI] [PubMed] [Google Scholar]
- 47.Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- 48.Heinke J, Patterson C, Moser M. Life is a pattern: vascular assembly within the embryo. Front Biosci (Elite Ed) 2012;4:2269–2288. doi: 10.2741/541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hua Z, Lv Q, Ye W, Wong CK, Cai G, Gu D, Ji Y, Zhao C, Wang J, Yang BB, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One. 2006;1:e116. doi: 10.1371/journal.pone.0000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kulshreshtha R, Ferracin M, Wojcik SE, Garzon R, Alder H, Agosto-Perez FJ, Davuluri R, Liu CG, Croce CM, Negrini M, et al. A microRNA signature of hypoxia. Mol Cell Biol. 2007;27:1859–1867. doi: 10.1128/MCB.01395-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Urbich C, Kuehbacher A, Dimmeler S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc Res. 2008;79:581–588. doi: 10.1093/cvr/cvn156. [DOI] [PubMed] [Google Scholar]
- 53.Fasanaro P, D’Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J Biol Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blick C, Ramachandran A, Wigfield S, McCormick R, Jubb A, Buffa FM, Turley H, Knowles MA, Cranston D, Catto J, et al. Hypoxia regulates FGFR3 expression via HIF-1α and miR-100 and contributes to cell survival in non-muscle invasive bladder cancer. Br J Cancer. 2013;109:50–59. doi: 10.1038/bjc.2013.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grundmann S, Hans FP, Kinniry S, Heinke J, Helbing T, Bluhm F, Sluijter JP, Hoefer I, Pasterkamp G, Bode C, et al. MicroRNA-100 regulates neovascularization by suppression of mammalian target of rapamycin in endothelial and vascular smooth muscle cells. Circulation. 2011;123:999–1009. doi: 10.1161/CIRCULATIONAHA.110.000323. [DOI] [PubMed] [Google Scholar]
- 56.Ghosh G, Subramanian IV, Adhikari N, Zhang X, Joshi HP, Basi D, Chandrashekhar YS, Hall JL, Roy S, Zeng Y, et al. Hypoxia-induced microRNA-424 expression in human endothelial cells regulates HIF-α isoforms and promotes angiogenesis. J Clin Invest. 2010;120:4141–4154. doi: 10.1172/JCI42980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan YC, Khanna S, Roy S, Sen CK. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem. 2011;286:2047–2056. doi: 10.1074/jbc.M110.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suárez Y, Fernández-Hernando C, Yu J, Gerber SA, Harrison KD, Pober JS, Iruela-Arispe ML, Merkenschlager M, Sessa WC. Dicer-dependent endothelial microRNAs are necessary for postnatal angiogenesis. Proc Natl Acad Sci USA. 2008;105:14082–14087. doi: 10.1073/pnas.0804597105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen Y, Gorski DH. Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood. 2008;111:1217–1226. doi: 10.1182/blood-2007-07-104133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Würdinger T, Tannous BA, Saydam O, Skog J, Grau S, Soutschek J, Weissleder R, Breakefield XO, Krichevsky AM. miR-296 regulates growth factor receptor overexpression in angiogenic endothelial cells. Cancer Cell. 2008;14:382–393. doi: 10.1016/j.ccr.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Smits M, Mir SE, Nilsson RJ, van der Stoop PM, Niers JM, Marquez VE, Cloos J, Breakefield XO, Krichevsky AM, Noske DP, et al. Down-regulation of miR-101 in endothelial cells promotes blood vessel formation through reduced repression of EZH2. PLoS One. 2011;6:e16282. doi: 10.1371/journal.pone.0016282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muramatsu F, Kidoya H, Naito H, Sakimoto S, Takakura N. microRNA-125b inhibits tube formation of blood vessels through translational suppression of VE-cadherin. Oncogene. 2013;32:414–421. doi: 10.1038/onc.2012.68. [DOI] [PubMed] [Google Scholar]
- 63.Nagineni CN, Samuel W, Nagineni S, Pardhasaradhi K, Wiggert B, Detrick B, Hooks JJ. Transforming growth factor-beta induces expression of vascular endothelial growth factor in human retinal pigment epithelial cells: involvement of mitogen-activated protein kinases. J Cell Physiol. 2003;197:453–462. doi: 10.1002/jcp.10378. [DOI] [PubMed] [Google Scholar]
- 64.Wang J, Wang Y, Wang Y, Ma Y, Lan Y, Yang X. Transforming growth factor β-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J Biol Chem. 2013;288:10418–10426. doi: 10.1074/jbc.M112.444463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Anand S, Majeti BK, Acevedo LM, Murphy EA, Mukthavaram R, Scheppke L, Huang M, Shields DJ, Lindquist JN, Lapinski PE, et al. MicroRNA-132-mediated loss of p120RasGAP activates the endothelium to facilitate pathological angiogenesis. Nat Med. 2010;16:909–914. doi: 10.1038/nm.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Anand S, Cheresh DA. MicroRNA-mediated regulation of the angiogenic switch. Curr Opin Hematol. 2011;18:171–176. doi: 10.1097/MOH.0b013e328345a180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kovacs B, Lumayag S, Cowan C, Xu S. MicroRNAs in early diabetic retinopathy in streptozotocin-induced diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:4402–4409. doi: 10.1167/iovs.10-6879. [DOI] [PubMed] [Google Scholar]
- 68.Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, Brizzi MF. microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol. 2010;30:1562–1568. doi: 10.1161/ATVBAHA.110.206201. [DOI] [PubMed] [Google Scholar]
- 69.Wilkinson-Berka JL, Deliyanti D, Rana I, Miller AG, Agrotis A, Armani R, Szyndralewiez C, Wingler K, Touyz RM, Cooper ME, et al. NADPH Oxidase, NOX1, Mediates Vascular Injury in Ischemic Retinopathy. Antioxid Redox Signal. 2013:Oct 30; Epub ahead of print. doi: 10.1089/ars.2013.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maulik N, Das DK. Redox signaling in vascular angiogenesis. Free Radic Biol Med. 2002;33:1047–1060. doi: 10.1016/s0891-5849(02)01005-5. [DOI] [PubMed] [Google Scholar]
- 71.Lin H, Qian J, Castillo AC, Long B, Keyes KT, Chen G, Ye Y. Effect of miR-23 on oxidant-induced injury in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2011;52:6308–6314. doi: 10.1167/iovs.10-6632. [DOI] [PubMed] [Google Scholar]
- 72.Magenta A, Cencioni C, Fasanaro P, Zaccagnini G, Greco S, Sarra-Ferraris G, Antonini A, Martelli F, Capogrossi MC. miR-200c is upregulated by oxidative stress and induces endothelial cell apoptosis and senescence via ZEB1 inhibition. Cell Death Differ. 2011;18:1628–1639. doi: 10.1038/cdd.2011.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menghini R, Casagrande V, Cardellini M, Martelli E, Terrinoni A, Amati F, Vasa-Nicotera M, Ippoliti A, Novelli G, Melino G, et al. MicroRNA 217 modulates endothelial cell senescence via silent information regulator 1. Circulation. 2009;120:1524–1532. doi: 10.1161/CIRCULATIONAHA.109.864629. [DOI] [PubMed] [Google Scholar]
- 74.Zhao T, Li J, Chen AF. MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab. 2010;299:E110–E116. doi: 10.1152/ajpendo.00192.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Liu X, Cheng Y, Yang J, Xu L, Zhang C. Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J Mol Cell Cardiol. 2012;52:245–255. doi: 10.1016/j.yjmcc.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doebele C, Bonauer A, Fischer A, Scholz A, Reiss Y, Urbich C, Hofmann WK, Zeiher AM, Dimmeler S. Members of the microRNA-17-92 cluster exhibit a cell-intrinsic antiangiogenic function in endothelial cells. Blood. 2010;115:4944–4950. doi: 10.1182/blood-2010-01-264812. [DOI] [PubMed] [Google Scholar]
- 77.Bai Y, Bai X, Wang Z, Zhang X, Ruan C, Miao J. MicroRNA-126 inhibits ischemia-induced retinal neovascularization via regulating angiogenic growth factors. Exp Mol Pathol. 2011;91:471–477. doi: 10.1016/j.yexmp.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 78.Liu B, Peng XC, Zheng XL, Wang J, Qin YW. MiR-126 restoration down-regulate VEGF and inhibit the growth of lung cancer cell lines in vitro and in vivo. Lung Cancer. 2009;66:169–175. doi: 10.1016/j.lungcan.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 79.McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee YB, Bantounas I, Lee DY, Phylactou L, Caldwell MA, Uney JB. Twist-1 regulates the miR-199a/214 cluster during development. Nucleic Acids Res. 2009;37:123–128. doi: 10.1093/nar/gkn920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Mil A, Grundmann S, Goumans MJ, Lei Z, Oerlemans MI, Jaksani S, Doevendans PA, Sluijter JP. MicroRNA-214 inhibits angiogenesis by targeting Quaking and reducing angiogenic growth factor release. Cardiovasc Res. 2012;93:655–665. doi: 10.1093/cvr/cvs003. [DOI] [PubMed] [Google Scholar]
- 82.Noveroske JK, Lai L, Gaussin V, Northrop JL, Nakamura H, Hirschi KK, Justice MJ. Quaking is essential for blood vessel development. Genesis. 2002;32:218–230. doi: 10.1002/gene.10060. [DOI] [PubMed] [Google Scholar]
- 83.Wang P, Luo Y, Duan H, Xing S, Zhang J, Lu D, Feng J, Yang D, Song L, Yan X. MicroRNA 329 suppresses angiogenesis by targeting CD146. Mol Cell Biol. 2013;33:3689–3699. doi: 10.1128/MCB.00343-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng C, Qiu Y, Zeng Q, Zhang Y, Lu D, Yang D, Feng J, Yan X. Endothelial CD146 is required for in vitro tumor-induced angiogenesis: the role of a disulfide bond in signaling and dimerization. Int J Biochem Cell Biol. 2009;41:2163–2172. doi: 10.1016/j.biocel.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 85.Fujita S, Ito T, Mizutani T, Minoguchi S, Yamamichi N, Sakurai K, Iba H. miR-21 Gene expression triggered by AP-1 is sustained through a double-negative feedback mechanism. J Mol Biol. 2008;378:492–504. doi: 10.1016/j.jmb.2008.03.015. [DOI] [PubMed] [Google Scholar]
- 86.Liu LZ, Li C, Chen Q, Jing Y, Carpenter R, Jiang Y, Kung HF, Lai L, Jiang BH. MiR-21 induced angiogenesis through AKT and ERK activation and HIF-1α expression. PLoS One. 2011;6:e19139. doi: 10.1371/journal.pone.0019139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guduric-Fuchs J, O’Connor A, Cullen A, Harwood L, Medina RJ, O’Neill CL, Stitt AW, Curtis TM, Simpson DA. Deep sequencing reveals predominant expression of miR-21 amongst the small non-coding RNAs in retinal microvascular endothelial cells. J Cell Biochem. 2012;113:2098–2111. doi: 10.1002/jcb.24084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ling H, Fabbri M, Calin GA. MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 2013;12:847–865. doi: 10.1038/nrd4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Séguin RM, Ferrari N. Emerging oligonucleotide therapies for asthma and chronic obstructive pulmonary disease. Expert Opin Investig Drugs. 2009;18:1505–1517. doi: 10.1517/13543780903179294. [DOI] [PubMed] [Google Scholar]
- 91.Thum T. MicroRNA therapeutics in cardiovascular medicine. EMBO Mol Med. 2012;4:3–14. doi: 10.1002/emmm.201100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Redis RS, Calin S, Yang Y, You MJ, Calin GA. Cell-to-cell miRNA transfer: from body homeostasis to therapy. Pharmacol Ther. 2012;136:169–174. doi: 10.1016/j.pharmthera.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 94.Zhu H, Fan GC. Extracellular/circulating microRNAs and their potential role in cardiovascular disease. Am J Cardiovasc Dis. 2011;1:138–149. [PMC free article] [PubMed] [Google Scholar]
- 95.Russo F, Di Bella S, Nigita G, Macca V, Laganà A, Giugno R, Pulvirenti A, Ferro A. miRandola: extracellular circulating microRNAs database. PLoS One. 2012;7:e47786. doi: 10.1371/journal.pone.0047786. [DOI] [PMC free article] [PubMed] [Google Scholar]


