Abstract
Background:
The consensus on most reliable supplemental test to predict the shunt responsiveness in patients with idiopathic normal pressure hydrocephalus (iNPH) is lacking. The aim of this study is to discuss the utility of external lumbar drain (ELD) in evaluation of shunt responsiveness for iNPH patients.
Methods:
A retrospective review of 66 patients with iNPH was conducted. All patients underwent 4-day ELD trial. ELD-positive patients were offered ventriculoperitoneal shunt (VPS) surgery. The primary outcome evaluation parameters were gait and mini mental status examination (MMSE) assessment. The family and patient perception of improvement was accounted for in the outcome evaluation.
Results:
There were 38 male and 28 female with mean age of 74 years (range 45-88 years). ELD trial was positive in 86% (57/66) of patients. No major complications were encountered with the ELD trial. A total of 60 patients (57 ELD-positive, 3 ELD-negative) underwent VPS insertion. The negative ELD trial (P = 0.006) was associated with poor outcomes following shunt insertion. The positive ELD trial predicted shunt responsiveness in 96% patients (P < 0.0001, OR = 96.2, CI = 11.6-795.3). A receiver operating characteristic (ROC) curve analysis revealed that the ELD trial is reasonably accurate in differentiating shunt responder from non-responder in iNPH patients (area under curve = 0.8 ± 0.14, P = 0.02, CI = 0.52-1.0). The mean follow-up period was 12-months (range 0.3-3 years). The significant overall improvement after VPS was seen in 92% (55/60). The improvement was sustained in 76% of patients at mean 3-year follow-up. The number of comorbid conditions (P = 0.034, OR = 4.15, CI = 1.2-9.04), and a history of cerebrovascular accident (CVA) (P = 0.035, OR = 4.4, CI = 1.9-14.6) were the predictors of poor outcome following shunt surgery.
Conclusion:
The positive ELD test predicted shunt responsiveness in 96% of patients. With adequate technique, maximal results with minimal complications can be anticipated. The number of comorbidities, history of CVA and negative ELD test were significantly associated with poor shunt outcomes.
Keywords: CSF tap test, external lumbar drain, idiopathic normal pressure hydrocephalus, supplemental test, valve pressure, ventriculoperitoneal shunt
INTRODUCTION
Since the seminal description of classical symptom triad by Hakim and Adams; gait disturbance, urinary incontinence, and cognitive impairment, the definition of normal pressure hydrocephalus (NPH) has evolved.[15] Idiopathic normal pressure hydrocephalus (iNPH) is defined as the presence of any one or more of the triad symptoms associated with normal cerebrospinal fluid (CSF) pressure (60-240 mm H2O) and computerized tomography (CT) scan evidence of ventriculomegaly. The incidence of iNPH is reported to be between 0.18 and 2.2 cases per 1,000,000 individuals; affecting most frequently the elderly between sixth and seventh decade of life.[11] CSF diversion procedures including shunt surgery and endoscopic third ventriculostomy provide an effective means of treatment. Ventriculoperitoneal shunting (VPS) is by far the most commonly employed CSF diversion procedure. Appropriate patient selection and prediction of the shunt responsive patients is paramount; given the high rate (30-40%) of post-VPS surgery complications, including shunt obstruction, infection, seizures, subdural hematoma, and less commonly intraparenchymal or intraventricular hemorrhage. Despite a number of studies, the expert consensus on most reliable predictors of favorable outcome after shunt surgery is still lacking.[7,12,16,18,20,24,30,33,44,45] The commonly used prognostic tools to select the patients for shunt surgery are the radionucleotide cisternography, high volume tap test (TT), CSF outflow resistance determination, external lumbar drain (ELD) trial, and long-term recording of ICP to identify B wave activity.[31] The current neurosurgical literature supports ELD. However, complications associated with ELD technique, lower negative predictive value (NPV) and high-cost reported in the literature has pacified the widespread application of ELD. At out institution, ELD was used as the sole supplemental test for prediction of shunt outcomes. The present study was conducted to discuss evolution of our experience in selecting optimal supplemental tests for prediction of shunt responsiveness. We aim to discuss our technique of ELD trial and to analyze if ELD is optimal supplemental test for prediction of shunt responsiveness. In addition, we aim to discuss our outcomes following VPS and analyze the clinical parameters that might affect the shunt responsiveness.
MATERIALS AND METHODS
A retrospective review of medical records for 108 patients presenting with NPH to our institution between August 2000 and July 2011 was conducted. The institutional review board approved the study. The inclusion criteria for study were: (1) Presence of any one of the symptom triad; gait disturbance, urinary incontinence, and cognitive impairment (2) normal CSF pressure (60-240 mm H2O) (3) radiographic evidence of ventriculomegaly (Evan's index > 0.3); and (4) No antecedent disorder that can be causally related to the secondary NPH.
The ELD trial was the main supplemental test employed in all patients with clinical diagnosis of iNPH. Other supplemental tests, including radionuceliotide cisternography, and high volume lumbar CSF TT were employed in the patients presenting early in the series.
ELD trial
Following initial examination, a lumbar puncture was performed employing sterile technique and a lumbar catheter was inserted. After demonstration of adequate drainage, the catheter was connected to an external CSF collection system and secured in place at the mattress level of patients’ bed. The ELD bag utilized in the trial was equipped with a control valve that allowed the drainage of CSF at 10 ml/h; which could be readjusted as needed. Nursing staff was trained to adjust the valve as necessary in order to obtain an average drainage of 10 ml of CSF per hour. If the amount of 10 ml/h was not reached, the level of drainage system was lowered to obtain drainage of 10 ml/h. This was required in 11% of the patients (7/66). This technique provided better control over the drainage. For assessment of ELD response, baseline videographic gait assessment, balance, muscle strength, fluency of speech, general behavior, and mini mental status examination (MMSE) were compiled on the day of admission. The gait assessment was performed by a physical therapist (PT) or occupational therapist (OT), a neurosurgeon, and a nurse practitioner. The assessment was based on balance and number of steps required to traverse a specified distance, valuated by timed 10-15 meter trial. The inputs on the balance, stance, and stride length were video recorded and noted on the patient's chart. No sophisticated grading of these parameters was employed. The assessment of patients, as on the day of admission, was repeated subsequently for 3 days and the final assessment was conducted on Day 4 following removal of the lumbar drain. The findings were recorded in the patient's chart. Patients were then discharged with instruction to follow up in the neurosurgery clinic 1-2 weeks later. The patient was considered positive on ELD trial if there was an increase of two or more points on the MMSE from the baseline to final exam or improvement in gait, changes in manner of sitting and standing up, balance and fluency of speech, which was assessed based on the videotape assessment and PT/OT documentation. The outcomes were evaluated after the 4-day ELD trial and the patients with positive ELD trial were then offered surgical treatment [Figure 2].
Figure 2.
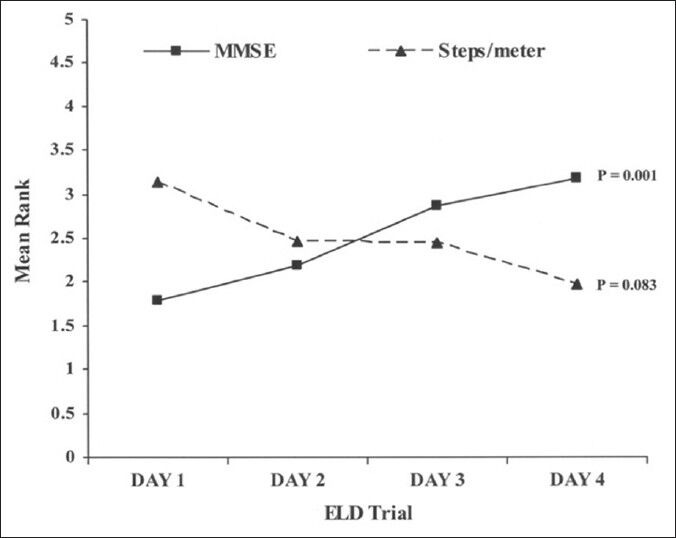
Mean Rank analysis of the MMSE and Steps/meter for the 4-day ELD trial. Values plotted on Y-axis are mean ranks of MMSE scores and Steps/meter for the 4-day ELD trial. P value obtained from Friedman's test for repeated measure analysis
VPS surgery
VPS insertion was carried out by posterior parietal approach. The programmable valve with antisiphon technology (Medtronic Strata, MN, USA) was installed in 31/60 patients shunted between March 2005 and February 2009. The valve was programmed to initial settings of 1.5, which corresponds to the opening pressure of 85-105 mm H2O. Codman valve (Codman, Johnson and Johnson, MA, USA), set to an initial value of 120 mm H2O, was installed in 13 patients before March 2005 and the new Codman programmable valve with initial pressure set to 200 mm H2O was installed in 16 patients after February 2009. Patients were discharged following shunt insertion and the follow-up was adjusted according to the symptomatology and need to modify the valve settings.
Follow-up and outcome assessment after VPS
The outcomes after VPS insertion were evaluated at each follow-up visit based on the clinical and functional assessment. The primary clinical criteria were gait improvement and cognitive evaluation. The functional outcome was assessed based on the patient and family perception of improvement in daily activity. Patients and families were interviewed regarding the degree of independence in terms of daily activities, improvement in overall general behavior, improvement in cognition, urinary control, changes in the dependence on assisted devices or person for movement, changes in balance and increase in the stride length. Follow up was conducted initially at 1 month after valve pressure modification. Later on, changes in valve pressure were made every 2-3 months until the maximal response is reached and was sustained during subsequent visits. Prior to each office visit the patients received head CT scan to look for changes in the ventricular size, transependymal CSF resorption and rule out any subdural collections [Figure 1]. The clinical parameters that might predict the outcomes after VPS insertion were analyzed.
Figure 1.
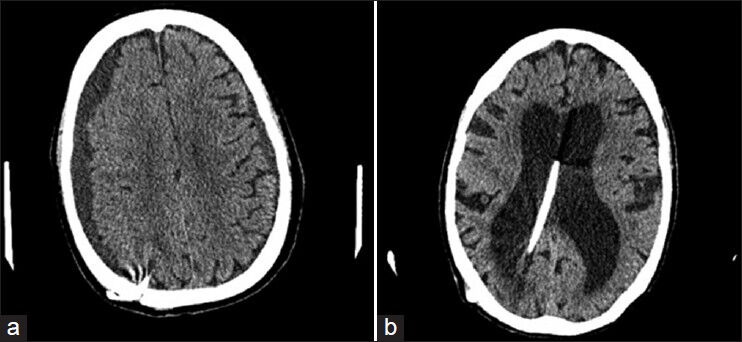
(a) Subdural hygroma on right measuring 1.8 cm in greatest thickness and on the left measuring 9 mm in greatest thickness. Regression of these hygromas was achieved by readjustment of the opening pressure of valve to a higher level (b) Enlarged ventricles with right lateral ventricle slightly larger than left, ventriculoperitoneal shunt in place in the medial aspect of the left lateral ventricle
Statistical analysis
All descriptive statistics calculated for categorical variable are reported as percentages and continuous variable as medians. Fisher exact test was used for testing association between two categorical variables. Mann-Whitney test was used for examining differences in continuous measures with deviation from the normality, and for the ordinal measures (MMSE scores) between the independent groups. Repeated measure analysis using Friedman's test was performed to test for change in the MMSE scores and steps/meter recorded during the ELD trial and for the final outcome evaluation. Logistic regression analysis was performed to examine the association of the outcome (improvement with VPS insertion) with the predictors, adjusting for age and gender of patients. Hosmer-Lemeshow statistic was used to assess the fit of the models. A receiver operating characteristic (ROC) curve was used to evaluate the prognostic performance of ELD trial as supplemental test for shunt responsiveness. All hypotheses were tested at 0.05 level of significance and the analysis was performed using the SPSS version 20 (IBM, Chicago, Inc).
RESULTS
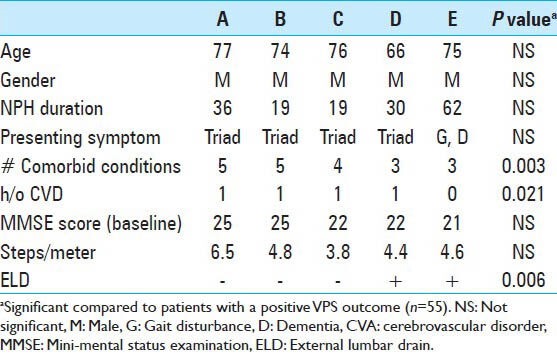
Sixty-six patients met inclusion criteria for the study. There were 38 male and 28 female patients, with mean age of 74 years (range 45-88 years). Table 1 demonstrates characteristics of the patients in the study. The mean duration of symptoms in this cohort of patients was 27.8 months (range 5-108 months). Abnormality in gait was the most common initial symptom (47%), however, at presentation, 64% of patients displayed the complete triad. Comorbidities were frequently present in this population, 18% had four or more comorbidities. The most frequent comorbidity was hypertension (68%), followed by other cardiac morbidities (36%), stroke or transient ischemic attack (TIA) (32%).
Table 1.
Characteristics of the patients in the study cohort
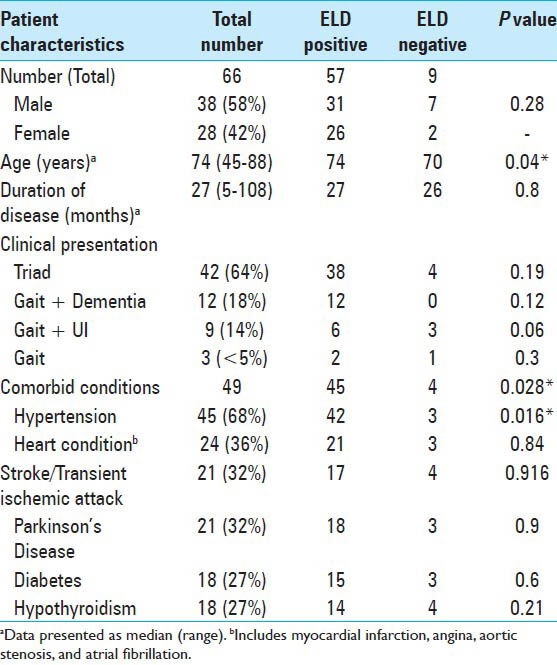
ELD trial
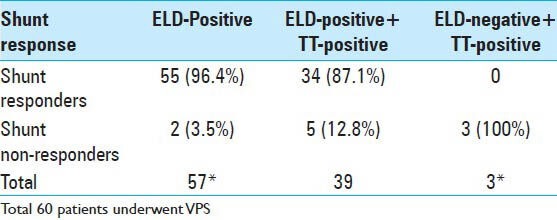
A total of 66 patients underwent ELD trial; 86% (57/66) of the patients were ELD positive. The mean age at presentation (P = 0.04), presence or absence of any comorbidity (P = 0.028), and positive history of hypertension (P = 0.016) was significantly different between ELD positive and negative patients [Table 1]. The baseline median MMSE score for patients undergoing ELD trial was 23.5 (range, 12-30). A significant improvement in the cognitive function of patients was observed, with the median MMSE score increasing to 27 on Day 4. Repeated measures analysis [Figure 2] indicated a significant difference in the MMSE scores (χ2= 15.74, P = 0.001). The gait assessment recorded as the median number of steps per meter covered was 3.7 (range, 1.7-8.1); number of steps per meter decreased during the course of ELD trial, but this improvement in gait did not reach the statistical significance (χ2= 6.67, P = 0.083). Improvement in balance was noted in 60% of patients. ELD positive patients were offered the surgical treatment with VPS insertion. ELD negative patients (9/66) were not offered surgery; however, 3 patients opted for VPS insertion regardless of the results of ELD trial. Figure 3 demonstrated the ROC curve for prognostic accuracy of ELD for shunt responsiveness. The ROC curve analysis revealed that the ELD trial is reasonably accurate in differentiating shunt responder from nonresponder in iNPH patients (area under curve = 0.8 ± 0.14, P = 0.02, CI = 0.52-1.0).
Figure 3.
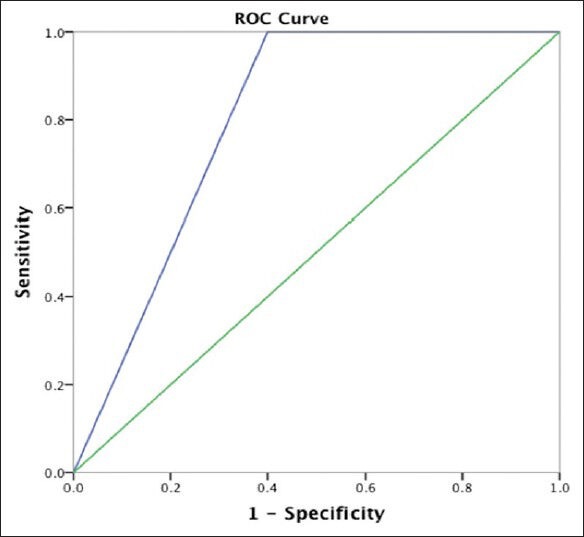
A ROC curve analyses of prognostic accuracy of ELD for shunt responsiveness in terms of sensitivity and specificity
No major complications were encountered with the ELD trial, however, 6/66 (10%) patients developed transient nerve root irritation. Catheter withdrawal of 5-10 mm followed by pain medication resolved the symptoms in all patients, and improvement in gait was noted on the third day of trial. One patient became agitated and confused subsequently removing the drain prematurely; however, the trial was completed successfully 2 months later. Another patient became disoriented after administration of the antihistamine confounding the precise trial findings; however, after careful evaluation the patient demonstrated definite improvement and was ultimately shunted.
Other supplemental tests
The patients managed during earlier years in the series (22/66) underwent radionuclide cisternogram as the supplemental test. Findings indicated type II activity (no ventricular activity with delayed migration) in 2 (9%); type IIIa (transient ventricular activity with clearance by usual migration) in 10 (45%); type IIIb (transient ventricular activity without normal clearance) in 3 (14%); and type IV activity (persistent ventricular activity without adequate clearance) in 7 (32%) patients. Those patients in categories III-IV were considered to have findings consistent with NPH. None of these patterns were significantly associated with outcome following VPS insertion.
High-volume CSF TT was employed earlier in the series and a total of 64% (42/66) patients underwent high volume TT. Symptomatic improvement was found in 97% (39/42) of patients. Gait was the most common domain to demonstrate improvement. The positive TT was not significantly associated with shunt outcomes; however, all the patients with poor outcomes had positive TT [Table 2].
Table 2.
Characteristics of patients having a poor outcome following VPS Insertion
VPS
VPS insertion was carried out in all 57 ELD trial positive and 3 ELD negative patients. With older valve, the overdrainage complications were encountered with setting at 1.5 (valve allowed setting from 0.5 to 2.5) and 120 mm H2O (the first generation Codman valve). To prevent complications including subdural hygroma and hematoma, and to have a unified start the initial opening pressure was set to 200 mm H2O with the newer valve. This allowed for adjustment to lower or higher valve settings based on the CT scan findings, videographic gait assessment, MMSE, general behavior of the patient, urinary control, and the patient and family perception of improvement. At the follow-up visit if the evaluation by clinical team, patient and family revealed a decline in functional status, the valve pressure was adjusted such that the maximum alleviation of symptoms could be obtained, at the same time minimizing the incidence of subdural fluid collections and other low pressure side effects. For instance, if a patient demonstrated decline in gait and/or cognition at valve setting of 200 mm H2O, the opening pressure of the valve was modified to 180 mm H2O and, in subsequent follow-up visits, the valve pressure was further lowered as needed. Although, with this methodology, more outpatient visits are anticipated, we found this as the safest measure that reduced the incidence of subdural hygromas to negligible. This was particularly seen in those patients who underwent VPS between 2009 and 2011, with the newer programmable Codman valves.
The over-drainage complication including subdural hygroma was present in 17% (10/60), which was treated by valve pressure adjustment. One patient (1.7%) patient had a subdural hematoma that required surgical intervention. Fifteen percent (9/60) patients with shunt obstruction (3) and subdural fluid collection (6) underwent revision surgery to change the shunt configuration. The patients who underwent VPS between 2009 and 2011 with new programmable Codman valve had lower incidence of subdural fluid collections (5%, 3/60). No cases of subdural hygroma requiring surgical intervention were encountered in the later group. No other major complications such as intraventricular or intraparenchymal hemorrhage or meningitis were encountered. The outcomes did not significantly differ between patients with or without over-drainage symptoms following shunt insertion.
Follow-up and outcome
The primary outcomes were assessed over a mean follow up of 12 months (0.3-3 years). The overall improvement in outcome after VPS insertion was evident in 55/60 (92%) patients and these were identified as shunt responders. The rate of improvement in patients with ELD trial positive and undergoing shunt surgery was 96% (55/57) [Table 4] (P < 0.0001, OR = 96.2, CI = 11.6-795.3). The characteristics of nonresponders are presented in Table 2. There was no significant difference in the age, gender, duration of disease, or presenting symptoms between the shunt responder group and nonresponders. The negative ELD trial was significantly associated with poor outcome following shunting (P = 0.006). The number of comorbidities present was significantly higher in shunt nonresponders (P = 0.002). Of the individual comorbidities, only the history of CVA was significantly associated with a poor outcome following shunting (P = 0.021). In this study, CVA was defined as those patients with a clinical history of symptomatic stroke or TIA. In separate logistic regression analysis models adjusted for age, gender, and disease duration, the number of comorbid conditions (P = 0.034, OR = 4.15, CI = 1.2-9.04), and a history of cerebrovascular accidents (CVA) (P = 0.035, OR = 4.4, CI = 1.9-14.6) were the predictors of poor outcome.
Table 4.
The results of supplemental test and shunt responsiveness
Long-term follow-up (>3 years) was available in 29/60 (48%) patients. The improvement was sustained in 76% (22/29) of the patients. Fifty-nine percent (17/29) of the patients improved substantially compared with their last follow-up; 21% (6/29) patients had improvement in both gait and MMSE (two points or more) and 38% (11/29) had gait improvement but no change in MMSE. In one patient, the overall improvement after VPS was maintained for 9 years. Forty-one percent (12/29) demonstrated no further improvement in symptoms at mean 3 year follow-up compared with the last post-VPS follow-up. Five patients (5/29;17%) demonstrated no change in gait and MMSE while 7/29 (24%) patients showed no change in gait but there was two points or more decline in MMSE compared with the last follow up. However, it was still higher than the preshunt MMSE.
DISCUSSION
Supplemental tests
Various supplemental tests have been employed to increase the predictive accuracy for shunt responsiveness to greater than 90%.[1,2,3,4,5,7,8,9,10,11,12,14,15,16,17,19,20,24,33,37,39,44,45] The predictive value of the cisternogram is reported to be identical to the combined clinical and CT criteria and is of little prognostic value.[32,41,42] In the present study, radionucleotide cisternography was initially performed for all patients diagnosed with iNPH. However, based on our experience and mounting evidence in literature concerning its predictability, this test was excluded from our institutional protocol. For those patients who underwent this test, the results of cisternography did not correlate with the outcomes following VPS surgery.
Another test used routinely for diagnosing iNPH is high volume CSF TT or Miller–Fisher test. The high volume TT is often adopted with the caveat that candidates for shunt surgery should not be excluded solely based on negative TT, because of its low sensitivity (26-61%).[14,22,24,44,45] Several alternative and adjunctive tests including brain magnetic resonance imaging (MRI) to predict the CSF TT, CSF pressure of ≥ 15 cm H2O,[16] CSF lumbar infusion test (3, 9-10, 16, 21), CSF flow rates measured by phase contrast MRI,[9] CSF aqueductal stroke volume[37] and ELD[12,14,24,33,45] have been employed to overcome the low sensitivity of high volume CSF TT. The positive predictive value (PPV) of CSF TT is reported in the range of 73-100%; the PPV of TT in present study was 87% as compared with that of 96% for the ELD trial [Table 4]. Three patients in our study had negative TT, however, these patients were never referred to the neurosurgical service for evaluation with ELD. We do not favor the use of high volume CSF TT for predicting shunt responsiveness, as a single high volume TT might give a false negative result. However, repeated lumbar puncture would increase sensitivity of TT. Repeated procedures might enhance the chances of infection and disabling pain from repeat lumbar punctures. Consequently, a temporary lumbar drain has been used in lieu of repeated lumbar punctures as the diagnostic and prognostic tool.[5,12,14,24,33,45] Conventionally, ELD has been used in combination with TT to predict the shunt responsiveness.[14] TT is used as the primary supplemental test and patients with negative TT are then offered ELD trial. We favor performing only ELD trial in all the patients with symptomatic iNPH.[14,25] The cost effectiveness of the ELD trial has been reported as a concern for adopting ELD as the first line supplemental test.[5,45] However, given the potential morbidity and mortality of the shunt surgery and reported high predictive value of ELD favors the reliance on ELD trial. The sensitivity of ELD in identifying shunt responsive patients for NPH has been reported ranging from 50% to 100%, specificity from 80% to 100%, PPV from 80% to 90% and NPV from 36% to 90%, with overall accuracy of 62%.[45] The sensitivity, specificity, NPV, and PPV ELD in present study was 100%, 60%, 100%, and 96%, respectively. The lower specificity can be explained by the fact that 6/9 patients with ELD-negative did not undergo VPS, which otherwise would have changed the test probabilities [Table 4]. The ROC curve analysis demonstrated reasonable prognostic accuracy of ELD for shunt responsiveness in iNPH patients [Figure 3]. As yet, the positive ELD test is a reliable criterion for preoperative selection of shunt responsiveness in probable iNPH patients; however, in case of negative ELD trial further investigation with additional supplemental tests might be beneficial.[21,26]
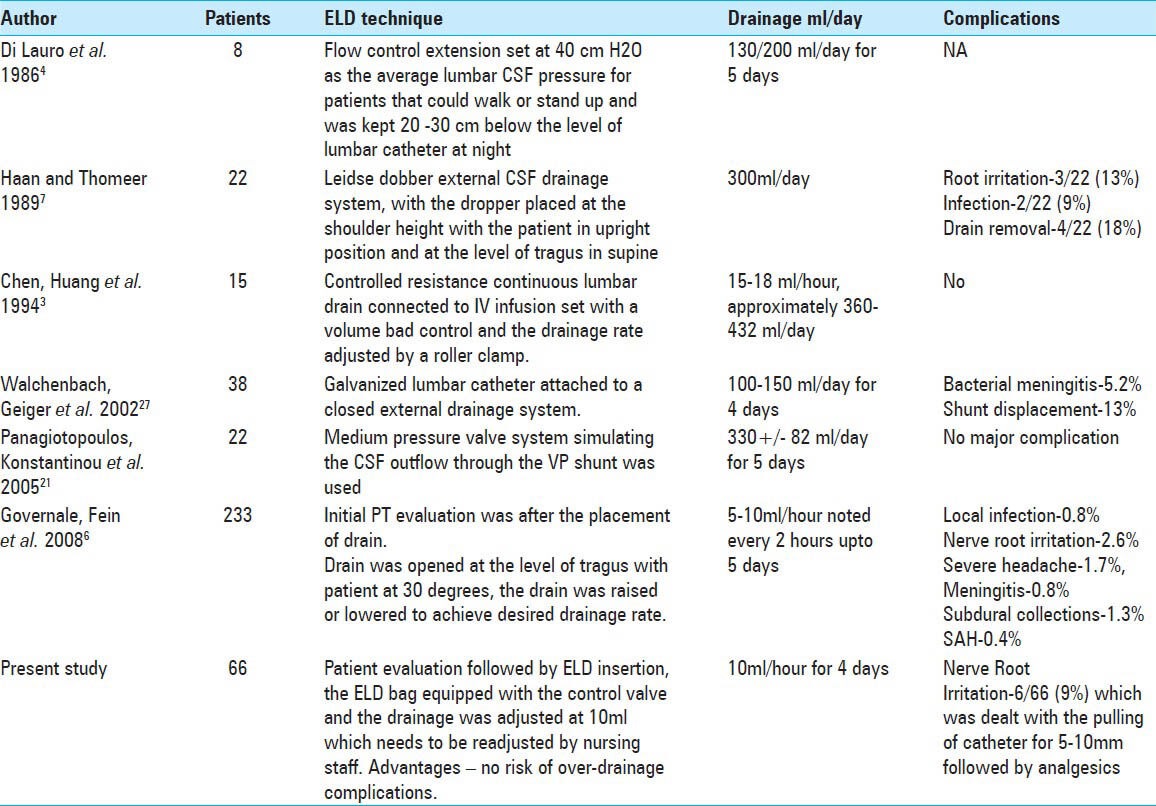
One of the major setbacks of ELD is the high rate of complications reported in the literature. An overall rate of complication reported in the early literature was as high as 40%. The recent study reports the rate of major complications (meningitis, retained catheter, intracranial subdural collections, and traumatic pattern subarachnoid hemorrhage) ranging from 0% to 5% and that of minor complications (local infection, headaches substantial enough to warrant drain removal, and nerve root irritation) ranging from 5.2% to 13%.[12,45] The only complication encountered in our study was transient nerve root irritation, which subsided after the ELD catheter was withdrawn by 5-10 mm. This declining complication rates, compared with past studies, might be attributive to the evolving techniques for performing the ELD trial. Table 3 presents various techniques reported in the literature. Our technique of titrating the CSF drainage at the rate of 10 ml/h, allowed controlled drainage bringing the post-ELD over-drainage complications including subdural collections and headache to zero.
Table 3.
Various techniques of ELD reported in the literature
Outcomes after VPS
The iNPH guidelines have reported favorable outcome rates ranging from 30% to 96% after VPS surgery.[5] In present study, the rate of improvement in patients undergoing VPS insertion was 92%. The number of comorbidities, history of CVA, and negative ELD were significantly associated with poor outcome. Various other studies have reported that clinical factors including age, severity of dementia, disease duration, number, and type of comorbidities influence and predict the outcome following shunt surgery.[25,27,28] The rate of sustained overall improvement for 3-7 years after the shunt surgery has been reported in the range of 26-91%.[29] The domain most responsive to shunt surgery reported in the literature is gait ataxia (80-88%) followed by cognitive impairment (30-65%), and the lowest rate of improvement is met with urinary incontinence (30-56%).[2,5,6,7,18,25,40,43] We observed sustained improvement in gait, MMSE, bladder control, and daily activities in 76% of the patients. The domain that deteriorated most was MMSE, whereas gait improved substantially in all the patients at mean 3 years follow-up. Similar trend of decline in improvement on long-term follow-up (>1 year) has been reported previously.[13,18,23,29,36,38] This decline in clinical improvement at long-term can be attributed to shunt dysfunction, progression of disease, or other comorbidities.[18,29] The shunt dysfunction necessitates revision surgery, the rate of which is reported equal to 53%, with indications of revision being shunt obstruction, infection, and change of shunt configuration.[35] In the present study, 15% of patients required revision surgery, primarily to change the valve configuration, in patients who were very sensitive to small pressure changes and required tight control of pressure. The reinstalled valve allowed a wide range of adjustment in the pressure changes. The technique of selecting an optimum initial pressure and adjustment during follow-up, based on the CT scan findings of low-pressure changes as well as amelioration or deterioration of the symptoms, has demonstrated decrease in the incidences of subdural fluid collections in our practice. Small subdural collection after shunt insertion has been reported in up to 30% of patients, but 10-15% have symptomatic collection requiring surgery.[34] In our study, the rate of subdural fluid collection was 17%. This was controlled by adjustment of the valve pressure until the maximal response was reached and the response was sustained during subsequent visits. The rate of subdural fluid collections varies depending on the type of valve used. The programmable valves have demonstrated beneficial effect in lowering the incidence of chronic subdural fluid collection. Recent studies have demonstrated that the use of gravitational valve is associated with better pressure control than the programmable valve.[21]
Finally, the ideal cost-effective supplementary test to predict the shunt responsiveness in patients with iNPH is still evolving. The present study provides preliminary results and demonstrates that ELD trial is the optimal supplemental test with high sensitivity, NPV, and PPV. However, the results of this study should be interpreted with caution due to retrospective study design with a relatively smaller number of patients. Further randomized prospective study with large sample size, where the patients undergoes a VPS regardless of ELD results are necessary to confirm the findings of this study and fully address the accuracy of ELD as the sole supplemental test for shunt responsiveness.
CONCLUSIONS
The overall outcome following VPS mainly depends on the selection of patients for CSF diversion surgery. The positive ELD trial predicted shunt responsiveness in 96% patients. The overall rate of improvement in patients undergoing VPS insertion was 92%. The number of comorbidities, history of CVA and negative ELD were significantly associated with poor outcome. The overall improvements were sustained in 76% of patients at mean 3 years follow-up. The ELD trial demonstrated reasonable prognostic accuracy for prediction of shunt-responsiveness in iNPH patients. In addition, low to negligible complications encountered in patients undergoing ELD trial is encouraging. However, further prospective randomized clinical trials are required to assess with certainty the predictive value of ELD.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2014/5/1/12/125860
REFERENCES
- 1.Algin O, Hakyemez B, Parlak M. The efficiency of PC-MRI in diagnosis of normal pressure hydrocephalus and prediction of shunt response. Acad Radiol. 2010;17:181–7. doi: 10.1016/j.acra.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Anile C, De Bonis P, Albanese A, Di Chirico A, Mangiola A, Petrella G, et al. Selection of patients with idiopathic normal-pressure hydrocephalus for shunt placement: A single-institution experience. J Neurosurg. 2010;113:64–73. doi: 10.3171/2010.1.JNS091296. [DOI] [PubMed] [Google Scholar]
- 3.Boon AJ, Tans JT, Delwel EJ, Egeler-Peerdeman SM, Hanlo PW, Wurzer JA, et al. Does CSF outflow resistance predict the response to shunting in patients with normal pressure hydrocephalus? Acta Neurochir Suppl. 1998;71:331–3. doi: 10.1007/978-3-7091-6475-4_96. [DOI] [PubMed] [Google Scholar]
- 4.Bradley WG, Safar FG, Furtado C, Ord J, Alksne JF. Increased intracranial volume: A clue to the etiology of idiopathic normal-pressure hydrocephalus? AJNR Am J Neuroradiol. 2004;25:1479–84. [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett MG, Sonnad SS, Stein SC. Screening tests for normal-pressure hydrocephalus: Sensitivity, specificity, and cost. J Neurosurg. 2006;105:823–9. doi: 10.3171/jns.2006.105.6.823. [DOI] [PubMed] [Google Scholar]
- 6.Cage TA, Auguste KI, Wrensch M, Wu YW, Gupta N. Self-reported functional outcome after surgical intervention in patients with idiopathic normal pressure hydrocephalus. J Clin Neurosci. 2011;18:649–54. doi: 10.1016/j.jocn.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 7.Chen IH, Huang CI, Liu HC, Chen KK. Effectiveness of shunting in patients with normal pressure hydrocephalus predicted by temporary, controlled-resistance, continuous lumbar drainage: A pilot study. J Neurol Neurosurg Psychiatry. 1994;57:1430–2. doi: 10.1136/jnnp.57.11.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Di Lauro L, Mearini M, Bollati A. The predictive value of 5 days CSF diversion for shunting in normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 1986;49:842–3. doi: 10.1136/jnnp.49.7.842-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dixon GR, Friedman JA, Luetmer PH, Quast LM, McClelland RL, Petersen RC, et al. Use of cerebrospinal fluid flow rates measured by phase-contrast MR to predict outcome of ventriculoperitoneal shunting for idiopathic normal-pressure hydrocephalus. Mayo Clin Proc. 2002;77:509–14. doi: 10.4065/77.6.509. [DOI] [PubMed] [Google Scholar]
- 10.Eide PK, Brean A. Cerebrospinal fluid pulse pressure amplitude during lumbar infusion in idiopathic normal pressure hydrocephalus can predict response to shunting. Cerebrospinal Fluid Res. 2010;7:5. doi: 10.1186/1743-8454-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallia GL, Rigamonti D, Williams MA. The diagnosis and treatment of idiopathic normal pressure hydrocephalus. Nat Clin Pract Neurol. 2006;2:375–81. doi: 10.1038/ncpneuro0237. [DOI] [PubMed] [Google Scholar]
- 12.Governale LS, Fein N, Logsdon J, Black PM. Techniques and complications of external lumbar drainage for normal pressure hydrocephalus. Neurosurgery. 2008;63(4 Suppl 2):379–84. doi: 10.1227/01.NEU.0000327023.18220.88. discussion 384. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg JO, Shenkin HA, Adam R. Idiopathic normal pressure hydrocephalus: A report of 73 patients. J Neurol Neurosurg Psychiatry. 1977;40:336–41. doi: 10.1136/jnnp.40.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haan J, Thomeer RT. [Temporary lumbar external drainage in normal pressure hydrocephalus] Tijdschr Gerontol Geriatr. 1989;20:25–8. [PubMed] [Google Scholar]
- 15.Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure. Observations on cerebrospinal fluid hydrodynamics. J Neurol Sci. 1965;2:307–27. doi: 10.1016/0022-510x(65)90016-x. [DOI] [PubMed] [Google Scholar]
- 16.Ishikawa M, Hashimoto M, Mori E, Kuwana N, Kazui H. The value of cerebrospinal fluid tap test for predicting shunt effectiveness in idiopathic normal pressure hydrocephalus. Fluids Barriers CNS. 2012;9:1. doi: 10.1186/2045-8118-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahlon B, Sundbarg G, Rehncrona S. Comparison between the lumbar infusion and CSF tap tests to predict outcome after shunt surgery in suspected normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2002;73:721–6. doi: 10.1136/jnnp.73.6.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilic K, Czorny A, Auque J, Berkman Z. Predicting the outcome of shunt surgery in normal pressure hydrocephalus. J Clin Neurosci. 2007;14:729–36. doi: 10.1016/j.jocn.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 19.Krauss JK, Regel JP, Vach W, Jungling FD, Droste DW, Wakhloo AK. Flow void of cerebrospinal fluid in idiopathic normal pressure hydrocephalus of the elderly: Can it predict outcome after shunting? Neurosurgery. 1997;40:67–73. doi: 10.1097/00006123-199701000-00015. discussion 73-4. [DOI] [PubMed] [Google Scholar]
- 20.Lee WJ, Wang SJ, Hsu LC, Lirng JF, Wu CH, Fuh JL. Brain MRI as a predictor of CSF tap test response in patients with idiopathic normal pressure hydrocephalus. J Neurol. 2010;257:1675–81. doi: 10.1007/s00415-010-5602-8. [DOI] [PubMed] [Google Scholar]
- 21.Lemcke J, Meier U, Muller C, Fritsch MJ, Kehler U, Langer N, et al. Safety and efficacy of gravitational shunt valves in patients with idiopathic normal pressure hydrocephalus: A pragmatic, randomised, open label, multicentre trial (SVASONA) J Neurol Neurosurg Psychiatry. 2013;84:850–7. doi: 10.1136/jnnp-2012-303936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malm J, Kristensen B, Karlsson T, Fagerlund M, Elfverson J, Ekstedt J. The predictive value of cerebrospinal fluid dynamic tests in patients with th idiopathic adult hydrocephalus syndrome. Arch Neurol. 1995;52:783–9. doi: 10.1001/archneur.1995.00540320059013. [DOI] [PubMed] [Google Scholar]
- 23.Malm J, Kristensen B, Stegmayr B, Fagerlund M, Koskinen LO. Three-year survival and functional outcome of patients with idiopathic adult hydrocephalus syndrome. Neurology. 2000;55:576–8. doi: 10.1212/wnl.55.4.576. [DOI] [PubMed] [Google Scholar]
- 24.Marmarou A, Bergsneider M, Klinge P, Relkin N, Black PM. The value of supplemental prognostic tests for the preoperative assessment of idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57(Suppl 3):S17–28. doi: 10.1227/01.neu.0000168184.01002.60. discussion ii-v. [DOI] [PubMed] [Google Scholar]
- 25.Marmarou A, Black P, Bergsneider M, Klinge P, Relkin N. Guidelines for management of idiopathic normal pressure hydrocephalus: Progress to date. Acta Neurochir Suppl. 2005;95:237–40. doi: 10.1007/3-211-32318-x_48. [DOI] [PubMed] [Google Scholar]
- 26.Marmarou A, Young HF, Aygok GA, Sawauchi S, Tsuji O, Yamamoto T, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: A prospective study in 151 patients. J Neurosurg. 2005;102:987–97. doi: 10.3171/jns.2005.102.6.0987. [DOI] [PubMed] [Google Scholar]
- 27.McGirt MJ, Woodworth G, Coon AL, Thomas G, Williams MA, Rigamonti D. Diagnosis, treatment, and analysis of long-term outcomes in idiopathic normal-pressure hydrocephalus. Neurosurgery. 2005;57:699–705. doi: 10.1093/neurosurgery/57.4.699. discussion 699-705. [DOI] [PubMed] [Google Scholar]
- 28.Meier U, Lemcke J. Co-morbidity as a predictor of outcome in patients with idiopathic normal-pressure hydrocephalus. Acta Neurochir Suppl. 2010;106:127–30. doi: 10.1007/978-3-211-98811-4_22. [DOI] [PubMed] [Google Scholar]
- 29.Mirzayan MJ, Luetjens G, Borremans JJ, Regel JP, Krauss JK. Extended long-term (>5 years) outcome of cerebrospinal fluid shunting in idiopathic normal pressure hydrocephalus. Neurosurgery. 2010;67:295–301. doi: 10.1227/01.NEU.0000371972.74630.EC. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima M, Arai H, Miyajima M. Diagnostic value of CSF biomarker profile in idiopathic normal pressure hydrocephalus; leucine-rich alpha-2-glycoprotein is a potential biological marker. Rinsho Shinkeigaku. 2010;50:973–76. doi: 10.5692/clinicalneurol.50.973. [DOI] [PubMed] [Google Scholar]
- 31.Paidakakos N, Borgarello S, Naddeo M. Indications for endoscopic third ventriculostomy in normal pressure hydrocephalus. Acta Neurochir Suppl. 2012;113:123–7. doi: 10.1007/978-3-7091-0923-6_25. [DOI] [PubMed] [Google Scholar]
- 32.Palm WM, Walchenbach R, Bruinsma B, Admiraal-Behloul F, Middelkoop HA, Launer LJ, et al. Intracranial compartment volumes in normal pressure hydrocephalus: Volumetric assessment versus outcome. AJNR Am J Neuroradiol. 2006;27:76–9. [PMC free article] [PubMed] [Google Scholar]
- 33.Panagiotopoulos V, Konstantinou D, Kalogeropoulos A, Maraziotis T. The predictive value of external continuous lumbar drainage, with cerebrospinal fluid outflow controlled by medium pressure valve, in normal pressure hydrocephalus. Acta Neurochir (Wien) 2005;147:953–8. doi: 10.1007/s00701-005-0580-9. discussion 958. [DOI] [PubMed] [Google Scholar]
- 34.Pople IK. Hydrocephalus and shunts: What the neurologist should know. J Neurol Neurosurg Psychiatry. 2002;73(Suppl 1):i17–22. doi: 10.1136/jnnp.73.suppl_1.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pujari S, Kharkar S, Metellus P, Shuck J, Williams MA, Rigamonti D. Normal pressure hydrocephalus: Long-term outcome after shunt surgery. J Neurol Neurosurg Psychiatry. 2008;79:1282–6. doi: 10.1136/jnnp.2007.123620. [DOI] [PubMed] [Google Scholar]
- 36.Raftopoulos C, Massager N, Baleriaux D, Deleval J, Clarysse S, Brotchi J. Prospective analysis by computed tomography and long-term outcome of 23 adult patients with chronic idiopathic hydrocephalus. Neurosurgery. 1996;38:51–9. doi: 10.1097/00006123-199601000-00014. [DOI] [PubMed] [Google Scholar]
- 37.Sankari SE, Fichten A, Gondry-Jouet C, Czosnyka M, Legars D, Deramond H, et al. Correlation between tap test and CSF aqueductal stroke volume in idiopathic normal pressure hydrocephalus. Acta Neurochir Suppl. 2012;113:43–6. doi: 10.1007/978-3-7091-0923-6_9. [DOI] [PubMed] [Google Scholar]
- 38.Stein SC, Langfitt TW. Normal-pressure hydrocephalus. Predicting the results of cerebrospinal fluid shunting. J Neurosurg. 1974;41:463–70. doi: 10.3171/jns.1974.41.4.0463. [DOI] [PubMed] [Google Scholar]
- 39.Tarnaris A, Toma AK, Chapman MD, Keir G, Kitchen ND, Watkins LD. Use of cerebrospinal fluid amyloid-beta and total tau protein to predict favorable surgical outcomes in patients with idiopathic normal pressure hydrocephalus. J Neurosurg. 2011;115:145–50. doi: 10.3171/2011.2.JNS101316. [DOI] [PubMed] [Google Scholar]
- 40.Toma AK, Papadopoulos MC, Stapleton S, Kitchen ND, Watkins LD. Conservative versus surgical management of idiopathic normal pressure hydrocephalus: A prospective double-blind randomized controlled trial: study protocol. Acta Neurochir Suppl. 2012;113:21–3. doi: 10.1007/978-3-7091-0923-6_4. [DOI] [PubMed] [Google Scholar]
- 41.Vanneste J, Augustijn P, Davies GA, Dirven C, Tan WF. Normal-pressure hydrocephalus. Is cisternography still useful in selecting patients for a shunt? Arch Neurol. 1992;49:366–70. doi: 10.1001/archneur.1992.00530280046021. [DOI] [PubMed] [Google Scholar]
- 42.Vanneste J, Augustijn P, Tan WF, Dirven C. Shunting normal pressure hydrocephalus: the predictive value of combined clinical and CT data. J Neurol Neurosurg Psychiatry. 1993;56:251–6. doi: 10.1136/jnnp.56.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanneste JA. Diagnosis and management of normal-pressure hydrocephalus. J Neurol. 2000;247:5–14. doi: 10.1007/s004150050003. [DOI] [PubMed] [Google Scholar]
- 44.Virhammar J, Cesarini KG, Laurell K. The CSF tap test in normal pressure hydrocephalus: Evaluation time, reliability and the influence of pain. Eur J Neurol. 2012;19:271–6. doi: 10.1111/j.1468-1331.2011.03486.x. [DOI] [PubMed] [Google Scholar]
- 45.Walchenbach R, Geiger E, Thomeer RT, Vanneste JA. The value of temporary external lumbar CSF drainage in predicting the outcome of shunting on normal pressure hydrocephalus. J Neurol Neurosurg Psychiatry. 2002;72:503–6. doi: 10.1136/jnnp.72.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]


