Abstract
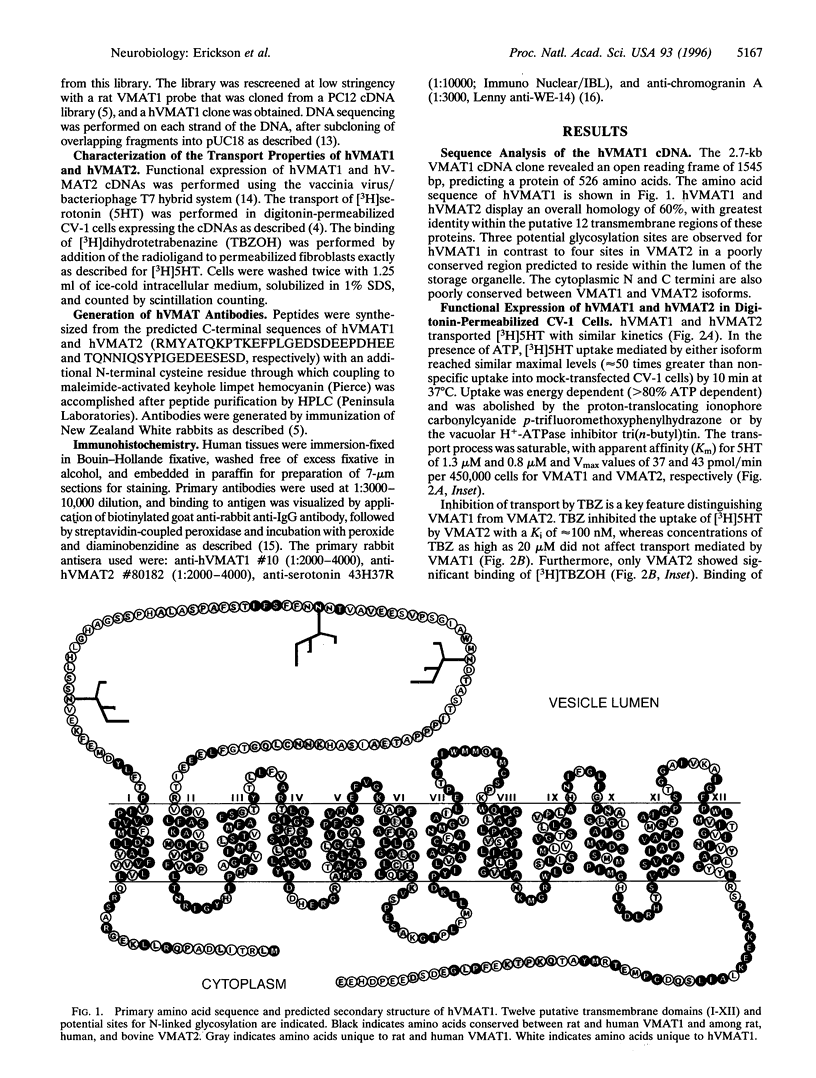
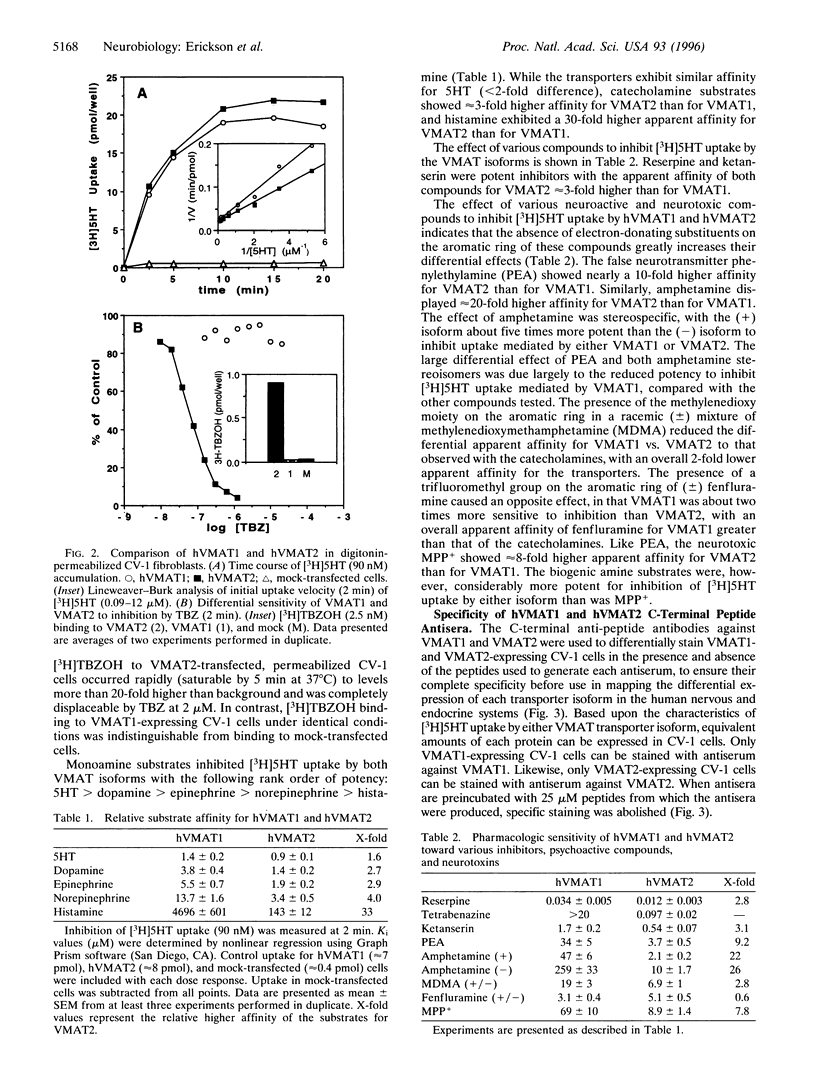
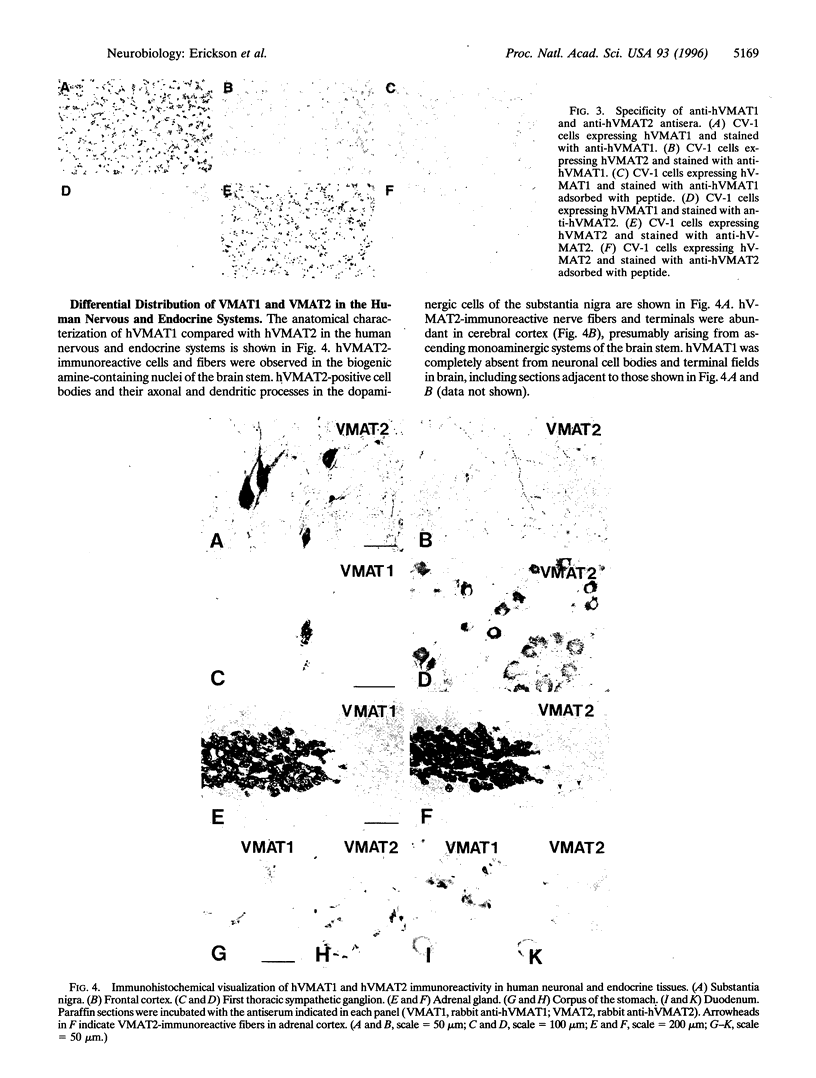
A second isoform of the human vesicular monoamine transporter (hVMAT) has been cloned from a pheochromocytoma cDNA library. The contribution of the two transporter isoforms to monoamine storage in human neuroendocrine tissues was examined with isoform-specific polyclonal antibodies against hVMAT1 and hVMAT2. Central, peripheral, and enteric neurons express only VMAT2. VMAT1 is expressed exclusively in neuroendocrine, including chromaffin and enterochromaffin, cells. VMAT1 and VMAT2 are coexpressed in all chromaffin cells of the adrenal medulla. VMAT2 alone is expressed in histamine-storing enterochromaffin-like cells of the oxyntic mucosa of the stomach. The transport characteristics and pharmacology of each VMAT isoform have been directly compared after expression in digitonin-permeabilized fibroblastic (CV-1) cells, providing information about substrate feature recognition by each transporter and the role of vesicular monoamine storage in the mechanism of action of psychopharmacologic and neurotoxic agents in human. Serotonin has a similar affinity for both transporters. Catecholamines exhibit a 3-fold higher affinity, and histamine exhibits a 30-fold higher affinity, for VMAT2. Reserpine and ketanserin are slightly more potent inhibitors of VMAT2-mediated transport than of VMAT1-mediated transport, whereas tetrabenazine binds to and inhibits only VMAT2. N-methyl-4-phenylpyridinium, phenylethylamine, amphetamine, and methylenedioxymethamphetamine are all more potent inhibitors of VMAT2 than of VMAT1, whereas fenfluramine is a more potent inhibitor of VMAT1-mediated monamine transport than of VMAT2-mediated monoamine transport. The unique distributions of hVMAT1 and hVMAT2 provide new markers for multiple neuroendocrine lineages, and examination of their transport properties provides mechanistic insights into the pharmacology and physiology of amine storage in cardiovascular, endocrine, and central nervous system function.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J. Molecular control of cell fate in the neural crest: the sympathoadrenal lineage. Annu Rev Neurosci. 1993;16:129–158. doi: 10.1146/annurev.ne.16.030193.001021. [DOI] [PubMed] [Google Scholar]
- Asher S. W., Aminoff M. J. Tetrabenazine and movement disorders. Neurology. 1981 Aug;31(8):1051–1054. doi: 10.1212/wnl.31.8.1051. [DOI] [PubMed] [Google Scholar]
- Axelrod J. Noradrenaline: fate and control of its biosynthesis. Science. 1971 Aug 13;173(3997):598–606. doi: 10.1126/science.173.3997.598. [DOI] [PubMed] [Google Scholar]
- Darchen F., Scherman D., Henry J. P. Reserpine binding to chromaffin granules suggests the existence of two conformations of the monoamine transporter. Biochemistry. 1989 Feb 21;28(4):1692–1697. doi: 10.1021/bi00430a040. [DOI] [PubMed] [Google Scholar]
- Darchen F., Scherman D., Laduron P. M., Henry J. P. Ketanserin binds to the monoamine transporter of chromaffin granules and of synaptic vesicles. Mol Pharmacol. 1988 Jun;33(6):672–677. [PubMed] [Google Scholar]
- Davis G. C., Williams A. C., Markey S. P., Ebert M. H., Caine E. D., Reichert C. M., Kopin I. J. Chronic Parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979 Dec;1(3):249–254. doi: 10.1016/0165-1781(79)90006-4. [DOI] [PubMed] [Google Scholar]
- Erickson J. D., Eiden L. E. Functional identification and molecular cloning of a human brain vesicle monoamine transporter. J Neurochem. 1993 Dec;61(6):2314–2317. doi: 10.1111/j.1471-4159.1993.tb07476.x. [DOI] [PubMed] [Google Scholar]
- Erickson J. D., Eiden L. E., Hoffman B. J. Expression cloning of a reserpine-sensitive vesicular monoamine transporter. Proc Natl Acad Sci U S A. 1992 Nov 15;89(22):10993–10997. doi: 10.1073/pnas.89.22.10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J. D., Eiden L. E., Schafer M. K., Weihe E. Reserpine- and tetrabenazine-sensitive transport of (3)H-histamine by the neuronal isoform of the vesicular monoamine transporter. J Mol Neurosci. 1995;6(4):277–287. doi: 10.1007/BF02736786. [DOI] [PubMed] [Google Scholar]
- Fuerst T. R., Niles E. G., Studier F. W., Moss B. Eukaryotic transient-expression system based on recombinant vaccinia virus that synthesizes bacteriophage T7 RNA polymerase. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8122–8126. doi: 10.1073/pnas.83.21.8122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasnier B., Krejci E., Botton D., Massoulié J., Henry J. P. Expression of a bovine vesicular monoamine transporter in COS cells. FEBS Lett. 1994 Apr 11;342(3):225–229. doi: 10.1016/0014-5793(94)80506-7. [DOI] [PubMed] [Google Scholar]
- Gonzalez A. M., Walther D., Pazos A., Uhl G. R. Synaptic vesicular monoamine transporter expression: distribution and pharmacologic profile. Brain Res Mol Brain Res. 1994 Mar;22(1-4):219–226. doi: 10.1016/0169-328x(94)90050-7. [DOI] [PubMed] [Google Scholar]
- Havu N. Enterochromaffin-like cell carcinoids of gastric mucosa in rats after life-long inhibition of gastric secretion. Digestion. 1986;35 (Suppl 1):42–55. doi: 10.1159/000199381. [DOI] [PubMed] [Google Scholar]
- Howell M., Shirvan A., Stern-Bach Y., Steiner-Mordoch S., Strasser J. E., Dean G. E., Schuldiner S. Cloning and functional expression of a tetrabenazine sensitive vesicular monoamine transporter from bovine chromaffin granules. FEBS Lett. 1994 Jan 24;338(1):16–22. doi: 10.1016/0014-5793(94)80108-8. [DOI] [PubMed] [Google Scholar]
- Håkanson R., Böttcher G., Ekblad E., Panula P., Simonsson M., Dohlsten M., Hallberg T., Sundler F. Histamine in endocrine cells in the stomach. A survey of several species using a panel of histamine antibodies. Histochemistry. 1986;86(1):5–17. doi: 10.1007/BF00492340. [DOI] [PubMed] [Google Scholar]
- Hörsch D., Weihe E., Müller S., Hancke E. Distribution and coexistence of chromogranin A-, serotonin- and pancreastatin-like immunoreactivity in endocrine-like cells of the human anal canal. Cell Tissue Res. 1992 Apr;268(1):109–116. doi: 10.1007/BF00338059. [DOI] [PubMed] [Google Scholar]
- Javitch J. A., D'Amato R. J., Strittmatter S. M., Snyder S. H. Parkinsonism-inducing neurotoxin, N-methyl-4-phenyl-1,2,3,6 -tetrahydropyridine: uptake of the metabolite N-methyl-4-phenylpyridine by dopamine neurons explains selective toxicity. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2173–2177. doi: 10.1073/pnas.82.7.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleven M. S., Schuster C. R., Seiden L. S. Effect of depletion of brain serotonin by repeated fenfluramine on neurochemical and anorectic effects of acute fenfluramine. J Pharmacol Exp Ther. 1988 Sep;246(3):822–828. [PubMed] [Google Scholar]
- Knepper S. M., Grunewald G. L., Rutledge C. O. Inhibition of norepinephrine transport into synaptic vesicles by amphetamine analogs. J Pharmacol Exp Ther. 1988 Nov;247(2):487–494. [PubMed] [Google Scholar]
- Krejci E., Gasnier B., Botton D., Isambert M. F., Sagné C., Gagnon J., Massoulié J., Henry J. P. Expression and regulation of the bovine vesicular monoamine transporter gene. FEBS Lett. 1993 Nov 29;335(1):27–32. doi: 10.1016/0014-5793(93)80432-t. [DOI] [PubMed] [Google Scholar]
- Langston J. W., Ballard P., Tetrud J. W., Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983 Feb 25;219(4587):979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Liu Y., Peter D., Roghani A., Schuldiner S., Privé G. G., Eisenberg D., Brecha N., Edwards R. H. A cDNA that suppresses MPP+ toxicity encodes a vesicular amine transporter. Cell. 1992 Aug 21;70(4):539–551. doi: 10.1016/0092-8674(92)90425-c. [DOI] [PubMed] [Google Scholar]
- McCann U., Hatzidimitriou G., Ridenour A., Fischer C., Yuan J., Katz J., Ricaurte G. Dexfenfluramine and serotonin neurotoxicity: further preclinical evidence that clinical caution is indicated. J Pharmacol Exp Ther. 1994 May;269(2):792–798. [PubMed] [Google Scholar]
- McMillen B. A., German D. C., Shore P. A. Functional and pharmacological significance of brain dopamine and norepinephrine storage pools. Biochem Pharmacol. 1980 Nov 15;29(22):3045–3050. doi: 10.1016/0006-2952(80)90444-x. [DOI] [PubMed] [Google Scholar]
- Peter D., Jimenez J., Liu Y., Kim J., Edwards R. H. The chromaffin granule and synaptic vesicle amine transporters differ in substrate recognition and sensitivity to inhibitors. J Biol Chem. 1994 Mar 11;269(10):7231–7237. [PubMed] [Google Scholar]
- Peter D., Liu Y., Sternini C., de Giorgio R., Brecha N., Edwards R. H. Differential expression of two vesicular monoamine transporters. J Neurosci. 1995 Sep;15(9):6179–6188. doi: 10.1523/JNEUROSCI.15-09-06179.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poynter D., Pick C. R., Harcourt R. A., Selway S. A., Ainge G., Harman I. W., Spurling N. W., Fluck P. A., Cook J. L. Association of long lasting unsurmountable histamine H2 blockade and gastric carcinoid tumours in the rat. Gut. 1985 Dec;26(12):1284–1295. doi: 10.1136/gut.26.12.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay R. R., Singer T. P. Energy-dependent uptake of N-methyl-4-phenylpyridinium, the neurotoxic metabolite of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine, by mitochondria. J Biol Chem. 1986 Jun 15;261(17):7585–7587. [PubMed] [Google Scholar]
- Reinhard J. F., Jr, Diliberto E. J., Jr, Viveros O. H., Daniels A. J. Subcellular compartmentalization of 1-methyl-4-phenylpyridinium with catecholamines in adrenal medullary chromaffin vesicles may explain the lack of toxicity to adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1987 Nov;84(22):8160–8164. doi: 10.1073/pnas.84.22.8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricaurte G. A., DeLanney L. E., Irwin I., Langston J. W. Toxic effects of MDMA on central serotonergic neurons in the primate: importance of route and frequency of drug administration. Brain Res. 1988 Apr 12;446(1):165–168. doi: 10.1016/0006-8993(88)91309-1. [DOI] [PubMed] [Google Scholar]
- Ricaurte G. A., Guillery R. W., Seiden L. S., Schuster C. R., Moore R. Y. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982 Mar 4;235(1):93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- Ricaurte G., Bryan G., Strauss L., Seiden L., Schuster C. Hallucinogenic amphetamine selectively destroys brain serotonin nerve terminals. Science. 1985 Sep 6;229(4717):986–988. doi: 10.1126/science.4023719. [DOI] [PubMed] [Google Scholar]
- Rindi G., Luinetti O., Cornaggia M., Capella C., Solcia E. Three subtypes of gastric argyrophil carcinoid and the gastric neuroendocrine carcinoma: a clinicopathologic study. Gastroenterology. 1993 Apr;104(4):994–1006. doi: 10.1016/0016-5085(93)90266-f. [DOI] [PubMed] [Google Scholar]
- Rudnick G., Wall S. C. The molecular mechanism of "ecstasy" [3,4-methylenedioxy-methamphetamine (MDMA)]: serotonin transporters are targets for MDMA-induced serotonin release. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1817–1821. doi: 10.1073/pnas.89.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuldiner S., Shirvan A., Linial M. Vesicular neurotransmitter transporters: from bacteria to humans. Physiol Rev. 1995 Apr;75(2):369–392. doi: 10.1152/physrev.1995.75.2.369. [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Steiner-Mordoch S., Yelin R., Wall S. C., Rudnick G. Amphetamine derivatives interact with both plasma membrane and secretory vesicle biogenic amine transporters. Mol Pharmacol. 1993 Dec;44(6):1227–1231. [PubMed] [Google Scholar]
- Schäfer M. K., Nohr D., Romeo H., Eiden L. E., Weihe E. Pan-neuronal expression of chromogranin A in rat nervous system. Peptides. 1994;15(2):263–279. doi: 10.1016/0196-9781(94)90012-4. [DOI] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. 1987 Oct 29-Nov 4Nature. 329(6142):840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y., Keen J. N., Bejerano M., Steiner-Mordoch S., Wallach M., Findlay J. B., Schuldiner S. Homology of a vesicular amine transporter to a gene conferring resistance to 1-methyl-4-phenylpyridinium. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9730–9733. doi: 10.1073/pnas.89.20.9730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzer D., Rayport S. Amphetamine and other psychostimulants reduce pH gradients in midbrain dopaminergic neurons and chromaffin granules: a mechanism of action. Neuron. 1990 Dec;5(6):797–808. doi: 10.1016/0896-6273(90)90339-h. [DOI] [PubMed] [Google Scholar]
- Varoqui H., Diebler M. F., Meunier F. M., Rand J. B., Usdin T. B., Bonner T. I., Eiden L. E., Erickson J. D. Cloning and expression of the vesamicol binding protein from the marine ray Torpedo. Homology with the putative vesicular acetylcholine transporter UNC-17 from Caenorhabditis elegans. FEBS Lett. 1994 Mar 28;342(1):97–102. doi: 10.1016/0014-5793(94)80592-x. [DOI] [PubMed] [Google Scholar]
- Weihe E., Schäfer M. K., Erickson J. D., Eiden L. E. Localization of vesicular monoamine transporter isoforms (VMAT1 and VMAT2) to endocrine cells and neurons in rat. J Mol Neurosci. 1994 Fall;5(3):149–164. doi: 10.1007/BF02736730. [DOI] [PubMed] [Google Scholar]