Abstract
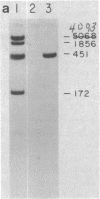
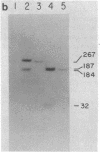
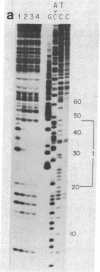
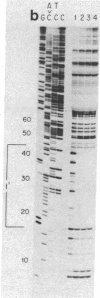
Nuclear factor I, a 47-kilodalton protein, purified from nuclear extracts of uninfected HeLa cells, is involved in the initiation and possibly the elongation of replicating adenovirus (Ad) DNA in vitro. The binding of nuclear factor I to DNA has been monitored by a filter binding assay of nuclear factor I to DNA has been monitored by a filter binding assay using plasmid pLA1 DNA, which contains a 3,290 base-pair fragment derived from the left-hand terminus (coordinates, 0-9.4 map units) of Ad serotype 5 DNA. Nuclear factor I binds selectively to a double-stranded fragment spanning nucleotides 0-451 to the Ad genome. The retention of the 451-base-pair DNA fragment-nuclear factor I complex on nitrocellulose filters does not require Mg2+ or ATP and is resistant to high ionic strength. DNase I protection experiments revealed that nuclear factor I binds to a nucleotide sequence located at position 17-48, close to the terminus of Ad DNA. This 32-nucleotide sequence contains four "consensus" sequences present in various serotypes of Ad DNA and is capable of forming higher ordered structures. The role of nuclear factor I and this DNA sequence in the generation of Ad preterminal protein-dCMP initiation complex is discussed.
Full text
PDF
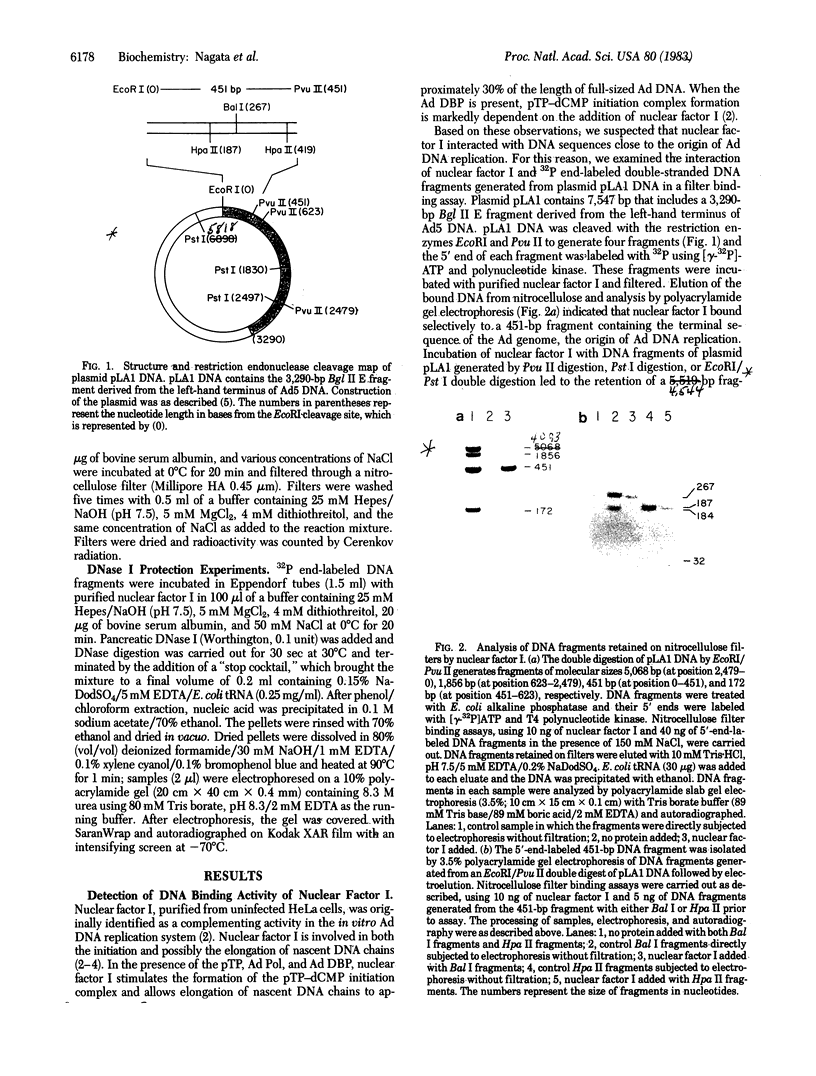
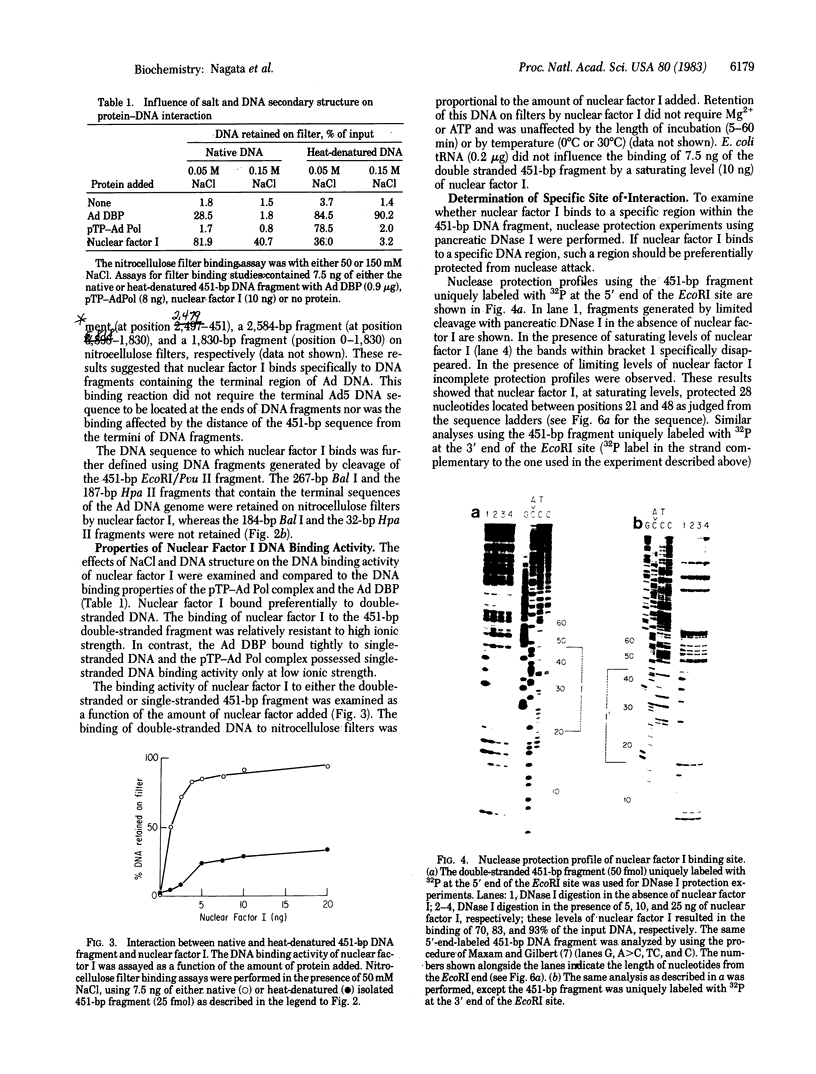


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aleström P., Stenlund A., Li P., Pettersson U. A common sequence in the inverted terminal repetitions of human and avian adenoviruses. Gene. 1982 May;18(2):193–197. doi: 10.1016/0378-1119(82)90117-2. [DOI] [PubMed] [Google Scholar]
- Arrand J. R., Roberts R. J. The nucleotide sequences at the termini of adenovirus-2 DNA. J Mol Biol. 1979 Mar 15;128(4):577–594. doi: 10.1016/0022-2836(79)90294-8. [DOI] [PubMed] [Google Scholar]
- Dijkema R., Dekker B. M. The inverted terminal repetition of the DNA of weakly oncogenic adenovirus type 7. Gene. 1979 Dec;8(1):7–15. doi: 10.1016/0378-1119(79)90003-9. [DOI] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Replication of adenovirus DNA-protein complex with purified proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):884–888. doi: 10.1073/pnas.78.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Lovett M. A., Helinski D. R. Strand and site specificity of the relaxation event for the relaxation complex of the antibiotic resistance plasmid R6K. Biochemistry. 1974 Dec 31;13(27):5484–5490. doi: 10.1021/bi00724a005. [DOI] [PubMed] [Google Scholar]
- Lichy J. H., Nagata K., Friefeld B. R., Enomoto T., Field J., Guggenheimer R. A., Ikeda J. E., Horwitz M. S., Hurwitz J. Isolation of proteins involved in the replication of adenoviral DNA in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):731–740. doi: 10.1101/sqb.1983.047.01.084. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Enomoto T., Lichy J. H., Hurwitz J. Adenovirus DNA replication in vitro: identification of a host factor that stimulates synthesis of the preterminal protein-dCMP complex. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6438–6442. doi: 10.1073/pnas.79.21.6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinagawa M., Padmanabhan R. Nucleotide sequence at the inverted terminal repetition of adenovirus type 2 DNA. Biochem Biophys Res Commun. 1979 Apr 13;87(3):671–678. doi: 10.1016/0006-291x(79)92011-4. [DOI] [PubMed] [Google Scholar]
- Steenbergh P. H., Maat J., van Ormondt H., Sussenbach J. S. The nucleotide sequence at the termini of adenovirus type 5 DNA. Nucleic Acids Res. 1977 Dec;4(12):4371–4389. doi: 10.1093/nar/4.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., Topp W. C., Engler J. A. Conserved sequences at the origin of adenovirus DNA replication. J Virol. 1982 Nov;44(2):530–537. doi: 10.1128/jvi.44.2.530-537.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki H., Sugimoto K., Takanami M., Shiroki K., Saito I., Shimojo H., Sawada Y., Uemizu Y., Uesugi S., Fujinaga K. Structure and gene organization in the transformed Hind III-G fragment of Ad12. Cell. 1980 Jul;20(3):777–786. doi: 10.1016/0092-8674(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Stillman B. W. Function of adenovirus terminal protein in the initiation of DNA replication. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2221–2225. doi: 10.1073/pnas.79.7.2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolun A., Aleström P., Pettersson U. Sequence of inverted terminal repetitions from different adenoviruses: demonstration of conserved sequences and homology between SA7 termini and SV40 DNA. Cell. 1979 Jul;17(3):705–713. doi: 10.1016/0092-8674(79)90277-0. [DOI] [PubMed] [Google Scholar]
- van Bergen B. G., van der Ley P. A., van Driel W., van Mansfeld A. D., van der Vliet P. C. Replication of origin containing adenovirus DNA fragments that do not carry the terminal protein. Nucleic Acids Res. 1983 Apr 11;11(7):1975–1989. doi: 10.1093/nar/11.7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]