Abstract
Background
Alveolar soft part sarcoma (ASPS) is a rare soft tissue tumor that usually affects young patients. Because of the rarity of the disease, most reports relating to ASPS are in the form of case reports or small series.
Methods
We performed a retrospective study to evaluate the clinicopathologic features, treatment, outcome and pattern of treatment failure in a consecutive series of patients with localized or metastatic ASPS between 1996 and 2011. Demographics, tumor sizes, sites and extent of disease, treatments provided, progression-free survival, and overall survival were evaluated.
Results
A total of 19 patients were identified. The clinical assumptive diagnosis of the first medical examination doctor was benign soft tissue tumor in 5 cases (26%) and benign hemangioma in 4 cases (21%), delaying treatment. The most common location of primary tumor was the thigh. The median diameter of the mass was 55 mm (range, 10 to 130 mm). An R0 resection was obtained in 11 cases. Adjuvant radiotherapy was delivered in 8 cases; postoperative systemic chemotherapy was delivered in 10 cases. Eight out of 15 patients (53%) exhibited metastases either at presentation or later. Median overall follow-up was 54 months.
Conclusions
The treatment principle for alveolar soft tissue sarcoma is massive resection, and when the surgical margin is questionable, radiation treatment can be added for prevention of local recurrence. Also, due to discovery of metastases and local recurrence, even after 5 years of general treatment, outpatient department follow-up is needed, and we need to keep in mind that lung, intracranial, and bone metastases are common.
Keywords: Alveolar soft part sarcoma, Neoplasm metastasis, Survival
Alveolar soft part sarcoma (ASPS) is a very clinically and morphologically unique soft tissue sarcoma which was first defined and named by Christopherson et al.1) in 1952. It is known to comprise 0.5%-1% of all soft tissue sarcomas, and it occurs in teenagers and young adults under forty years of age.2,3) It usually occurs in the trunk and lower extremities, but is also found in the genital area, breasts, mediastinum, bladder, gastrointestinal, orbital area, and tongue.4) ASPS is found as a slowly growing soft tissue mass. It is first diagnosed by symptoms produced by metastasis into the lungs and brain because it is diagnosed late in time since it has no related symptoms. The term "alveolar" originated from the polygonal cells histologically gathered to form a pseudo-alveolar shape. Pathologically, it is not difficult to diagnose because the rhomboid-shaped crystalline materials are observed in the cytoplasm when it is dyed with periodic acid schiff (PAS), but in magnetic resonance imaging or contrast-enhanced computed tomography (CT), it can be confused as a hemangioma which is much more common and has a rich supply of blood vessels. In this manner, ASPS can occur anywhere in the trunk or extremities, but its occurrence is very rare, and clinicians have little experience of treatment. Because of the rarity of the disease, most reports relating to ASPS are in the form of case reports or small series. In this study, through 19 cases of pathologically confirmed ASPS, we analyzed the clinical aspect, therapeutic method and therapy results.
METHODS
We performed a comprehensive retrospective review of the medical records of patients who were pathologically confirmed between 1996 and 2011 (n = 19), and we performed an investigation of image tests such as simple X-rays, angiographies, contrast-enhanced CTs and magnetic resonance images (MRIs), as well as of pathological biopsy results and follow-up evaluations. This study was approved by Yonsei University Health System, Severence Hospital, Institutional Review Board (IRB# 4-2012-0699). The anatomical location and depth of the mass, clinically assumed diagnosis of the first medical examination doctor, size of mass, surgical method, whether it metastasized at the time of diagnosis or during follow-up and the site of metastasis, and survival rate were studied.
The depth of the mass was classified as "superficial" when the mass didn't invade the deep fascia and as "deep" when it invaded the deep fascia in image tests (MRI, CT, or ultrasonography) performed before the operation. The size of the mass was measured by the longest axis in centimeters in pathological biopsy tests after the surgery (Fig. 1). All macroscopically complete resections were classified according to the closest surgical margin, which was microscopically categorized as positive (tumor within 1 mm from the inked surface, R1) or negative (absence of tumor within 1 mm from the inked surface, R0). Locally recurrent disease was defined as tumor occurrence at a site previously treated for ASPS. Recurrance and survival was analyzed via the Kaplan-Meier method.
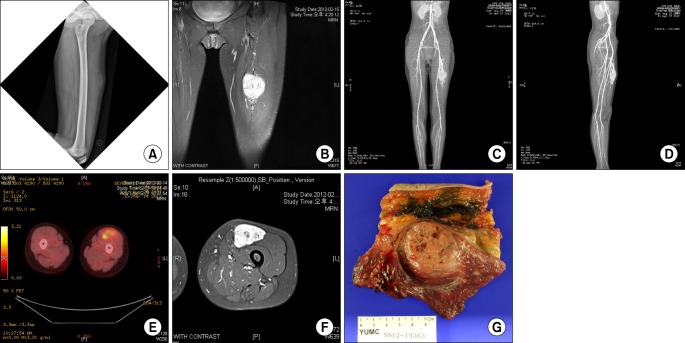
Fig. 1.
A 14-year-old female patient presented with a chief complaint of growing palpable mass in the anterior aspect of the left mid thigh. (A) On the plain radiography of the left femur, soft tissue shadow was seen in the anterior aspect of the midshaft of the femur. (B) On T2-weighted coronal magnetic resonance image, there was a 7 × 5 × 3 mm-sized, well-enhanced soft tissue mass at the same site. (C,D) On computed tomography angiogram, a highly vascularized mass lesion was observed, and tortuous prominent blood vessels were seen on superior and inferior margins of the mass. (E,F) There was no metastasis seen on positron emission tomography computed tomography. (G) Incisional biopsy was performed and the mass was diagnosed as alveolar soft part sarcoma. A wide marginal excision was performed.
RESULTS
Clinical Features
In the total of 19 patients, 6 were men and 13 were women, so the group of women was a little larger. The mean age of patients was 30.3 years old, ranging from 10 to 64 years old. The patients had follow-up at longest for 14 years and 8 months with a mean of 54 months. Fifteen cases (78.9%) visited with a painless mass as a chief complaint, 4 cases (21.1%) complained of a mass accompanied by pain and discomfort. From the start of the symptoms to the hospital visit, the longest time lasted 60 months with 15 months as the average.
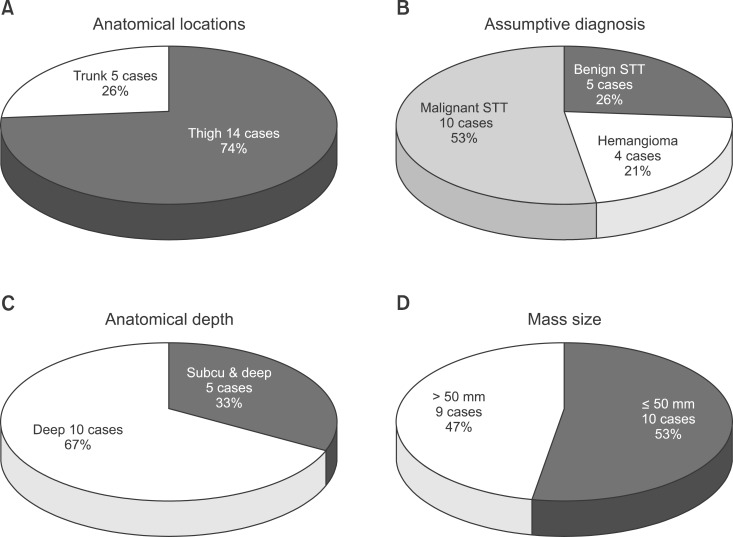
The tumor was located in the trunk in 5 cases (26%) and in a lower extremity in 14 cases (74%). The clinical assumptive diagnosis made by the first medical examiner was a benign soft tissue tumor in 5 cases (26%) and benign hemangioma in 4 cases (21%). In the 15 cases where we could do the image test before the surgery, the mass was located in deep areas in 10 cases (67%), throughout the deep and superficial areas in 5 cases (33%), and none were located in superficial area only. The tumor size was 55 mm (10-130 mm) on average. It exceeded 50 mm in 9 cases (47%) and it was the same or less than 50 mm in 10 cases (53%) (Fig. 2).
Fig. 2.
(A) From a total of 19 cases, the mass was located in the trunk in 5 cases (26%) and thigh in 14 cases (74%). (B) During the first medical examination, benign soft tissue tumor was diagnosed in 5 cases (26%) and benign hemangioma in 4 cases (21%). (C) In the 15 cases in which magnetic resonance imaging was performed before the surgery, the mass was deep seated in 10 cases (67%) and located from subcutaneous layer to deep layer in 5 cases (33%). (D) Mass size exceeded 50 mm in 9 cases (47%), and it was less than or the same as that in 10 cases (53%). STT: soft tissue tumor.
Therapeutic Results
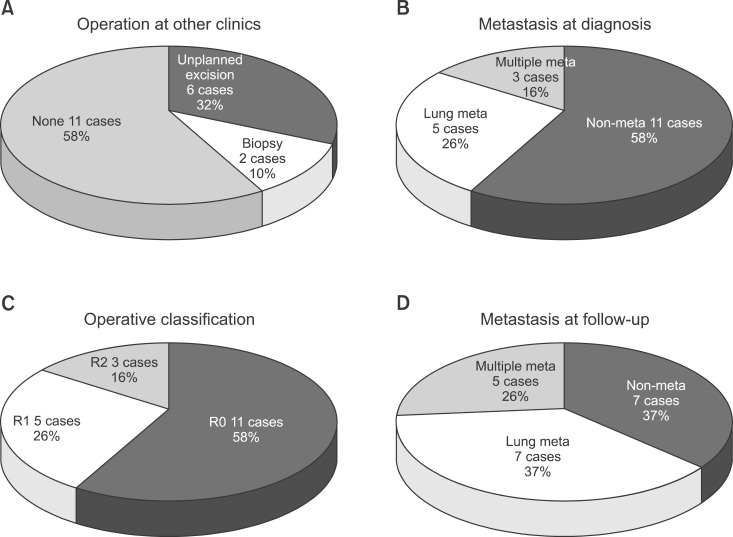
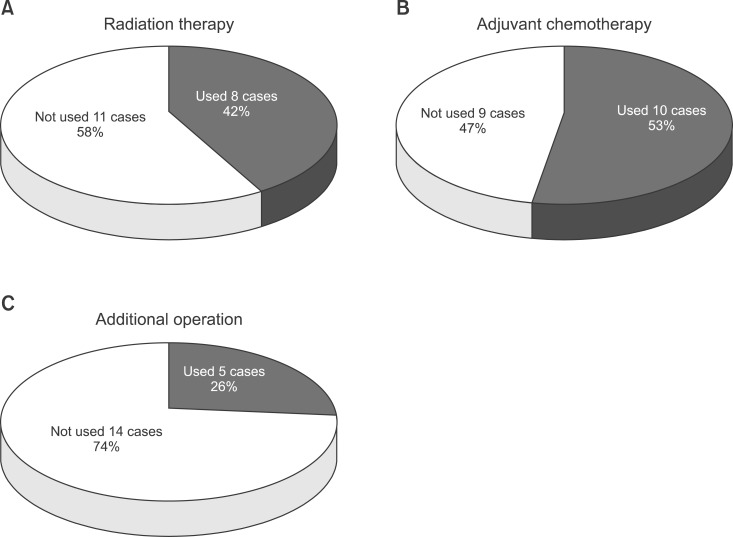
In 8 cases, patients were transferred from other hospitals or institutes after surgery. Among those, 6 cases (32%) had an unplanned mass excision, and 2 cases (10%) had a biopsy and transferred afterwards. Eight cases already had metastasis in other organs at the time of diagnosis. Among those, 5 cases (26%) had lung metastasis, and 3 cases (16%) had 2 or more multiorgan metastases including those in the bone and intracranial space. Among patients who had surgery performed in our hospital, 11 cases (16%) had R0, 5 cases (26%) had R1, 3 cases (16%) had R2. Among 11 cases that had no metastases, 4 cases had metastasis observed during outpatient department follow-up. Lung metastases were found in 2 cases, and multiorgan metastases was found in 2 cases (Fig. 3). In 5 cases, additional surgical intervention was performed after the first surgery. In 8 cases (42.1%), radiation was added (total radiation 60 Gy), and in 10 cases (52.6%), chemotherapy was performed (Fig. 4).
Fig. 3.
(A) Eight cases were transferred from other hospitals after surgery: unplanned excision in 6 cases and biopsy only in 2 cases. (B) Eight cases had metastases at the time of diagnosis: lung metastasis in 5 cases and multiple metastases in 3 cases (16%). (C) Among the cases with an operation at our hospital, the mass was classified as R0 in 11, R1 in 5, and R2 in 3. (D) In the 11 cases without metastases, lung metastasis was found in 2 and multiple metastases in another 2 during the outpatient follow-up. meta: metastasis.
Fig. 4.
(A) Eight cases (42%) had radiation therapy after operation. (B) Ten cases (53%) had adjuvant chemotherapy. (C) Five cases (26%) had an additional operation.
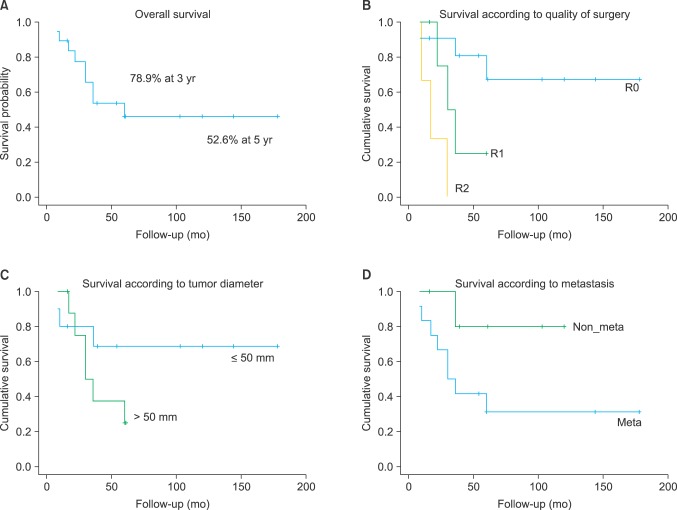
In total, 3-year survival was 78.9% and 5-year survival was 52.6%. In survival classified by surgical resection margin, RO 3-year survival was 100%, and 5-year survival was 80%, which were higher than those of R1 or R2 in terms of long-term survival. In tumor size, survival was 70% in patients with smaller than 50 mm at final follow-up, which was better than tumors sized more than 42.9%. Also when there is no other organ metastasis, long-term survival was better than in cases with metastasis (Fig. 5). There was no meaningful difference in final follow-up survival according to depth of the tumor.
Fig. 5.
(A) Overall 3-year survival rate was 78.9%. (B) The long-term survival rate was higher in R0 than R1 and R2 classified according to the quality of surgery. (C) The long-term survival rate was higher in patients with a tumor ≤ 50 mm in diameter than those with > 50 mm. (D) The long-term survival rate was higher in cases without metastases than in cases with metastases.
DISCUSSION
Alveolar soft tissue sarcoma has low occurrence, and there is a report that states it is more common in female patients.3) It is known to most frequently invade lower extremities, second frequently the trunk, and lower extremities the least. In this study, female patients were more than double in number than male patients, and the lower extremities were the most common sites of invasion, the trunk the second most common. Alveolar soft tissue sarcoma has slow-growing characteristics, and because of this metastasis to other organs were found in 20%-40% at the time of diagnosis. Metastasis usually invades the lung, and there is a report of lung metastasis found 35 years after surgical removal of the primary cancer.5) Following lung metastases, bone and intracranial metastases are common, but lymph node metastases are not common.3,6)
Alveolar soft tissue sarcoma usually shows high signal in T1-weighted and T2-weighted MRI and features internal and external multilobulated signal change.7-9) The part with a high signal in T1-weighted MRI has low blood flow rate, and the part with multilobulated signal change in T1-weighted and T2-weighted MRI has high blood flow rate, relatively. This is a characteristic feature of alveolar soft tissue sarcoma.8) In angiography, expended feeding and efferent vessels are seen inside and around the tumor, and these findings are similar to arteriovenous malformation, so it is easy to misdiagnose.6,8) When doing image studies for alveolar soft tissue sarcoma, hemangioma, arteriovenous malformation, clear-cell sarcoma, metastatic malignant melanoma, liposarcoma, and soft tissue sarcoma with internal bleeding are needed for different diagnoses. These lesions have high signal intensity in T1-weighted MRI or have similar signal intensity as muscle. In clear-cell sarcoma, multilobulated signal change is not seen, and it is not easy to differentiate with hemangioma or arteriovenous malformation, but the fact that the solid tissue component seen in alveolar soft tissue sarcoma is not seen in these lesions helps to differentiate alveolar soft tissue sarcoma.8)
According to the depiction first performed by Christopherson et al.,1) histologically, alveolar soft tissue sarcoma is relatively big and has a tube shaped nucleolus and eosinophilic cytoplasm, and, structurally, alveolar soft tissue sarcoma has polygonal tumor cells forming an organoid nest and has a unique morphological pattern, called Pseudoalveolar pattern, which is divided by capillary sized vascular channels and tube shaped septum. Clear cell change is definite and this sometimes needs differential diagnosis with metastatic renal cell carcinoma. Renal cell carcinoma can be distinguished by associated past history, clinical symptom, radiologic evaluation, etc. Renal cell carcinoma is positive in immunohistochemical staining to cytokeratin and vimentine, so it is not difficult to differentially diagnose from alveolar soft tissue sarcoma. Diseases to differentially diagnose histologically are paraganglioma, adrenal cortical carcinoma, hepatocellular carcinoma, alveolar rhabdomyosarcoma, malignant melanoma, granular cell tumor, etc.10,11) Paraganglima has an alveolar structure and is positive in immunohistochemical staining to chromogranin and synaptophysin. Metastatic adrenal cortical carcinoma and hepatocellular carcinoma can be differentiated each by melan-A-cross reactivity and Hep Par staining. Alveolar rhabdomyosarcoma can be distinguished by myogenin and MyoD1 staining in skeletal muscles, and malignant melanoma can be distinguished by staining in melanocytes such as S-100 protein and HMB45. Granular cell tumor doesn't have crystalline bundles specifically seen in alveolar soft tissue sarcoma and has positive reaction to S-100 protein.
Pennacchioli et al.12) reported that 10-year survival after surgical resection of alveolar soft tissue sarcoma is 53.4%, informing that tumor size and classification of surgical resection margin are related with patient prognosis. Ogose et al.13) reported that when tumor size is less than 5 mm, 5-year, 10-year, 15-year survival are 72%, 65%, and 65%, respectively, and when it is larger than 5 mm, each are 46%, 9%, and 0%, respectively. Alveolar soft tissue sarcoma is a relatively slow growing tumor, and the larger the tumor size, the longer ago it started and had had more opportunity to metastatize into other organs. Reports of a larger tumor size with more metastases found in other organs support this assertion.14,15) With respect to the surgical margin of alveolar soft tissue sarcoma, the influencing prognosis is known from many previous reports, and most tumor specialists agree that it is most important for the full recovery of a local tumor. For this, en-bloc of the tumor with sufficient amount of normal cells surrounding the tumor should be possible. The larger the size of the tumor, the lesser the opportunity of en-bloc including the tumor, so tumor size can influence surgical margin. Discovery of tumor cells in the surgical margin means active tumor cells are left and need more aggressive treatment. Recurrence of alveolar soft tissue sarcoma is reported to be 20%.3) Sherman et al.16) reported radiation treatment of alveolar soft tissue sarcoma, suggesting radiation treatment as the treatment rule for alveolar soft tissue sarcoma. Ogose et al.13) suggested supplemental radiation treatment is needed when the surgical margin is insuffient, but until now there are no reports of radiation treatment and anticancer treatment influencing prognosis.13,17) In this study, 3 cases of R2 resection were carried out with radiation treatment and anticancer treatment after surgery, but after follow-up of 5 years, all had expired. Whereas the 5-year, 10-year, and 15-year survival of patients without metastasis at the time of diagnosis were all 81%, 5-year survival of patients with metastasis at the time of diagnosis was 46% and 10-year survival was 0%.13)
Alveolar soft tissue sarcoma patients mostly came to the hospital due to a painless mass as the chief complaint and presented symptoms clinically similar to benign hemangioma, so biopsies were performed as needed. Treatment principle was of a massive resection, and when the surgical margin is questionable, radiation treatment can be added for prevention of local recurrence. Also, with the discovery of metastasis and local recurrence, even after 5 years of general treatment, outpatient department follow-up is needed, and we need to keep in mind that lung, intracranial, and bone metastases are common.
Footnotes
An abstract of this work was presented at Daejeon, The 56th Spring Congress of the Korean Orthopaedic Association, 2012.
No potential conflict of interest relevant to this article was reported.
References
- 1.Christopherson WM, Foote FW, Jr, Stewart FW. Alveolar soft-part sarcomas; structurally characteristic tumors of uncertain histogenesis. Cancer. 1952;5(1):100–111. doi: 10.1002/1097-0142(195201)5:1<100::aid-cncr2820050112>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 2.Ekfors TO, Kalimo H, Rantakokko V, Latvala M, Parvinen M. Alveolar soft part sarcoma: a report of two cases with some histochemical and ultrastructural observations. Cancer. 1979;43(5):1672–1677. doi: 10.1002/1097-0142(197905)43:5<1672::aid-cncr2820430517>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 3.Lieberman PH, Brennan MF, Kimmel M, Erlandson RA, Garin-Chesa P, Flehinger BY. Alveolar soft-part sarcoma: a clinico-pathologic study of half a century. Cancer. 1989;63(1):1–13. doi: 10.1002/1097-0142(19890101)63:1<1::aid-cncr2820630102>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 4.Fanburg-Smith JC, Miettinen M, Folpe AL, Weiss SW, Childers EL. Lingual alveolar soft part sarcoma; 14 cases: novel clinical and morphological observations. Histopathology. 2004;45(5):526–537. doi: 10.1111/j.1365-2559.2004.01966.x. [DOI] [PubMed] [Google Scholar]
- 5.Kayton ML. Alveolar soft part sarcoma. In: Enzinger FM, Weiss SW, editors. Soft tissue tumor. 3rd ed. St. Louis, MI: Mosby; 1995. pp. 1067–1074. [Google Scholar]
- 6.Pang LM, Roebuck DJ, Griffith JF, Kumta SM, Metreweli C. Alveolar soft-part sarcoma: a rare soft-tissue malignancy with distinctive clinical and radiological features. Pediatr Radiol. 2001;31(3):196–199. doi: 10.1007/s002470000388. [DOI] [PubMed] [Google Scholar]
- 7.Iwamoto Y, Morimoto N, Chuman H, Shinohara N, Sugioka Y. The role of MR imaging in the diagnosis of alveolar soft part sarcoma: a report of 10 cases. Skeletal Radiol. 1995;24(4):267–270. doi: 10.1007/BF00198412. [DOI] [PubMed] [Google Scholar]
- 8.Suh JS, Cho J, Lee SH, et al. Alveolar soft part sarcoma: MR and angiographic findings. Skeletal Radiol. 2000;29(12):680–689. doi: 10.1007/s002560000285. [DOI] [PubMed] [Google Scholar]
- 9.Lorigan JG, O'Keeffe FN, Evans HL, Wallace S. The radiologic manifestations of alveolar soft-part sarcoma. AJR Am J Roentgenol. 1989;153(2):335–339. doi: 10.2214/ajr.153.2.335. [DOI] [PubMed] [Google Scholar]
- 10.Daigeler A, Kuhnen C, Hauser J, et al. Alveolar soft part sarcoma: clinicopathological findings in a series of 11 cases. World J Surg Oncol. 2008;6:71. doi: 10.1186/1477-7819-6-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Günay C, Atalar H, Kaygusuz G, Yildiz Y, Saglik Y. Alveolar soft part sarcoma of the extremities: an evaluation of four cases. Acta Orthop Traumatol Turc. 2007;41(4):326–331. [PubMed] [Google Scholar]
- 12.Pennacchioli E, Fiore M, Collini P, et al. Alveolar soft part sarcoma: clinical presentation, treatment, and outcome in a series of 33 patients at a single institution. Ann Surg Oncol. 2010;17(12):3229–3233. doi: 10.1245/s10434-010-1186-x. [DOI] [PubMed] [Google Scholar]
- 13.Ogose A, Yazawa Y, Ueda T, et al. Alveolar soft part sarcoma in Japan: multi-institutional study of 57 patients from the Japanese Musculoskeletal Oncology Group. Oncology. 2003;65(1):7–13. doi: 10.1159/000071199. [DOI] [PubMed] [Google Scholar]
- 14.Pisters PW, Leung DH, Woodruff J, Shi W, Brennan MF. Analysis of prognostic factors in 1,041 patients with localized soft tissue sarcomas of the extremities. J Clin Oncol. 1996;14(5):1679–1689. doi: 10.1200/JCO.1996.14.5.1679. [DOI] [PubMed] [Google Scholar]
- 15.Coindre JM, Terrier P, Bui NB, et al. Prognostic factors in adult patients with locally controlled soft tissue sarcoma: a study of 546 patients from the French Federation of Cancer Centers Sarcoma Group. J Clin Oncol. 1996;14(3):869–877. doi: 10.1200/JCO.1996.14.3.869. [DOI] [PubMed] [Google Scholar]
- 16.Sherman N, Vavilala M, Pollock R, Romsdahl M, Jaffe N. Radiation therapy for alveolar soft-part sarcoma. Med Pediatr Oncol. 1994;22(6):380–383. doi: 10.1002/mpo.2950220605. [DOI] [PubMed] [Google Scholar]
- 17.Pappo AS, Parham DM, Cain A, et al. Alveolar soft part sarcoma in children and adolescents: clinical features and outcome of 11 patients. Med Pediatr Oncol. 1996;26(2):81–84. doi: 10.1002/(SICI)1096-911X(199602)26:2<81::AID-MPO2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]